Microvascular Changes in the Retina Correlate with MRI Markers in Patients with Early-Onset Dementia
Abstract
1. Introduction
2. Methods
2.1. Participants and Study Design
2.2. MRI Protocols and Imaging Analysis
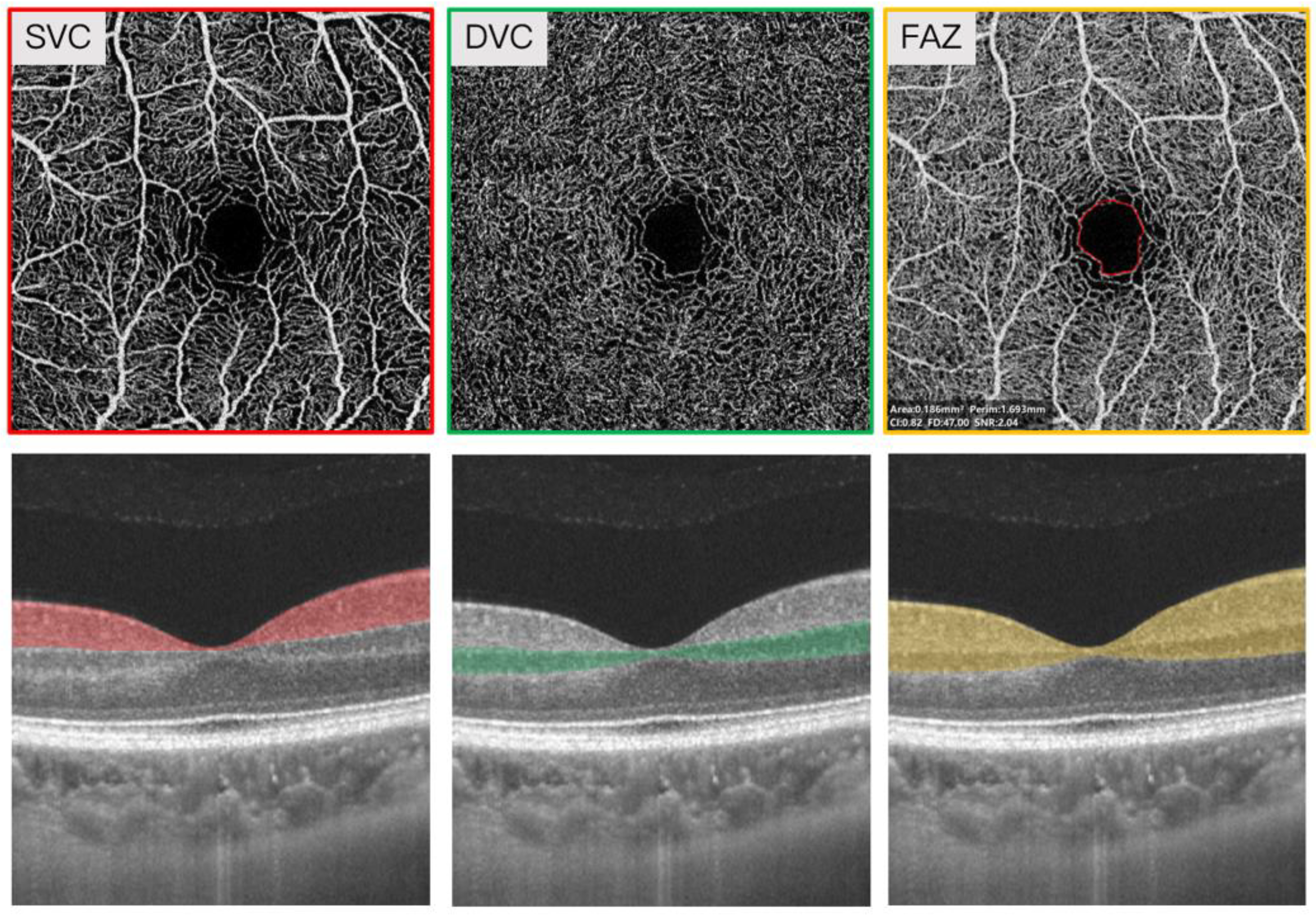
2.3. Swept-Source Optical Coherence Tomography Angiography Imaging
2.4. Statistical analyses
3. Results
4. Discussion
5. Limitation and Strengths
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frisoni, G.B.; Fox, N.C.; Jack, C.R.; Scheltens, P.; Thompson, P.M. The clinical use of structural MRI in Alzheimer disease. Nat. Rev. Neurol. 2010, 6, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Trejo-Lopez, J.A.; Yachnis, A.T.; Prokop, S. Neuropathology of Alzheimer’s Disease. Neurotherapeutics 2022, 19, 173–185. [Google Scholar] [CrossRef]
- Tondelli, M.; Wilcock, G.K.; Nichelli, P.; De Jager, C.A.; Jenkinson, M.; Zamboni, G. Structural MRI changes detectable up to ten years before clinical Alzheimer’s disease. Neurobiol. Aging 2012, 33, 825.e25–825.e36. [Google Scholar] [CrossRef] [PubMed]
- Woodworth, D.C.; Sheikh-Bahaei, N.; Scambray, K.A.; Phelan, M.J.; Perez-Rosendahl, M.; Corrada, M.M.; Kawas, C.H.; Sajjadi, S.A.; Alzheimer’s Disease Neuroimaging Initiative. Dementia is associated with medial temporal atrophy even after accounting for neuropathologies. Brain Commun. 2022, 4, fcac052. [Google Scholar] [CrossRef] [PubMed]
- Visser, P.J.; Verhey, F.R.J.; Hofman, P.A.M.; Scheltens, P.; Jolles, J. Medial temporal lobe atrophy predicts Alzheimer’s disease in patients with minor cognitive impairment. J. Neurol. Neurosurg. Psychiatry 2002, 72, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Brickman, A.M.; Honig, L.S.; Scarmeas, N.; Tatarina, O.; Sanders, L.; Albert, M.S.; Brandt, J.; Blacker, D.; Stern, Y. Measuring Cerebral Atrophy and White Matter Hyperintensity Burden to Predict the Rate of Cognitive Decline in Alzheimer Disease. Arch. Neurol. 2008, 65, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Garnier-Crussard, A.; Bougacha, S.; Wirth, M.; Dautricourt, S.; Sherif, S.; Landeau, B.; Gonneaud, J.; De Flores, R.; de la Sayette, V.; Vivien, D.; et al. White matter hyperintensity topography in Alzheimer’s disease and links to cognition. Alzheimers Dement. 2022, 18, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Appleton, J.P.; Woodhouse, L.J.; Adami, A.; Becker, J.L.; Berge, E.; Cala, L.A.; Casado, A.M.; Caso, V.; Christensen, H.K.; Dineen, R.A.; et al. Imaging markers of small vessel disease and brain frailty, and outcomes in acute stroke. Neurology 2020, 94, e439–e452. [Google Scholar] [CrossRef] [PubMed]
- Raman, M.R.; Kantarci, K.; Murray, M.; Jack, C.R., Jr.; Vemuri, P. Imaging markers of cerebrovascular pathologies: Pathophysiology, clinical presentation, and risk factors. Alzheimers Dementia Diagn. Assess. Dis. Monit. 2016, 5, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C. The Pathobiology of Vascular Dementia. Neuron 2013, 80, 844–866. [Google Scholar] [CrossRef] [PubMed]
