Declarative Memory Impairment and Emotional Bias in Recurrent Depression with a Seasonal Pattern: The Interplay between Emotion and Cognition in Seasonal Affective Disorder
Abstract
1. Introduction
2. Materials and Methods
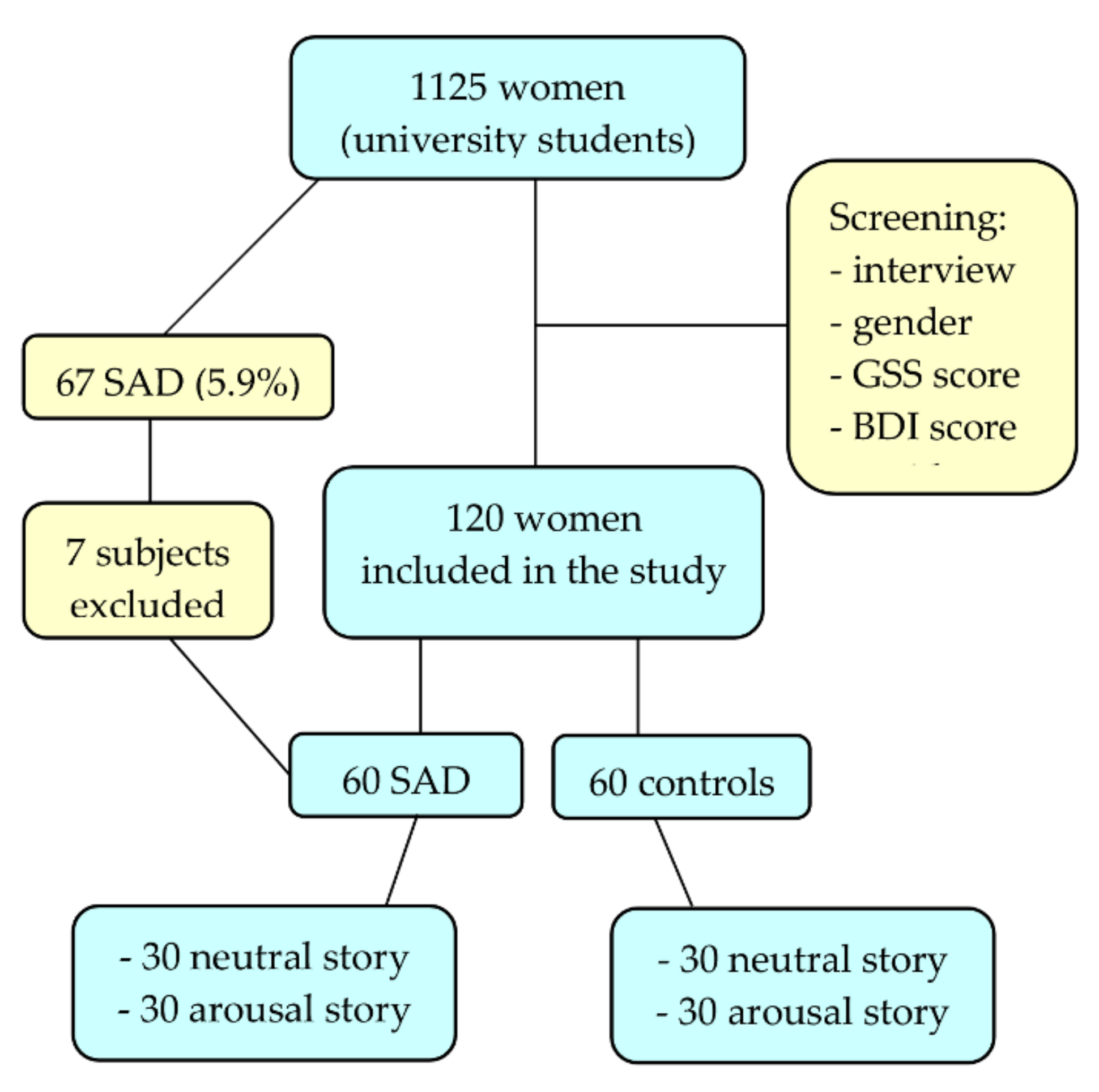
2.1. Participants
2.2. Stimulus Material
2.3. Procedure
2.3.1. Emotional Rating
2.3.2. Memory Test
Free-Recall Test
Recognition Memory Test
2.3.3. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics of the Sample
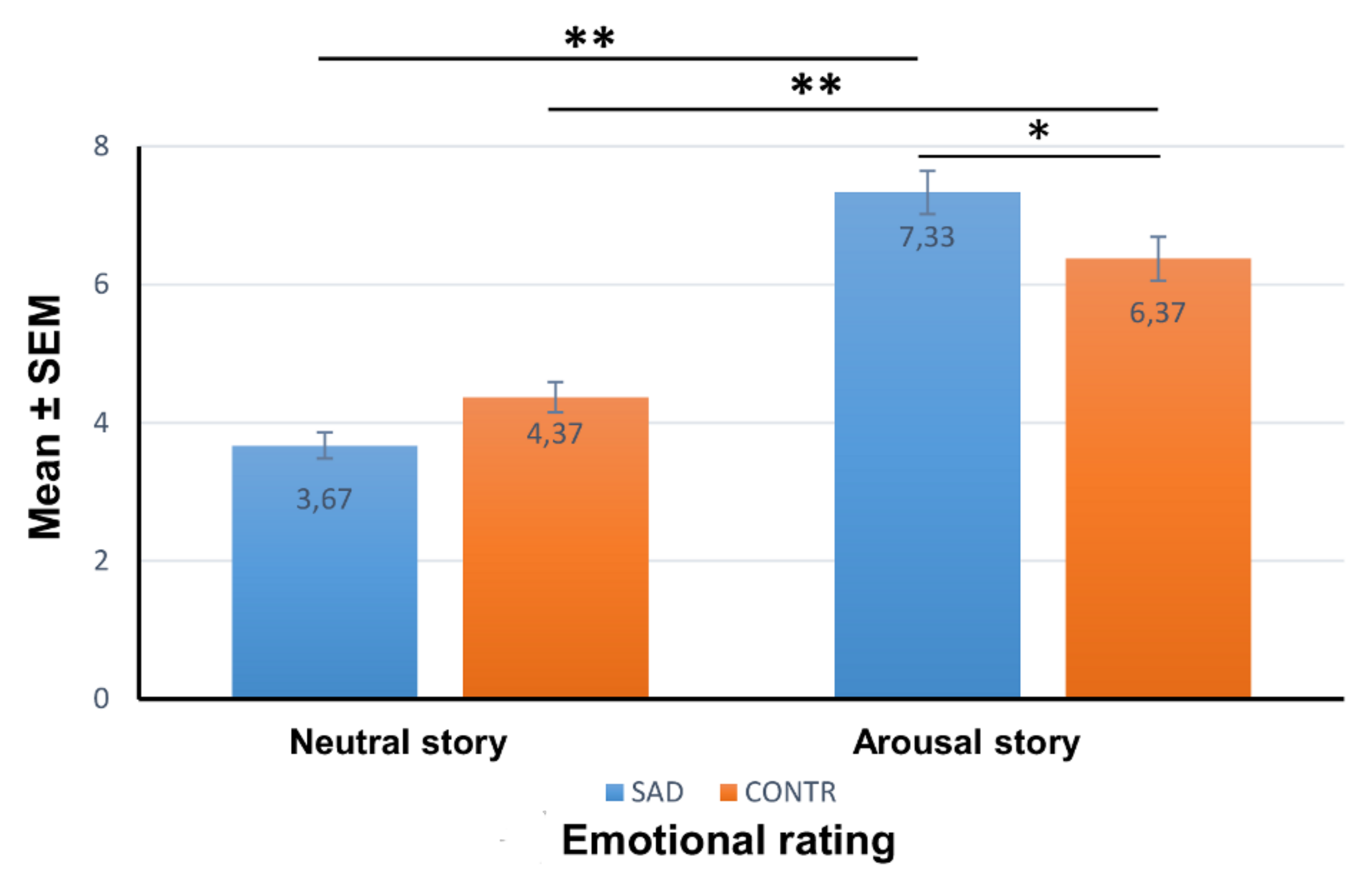
3.2. Emotional Rating of the Stories
3.3. Free Recall of the Stories
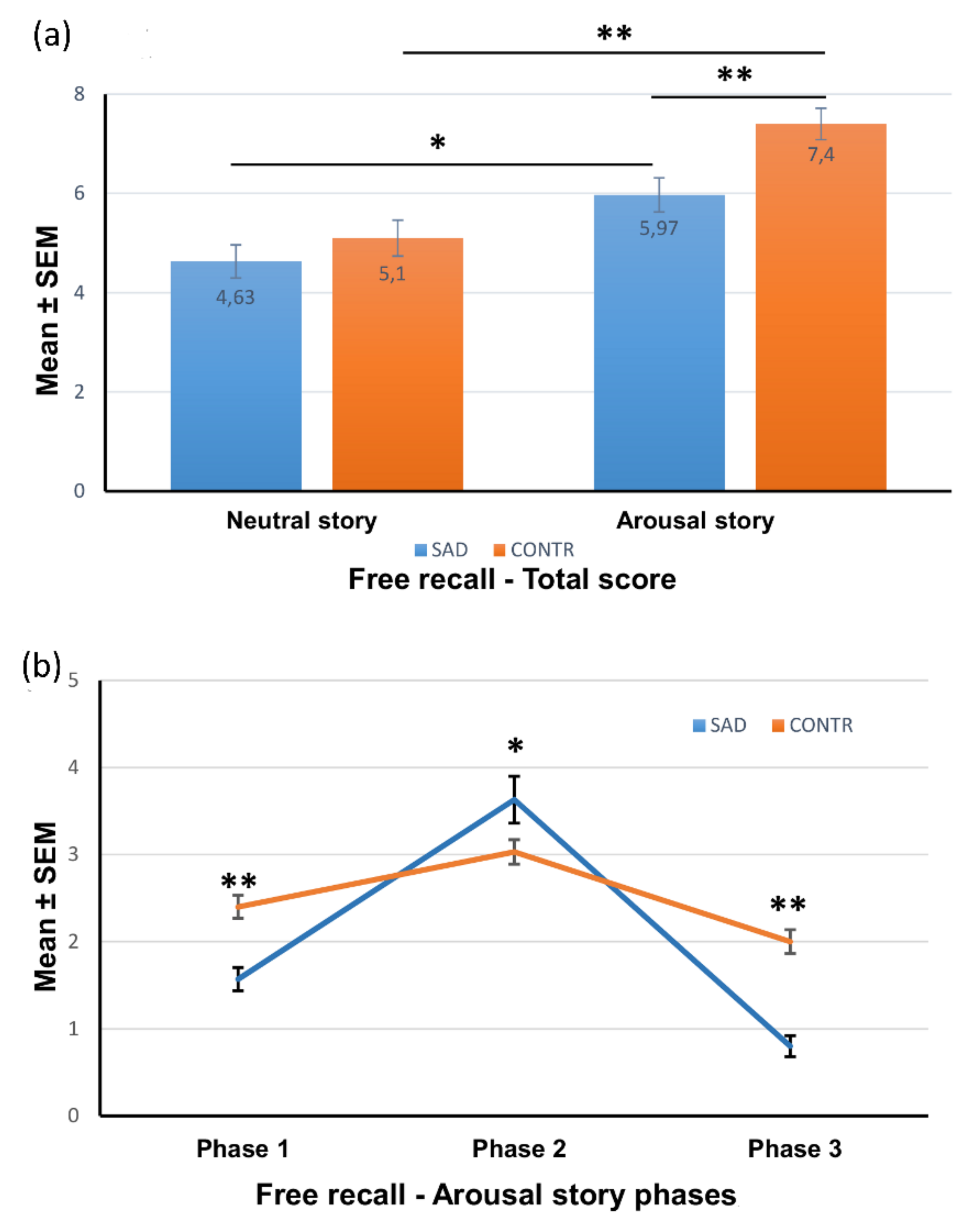
3.3.1. Total Free Recall
3.3.2. Free Recall for Each Phase
3.4. Recognition Memory of the Stories (Questionnaire)
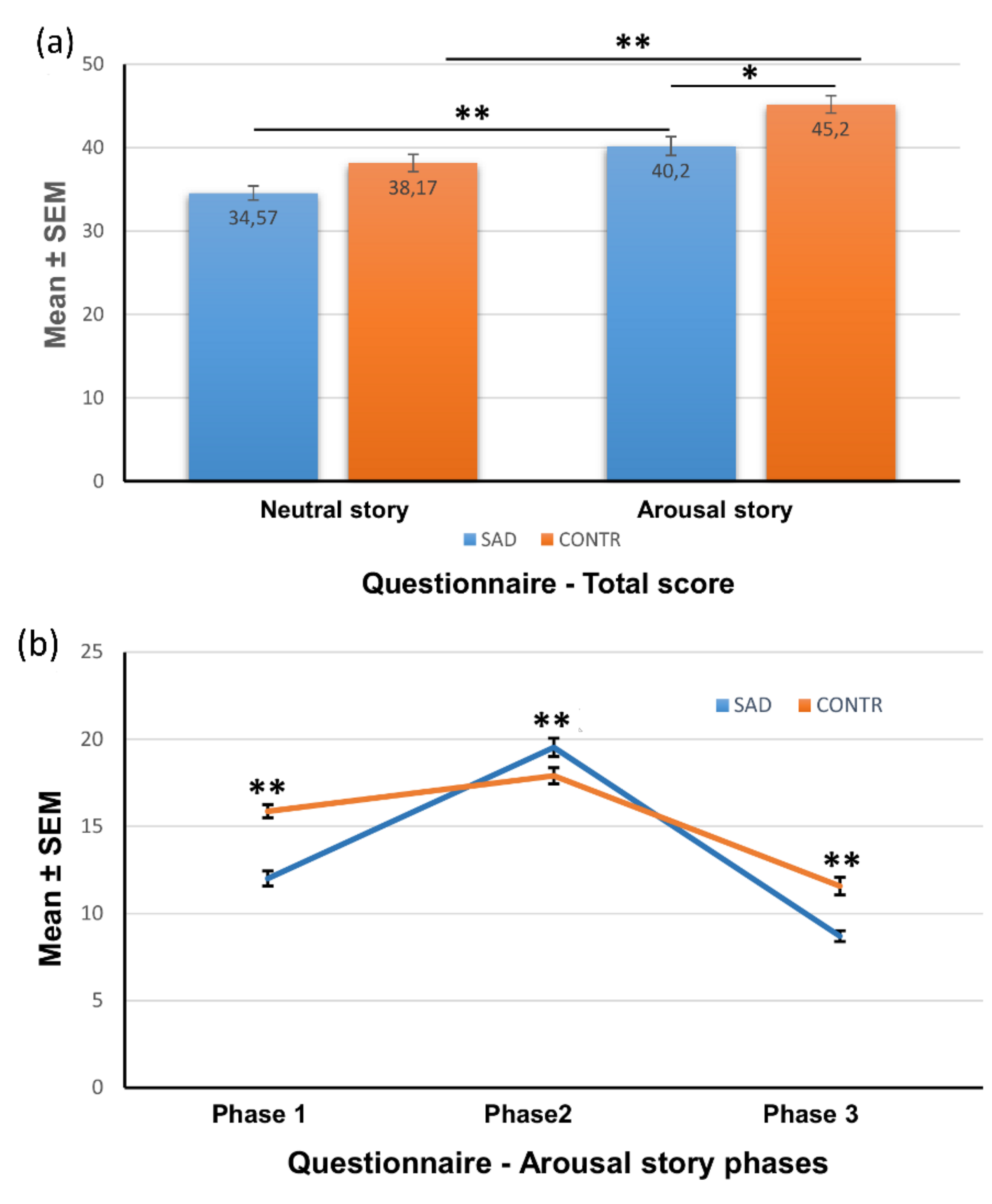
3.4.1. Total Recognition Memory
3.4.2. Recognition Memory for Each Phase
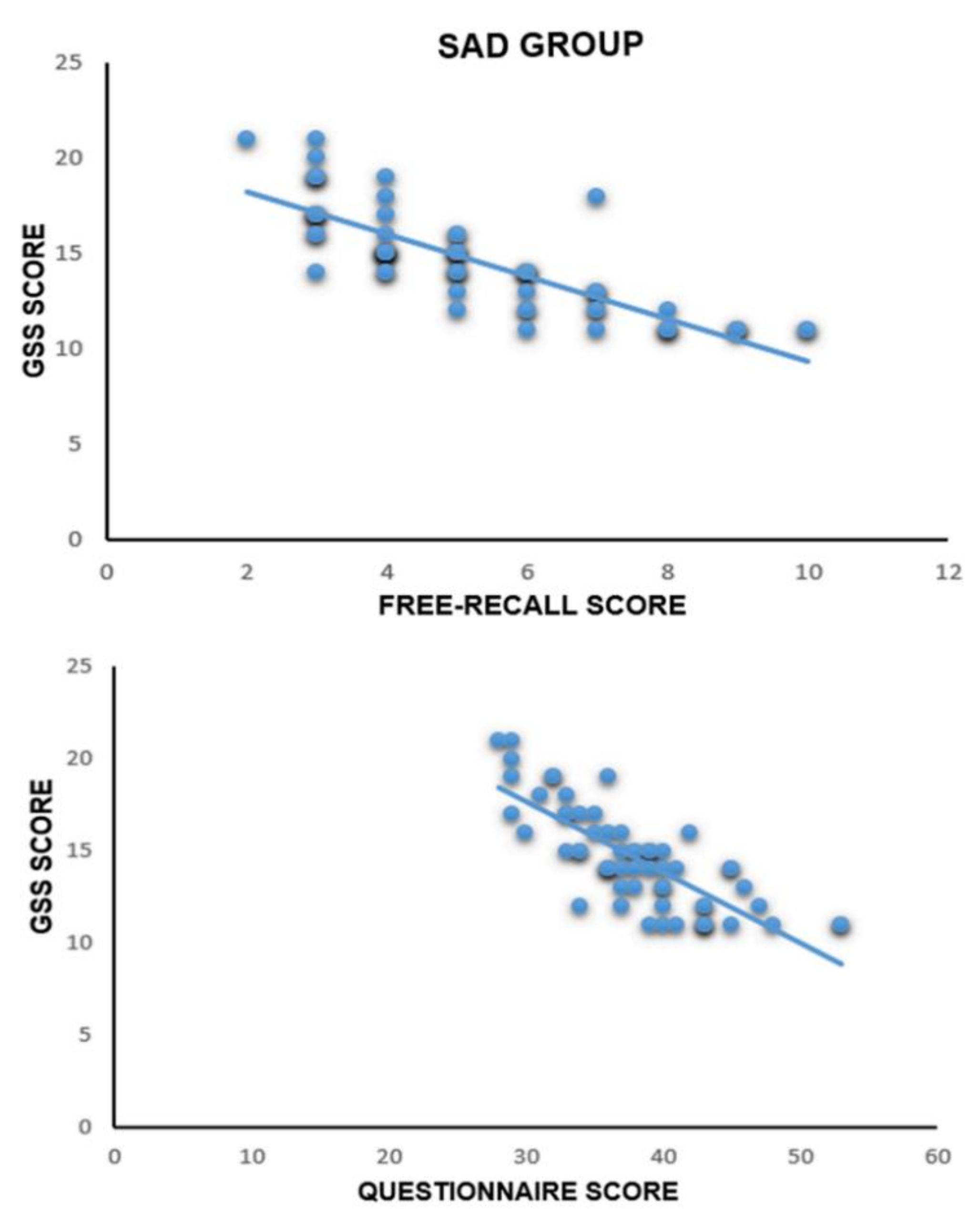
3.5. Correlational Analysis between GSS Values and Free-Recall Test and Recognition Test
4. Discussion
4.1. This Work
4.2. Contributions
4.3. Limitations
4.4. Future Works
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SAD | Seasonal affective disorder |