- De Silva, T.; Faraci, F.M. Microvascular Dysfunction and Cognitive Impairment. Cell Mol. Neurobiol. 2016, 36, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Raz, L.; Knoefel, J.E.; Bhaskar, K. The neuropathology and cerebrovascular mechanisms of dementia. J. Cereb. Blood Flow Metab. 2016, 36, 172–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Murphy, O.; Caldito, N.G.; Calabresi, P.A.; Saidha, S. Emerging Applications of Optical Coherence Tomography Angiography (OCTA) in neurological research. Eye Vis. 2018, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Alber, J.; Goldfarb, D.; Thompson, L.I.; Arthur, E.; Hernandez, K.; Cheng, D.; DeBuc, D.C.; Cordeiro, F.; Provetti-Cunha, L.; Haan, J.D.; et al. Developing retinal biomarkers for the earliest stages of Alzheimer’s disease: What we know, what we don’t, and how to move forward. Alzheimers Dement. 2020, 16, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.Y.; Mok, V.; Foster, P.J.; Trucco, E.; Chen, C.; Wong, T.Y. Retinal imaging in Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2021, 92, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.B.; Chitranshi, N.; den Haan, J.; Mirzaei, M.; You, Y.; Lim, J.K.; Basavarajappa, D.; Godinez, A.; Di Angelantonio, S.; Sachdev, P.; et al. Retinal changes in Alzheimer’s disease- integrated prospects of imaging, functional and molecular advances. Prog. Retin. Eye Res. 2020, 82, 100899. [Google Scholar] [CrossRef] [PubMed]
- Vij, R.; Arora, S. A systematic survey of advances in retinal imaging modalities for Alzheimer’s disease diagnosis. Metab. Brain Dis. 2022, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, X.; Azhati, G.; Li, T.; Xu, G.; Liu, F. Retinal microvascular attenuation in mental cognitive impairment and Alzheimer’s disease by optical coherence tomography angiography. Acta Ophthalmol. 2020, 98, e781–e787. [Google Scholar] [CrossRef] [PubMed]
- Chua, J.; Hu, Q.; Ke, M.; Tan, B.; Hong, J.; Yao, X.; Hilal, S.; Venketasubramanian, N.; Garhöfer, G.; Cheung, C.Y.; et al. Retinal microvasculature dysfunction is associated with Alzheimer’s disease and mild cognitive impairment. Alzheimers Res. Ther. 2020, 12, 161. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, Q.; Tao, R.; Lu, H.; Xiao, Z.; Zheng, L.; Ding, D.; Ding, S.; Ma, Y.; Lu, Z.; et al. Decreased Retinal Vascular Density in Alzheimer’s Disease (AD) and Mild Cognitive Impairment (MCI): An Optical Coherence Tomography Angiography (OCTA) Study. Front. Aging Neurosci. 2021, 12, 572484. [Google Scholar] [CrossRef] [PubMed]
- Hui, J.; Zhao, Y.; Yu, S.; Liu, J.; Chiu, K.; Wang, Y. Detection of retinal changes with optical coherence tomography angiography in mild cognitive impairment and Alzheimer’s disease patients: A meta-analysis. PLoS ONE 2021, 16, e0255362. [Google Scholar] [CrossRef] [PubMed]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. J. Alzheimers Assoc. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Masellis, M.; Sherborn, K.; Rosa-Neto, P.; Sadovnick, D.A.; Hsiung, G.-Y.R.; Black, S.; Prasad, S.; Williams, M.; Gauthier, S. Early-onset dementias: Diagnostic and etiological considerations. Alzheimers Res. Ther. 2013, 5 (Suppl. S1), S7–S22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Kwapong, W.R.; Yang, T.; Liu, P.; Tuo, Q.; Cheng, Y.; Li, X.; Liu, M.; Lei, P.; Wu, B. Choriocapillaris Changes Are Correlated With Disease Duration and MoCA Score in Early-Onset Dementia. Front. Aging Neurosci. 2021, 13, 656750. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef]
- Fazekas, F.; Chawluk, J.B.; Alavi, A.; Hurtig, H.I.; Zimmerman, R.A. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am. J. Roentgenol. 1987, 149, 351–356. [Google Scholar] [CrossRef]
- Frisoni, G.B.; Scheltens, P.; Galluzzi, S.; Nobili, F.; Fox, N.; Robert, P.; Soininen, H.; Wahlund, L.-O.; Waldemar, G.; Salmon, E. Neuroimaging tools to rate regional atrophy, subcortical cerebrovascular disease, and regional cerebral blood flow and metabolism: Consensus paper of the EADC. J. Neurol. Neurosurg. Psychiatry 2003, 74, 1371–1381. [Google Scholar] [CrossRef]
- Koedam, E.L.G.E.; Lehmann, M.; Van Der Flier, W.M.; Scheltens, P.; Pijnenburg, Y.A.L.; Fox, N.; Barkhof, F.; Wattjes, M.P. Visual assessment of posterior atrophy development of a MRI rating scale. Eur. Radiol. 2011, 21, 2618–2625. [Google Scholar] [CrossRef]
- Sharma, R.; DeAraugo, S.; Infeld, B.; O’Sullivan, R.; Gerraty, R.P. Cerebral amyloid angiopathy: Review of clinico-radiological features and mimics. J. Med. Imaging Radiat. Oncol. 2018, 62, 451–463. [Google Scholar] [CrossRef]
- Kwapong, W.R.; Jiang, S.; Yan, Y.; Wan, J.; Wu, B. Macular Microvasculature Is Associated with Total Cerebral Small Vessel Disease Burden in Recent Single Subcortical Infarction. Front. Aging Neurosci. 2022, 13, 787775. [Google Scholar] [CrossRef]
- Leite, A.B.; Scheltens, P.; Barkhof, F. Pathological Aging of the Brain. Top. Magn. Reson. Imaging 2004, 15, 369–389. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Kalaria, R.N. The role of cerebral ischemia in Alzheimer’s disease. Neurobiol. Aging 2000, 21, 321–330. [Google Scholar] [CrossRef]
- O’Bryhim, B.E.; Lin, J.B.; Van Stavern, G.P.; Apte, R.S. OCT Angiography Findings in Preclinical Alzheimer’s Disease: 3-Year Follow-Up. Ophthalmology 2021, 128, 1489–1491. [Google Scholar] [CrossRef] [PubMed]
- Zabel, P.; Kaluzny, J.J.; Wilkosc-Debczynska, M.; Gebska-Toloczko, M.; Suwala, K.; Zabel, K.; Zaron, A.; Kucharski, R.; Araszkiewicz, A. Comparison of Retinal Microvasculature in Patients with Alzheimer’s Disease and Primary Open-Angle Glaucoma by Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3447–3455. [Google Scholar] [CrossRef]