| MDD | Major depressive disorder |
| SPAQ | Seasonal Pattern Assessment Questionnaire |
| BDI-II | Beck Depression Inventory-II |
| NS | Neutral story |
| AS | Arousal story |
| GSS | Global seasonality score |
| ANOVA | Analysis of variance |
References
- Kasper, S.; Wehr, T.A.; Bartko, J.J.; Gaist, P.A.; Rosenthal, N.E. Epidemiological findings of seasonal changes in mood and behavior: A telephone survey of Montgomery County, Maryland. Arch. Gen. Psychiatry 1989, 46, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Fellinger, M.; Waldhoer, T.; König, D.; Hinterbuchinger, B.; Pruckner, N.; Baumgartner, J.; Vyssoki, S.; Vyssoki, B. Seasonality in bipolar disorder: Effect of sex and age. J. Affect. Disord. 2019, 243, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Hinterbuchinger, B.; König, D.; Gmeiner, A.; Listabarth, S.; Fellinger, M.; Thenius, C.; Baumgartner, J.S.; Vyssoki, S.; Waldhoer, T.; Vyssoki, B.; et al. Seasonality in schizophrenia-An analysis of a nationwide registry with 110,735 hospital admissions. Eur. Psychiatry 2020, 63, e55. [Google Scholar] [CrossRef]
- Riccobono, G.; Iannitelli, A.; Pompili, A.; Iorio, C.; Stratta, P.; Rossi, R.; Bersani, G.; Pacitti, F. Night Eating Syndrome, circadian rhythms and seasonality: A study in a population of Italian university students. Riv. Psichiatr. 2020, 55, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Winthorst, W.H.; Bos, E.H.; Roest, A.M.; de Jonge, P. Seasonality of mood and affect in a large population sample. PLoS ONE 2020, 15, e0239033. [Google Scholar] [CrossRef] [PubMed]
- Riccobono, G.; Pompili, A.; Iannitelli, A.; Pacitti, F. The relationship between Night Eating Syndrome, depression and chronotype in a non-clinical adolescent population. Riv. Psichiatr. 2019, 10, e01666. [Google Scholar] [CrossRef]
- Ajdacic-Gross, V.; Bopp, M.; Ring, M.; Gutzwiller, F.; Rossler, W. Seasonality in suicide—A review and search of new concepts for explaining the heterogeneous phenomena. Soc. Sci. Med. 2010, 71, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Pacitti, F.; Russo, D.; Iannitelli, A.; Bersani, G.; Pancheri, P. Seasonal affective disorder and premenstrual syndrome. Ital. J. Psychopatol. 2006, 12, 186–193. [Google Scholar]