- O’Bryhim, B.E.; Apte, R.S.; Kung, N.; Coble, D.; Van Stavern, G.P. Association of Preclinical Alzheimer Disease With Optical Coherence Tomographic Angiography Findings. JAMA Ophthalmol. 2018, 136, 1242–1248. [Google Scholar] [CrossRef]
- Pantoni, L.; Poggesi, A.; Inzitari, D. The relation between white-matter lesions and cognition. Curr. Opin. Neurol. 2007, 20, 390–397. [Google Scholar] [CrossRef]
- Fazekas, F.; Kleinert, R.; Offenbacher, H.; Schmidt, R.; Payer, F.; Radner, H.; Lechner, H. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology 1993, 43, 1683–1689. [Google Scholar] [CrossRef]
- Snyder, P.J.; Alber, J.; Alt, C.; Bain, L.J.; Bouma, B.E.; Bouwman, F.H.; DeBuc, D.C.; Campbell, M.C.; Carrillo, M.C.; Chew, E.Y.; et al. Retinal imaging in Alzheimer’s and neurodegenerative diseases. Alzheimers Dement. 2021, 17, 103–111. [Google Scholar] [CrossRef]
- Lin, J.; Wang, D.; Lan, L.; Fan, Y. Multiple Factors Involved in the Pathogenesis of White Matter Lesions. BioMed. Res. Int. 2017, 2017, 9372050. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Hernández, M.C.V.; Muñoz-Maniega, S. What are White Matter Hyperintensities Made of? J. Am. Hear Assoc. 2015, 4, 001140. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, H.; Ikeda, M.; Nagashima, K.; Fujita, Y.; Makioka, K.; Tsukagoshi, S.; Yamazaki, T.; Takai, E.; Sanada, E.; Kobayashi, A.; et al. Deep White Matter Lesions Are Associated with Early Recognition of Dementia in Alzheimer’s Disease. J. Alzheimers Dis. 2019, 68, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Koronyo, Y.; Biggs, D.; Barron, E.; Boyer, D.S.; Pearlman, J.A.; Au, W.J.; Kile, S.J.; Blanco, A.; Fuchs, D.-T.; Ashfaq, A.; et al. Retinal amyloid pathology and proof-of-concept imaging trial in Alzheimer’s disease. JCI Insight 2017, 2, e93621. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.; Georgakis, M.K.; Neitzel, J.; Rannikmäe, K.; Ewers, M.; Seshadri, S.; Sudlow, C.L.; Dichgans, M. Midlife vascular risk factors and risk of incident dementia: Longitudinal cohort and Mendelian randomization analyses in the UK Biobank. Alzheimers Dement. 2021, 17, 1422–1431. [Google Scholar] [CrossRef]
- Lee, C.J.; Lee, J.-Y.; Han, K.; Kim, D.H.; Cho, H.; Kim, K.J.; Kang, E.S.; Cha, B.-S.; Lee, Y.-H.; Park, S. Blood Pressure Levels and Risks of Dementia: A Nationwide Study of 4.5 Million People. Hypertension 2022, 79, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Reinke, C.; Buchmann, N.; Fink, A.; Tegeler, C.; Demuth, I.; Doblhammer, G. Diabetes duration and the risk of dementia: A cohort study based on German health claims data. Age Ageing 2022, 51, afab231. [Google Scholar] [CrossRef] [PubMed]