- Rosenthal, N.E.; Sack, D.A.; Gillin, J.C.; Lewy, A.J.; Goodwin, F.K.; Davenport, Y.; Mueller, P.S.; Newsome, D.A.; Wehr, T.A. Seasonal affective disorder. A description of the syndrome and preliminary findings with light therapy. Arch. Gen. Psychiatry 1984, 41, 72–80. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar]
- Magnusson, A. An overview of epidemiological studies on seasonal affective disorder. Acta Psychiatr. Scand. 2000, 101, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.D.; Miller, A.M.; Woesner, M.E. Bright Light Therapy: Seasonal Affective Disorder and Beyond. Einstein J. Biol. Med. 2017, 32, E13–E25. [Google Scholar] [PubMed]
- Tonon, A.C.; Pilz, L.K.; Markus, R.P.; Hidalgo, M.P.; Elisabetsky, E. Melatonin and depression: A translational perspective fron animal Models to clinical. Front. Psychiatry 2021, 12, 638981. [Google Scholar] [CrossRef]
- Melrose, S. Seasonal Affective Disorder: An Overview of Assessment and Treatment approaches. Depress Res. Treat. 2015, 2015, 178564. [Google Scholar] [CrossRef]
- Lewy, A.J. Melatonin and human chronobiology. Cold Spring. Harb. Symp. Quant. Biol. 2007, 72, 623–636. [Google Scholar] [CrossRef]
- Garbazza, C.; Benedetti, F. Genetic Factors Affecting Seasonality, Mood, and the Circadian Clock. Front. Endocrinol. 2018, 9, 481. [Google Scholar] [CrossRef] [PubMed]
- Roecklein, K.A.; Wong, P.M.; Miller, M.A.; Donofry, S.D.; Kamarck, M.L.; Brainard, G.C. Melanopsin, photosensitive ganglion cells, and seasonal affective disorder. Neurosci. Biobehav. Rev. 2013, 37, 229–239. [Google Scholar] [CrossRef]
- Mc Mahon, B.; Andersen, S.B.; Madsen, M.K.; Hjordt, L.V.; Hageman, I.; Dam, H.; Svarer, C.; da Cunha-Bang, S.; Baaré, W.; Madsen, J.; et al. Seasonal difference in brain serotonin transporter binding predicts symptom severity in patients with seasonal affective disorder. Brain 2016, 139, 1605–1614. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Sharma, P.K.; Garg, V.K.; Singh, A.K.; Mondal, S.C. Role of serotonin in seasonal affective disorder. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 49–55. [Google Scholar]
- Praschak-Rieder, N.; Willeit, M.; Wilson, A.A.; Houle, S.; Meyer, J.H. Seasonal variation in human brain serotonin transporter binding. Arch. Gen. Psychiatry 2008, 65, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Lambert, G.W.; Reid, C.; Kaye, D.M.; Jennings, G.L.; Esler, M.D. Effect of sunlight and season on serotonin turnover in the brain. Lancet 2002, 360, 1840–1842. [Google Scholar] [CrossRef]