- Amidei, C.B.; Fayosse, A.; Dumurgier, J.; Machado-Fragua, M.D.; Tabak, A.G.; van Sloten, T.; Kivimäki, M.; Dugravot, A.; Sabia, S.; Singh-Manoux, A. Association Between Age at Diabetes Onset and Subsequent Risk of Dementia. JAMA 2021, 325, 1640–1649. [Google Scholar] [CrossRef]
- Gouliopoulos, N.; Siasos, G.; Moschos, M.M.; Oikonomou, E.; Rouvas, A.; Bletsa, E.; Stampouloglou, P.; Siasou, G.; Paraskevopoulos, T.; Vlasis, K.; et al. Endothelial dysfunction and impaired arterial wall properties in patients with retinal vein occlusion. Vasc. Med. 2020, 25, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.P.; Zhang, M.; Hwang, T.; Bailey, S.T.; Wilson, D.J.; Jia, Y.; Huang, D. Detailed Vascular Anatomy of the Human Retina by Projection-Resolved Optical Coherence Tomography Angiography. Sci. Rep. 2017, 7, srep42201. [Google Scholar] [CrossRef] [PubMed]
| Descriptive | |
|---|---|
| Number | 63 |
| Gender, M | 27 |
| Age, years | 60.43 ± 5.80 |
| Systolic blood pressure, mmHg | 126.84 ± 11.09 |
| Diastolic blood pressure, mmHg | 78.39 ± 7.21 |
| Hypertension, n | 11 |
| Diabetes, n | 4 |
| Education, years | 9 (6 -12) |
| Duration, years | 2 (1–3) |
| MMSE | 14 (9–20) |
| MoCA | 9 (6–15) |
| FAZ area, mm² | 0.35 ± 0.13 |
| SVC, % | 42.37 ± 5.52 |
| DVC, % | 48.47 ± 4.50 |
| MTA | 2 (1–2) |
| PCA | 1 (1–2) |
| GCA | 1 (1–2) |
| PWMH | 1 (0–1) |
| DWMH | 1 (0–1) |
| CAA, n | 5 |
| SVC | DVC | FAZ | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B | SE | p-Value | B | SE | p-Value | B | SE | p-Value | |
| MTA | −0.024 | 0.027 | 0.374 | −0.046 | 0.037 | 0.208 | 2.362 | 1.095 | 0.031 |
| PCA | 0.001 | 0.024 | 0.953 | 0.025 | 0.03 | 0.398 | −0.637 | 0.924 | 0.491 |
| GCA | −0.021 | 0.02 | 0.285 | −0.013 | 0.023 | 0.562 | 0.894 | 0.584 | 0.126 |
| PWMH | 0.027 | 0.013 | 0.032 | 0.032 | 0.017 | 0.052 | −0.5 | 0.686 | 0.466 |
| DWMH | 0.037 | 0.014 | 0.007 | 0.038 | 0.016 | 0.018 | −1.715 | 0.535 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Liu, P.; Kwapong, W.R.; Wu, B.; Liu, M.; Zhang, S. Microvascular Changes in the Retina Correlate with MRI Markers in Patients with Early-Onset Dementia. Brain Sci. 2022, 12, 1391. https://doi.org/10.3390/brainsci12101391
Zhang Z, Liu P, Kwapong WR, Wu B, Liu M, Zhang S. Microvascular Changes in the Retina Correlate with MRI Markers in Patients with Early-Onset Dementia. Brain Sciences. 2022; 12(10):1391. https://doi.org/10.3390/brainsci12101391
Chicago/Turabian StyleZhang, Ziyi, Peng Liu, William Robert Kwapong, Bo Wu, Ming Liu, and Shuting Zhang. 2022. "Microvascular Changes in the Retina Correlate with MRI Markers in Patients with Early-Onset Dementia" Brain Sciences 12, no. 10: 1391. https://doi.org/10.3390/brainsci12101391
APA StyleZhang, Z., Liu, P., Kwapong, W. R., Wu, B., Liu, M., & Zhang, S. (2022). Microvascular Changes in the Retina Correlate with MRI Markers in Patients with Early-Onset Dementia. Brain Sciences, 12(10), 1391. https://doi.org/10.3390/brainsci12101391