- Golden, A.M.; Dalgleish, T.; Spinks, H. Dysfunctional attitudes in seasonal affective disorder. Behav. Res. Ther. 2006, 44, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Sigmon, S.T.; Pells, J.J.; Schartel, J.G.; Hermann, B.A.; Endfield, T.M.; LaMattina, S.M.; Boulard, N.E.; Withcomb-Smith, S.R. Stress reactivity and coping in seasonal and nonseasonal depression. Behav. Res. Ther. 2007, 45, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Rohan, K.J.; Sigmon, S.T.; Dorhofer, D.M. Cognitive-behavioral factors in seasonal affective disorder. J. Consult. Clin. Psychol. 2003, 71, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Young, M.A.; Azam, O.A. Ruminative response style and the severity of Seasonal Affective Disorder. Cogn. Ther. Res. 2003, 27, 223–232. [Google Scholar] [CrossRef]
- Perini, G.; Cotta Ramusino, M.; Sinforiani, E.; Bernini, S.; Petrachi, R.; Costa, A. Cognitive impairment in depression: Recent advances and novel treatments. Neuropsychiatr. Dis. Treat. 2019, 15, 1249–1258. [Google Scholar] [CrossRef]
- Rock, P.L.; Roiser, J.P.; Riedel, W.J.; Blackwell, A.D. Cognitive impairment in depression: A systematic review and meta-analysis. Psychol. Med. 2014, 44, 2029–2040. [Google Scholar] [CrossRef]
- Hjordt, L.V.; Stenbæk, D.S.; Ozenne, B.; Mc Mahon, B.; Hageman, I.; Hasselbalch, S.G.; Knudsen, G.M. Season-independent cognitive deficits in seasonal affective disorder and their relation to depressive symptoms. Psychiatry Res. 2017, 257, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Hjordt, L.V.; Stenbaek, D.S.; Madsen, K.S.; Mc Mahon, B.; Jensen, C.G.; Vestergaard, M.; Hageman, I.; Meder, D.; Hasselbalch, S.G.; Knudsen, G.M. State-dependent alterations in inhibitory control and emotional face identification in seasonal affective disorder. J. Abnorm. Psychol. 2017, 126, 291–300. [Google Scholar] [CrossRef]
- Jensen, C.G.; Hjordt, L.V.; Stenbæk, D.S.; Andersen, E.; Back, S.K.; Lansner, J.; Hageman, I.; Dam, H.; Nielsen, A.P.; Knudsen, G.M.; et al. Development and psychometric validation of the verbal affective memory test. Memory 2015, 24, 1208–1223. [Google Scholar] [CrossRef]
- Merikanto, I.; Lahti, T.; Castaneda, A.E.; Tuulio-Henriksson, A.; Aalto-Setälä, T.; Suvisaari, J.; Partonen, T. Influence of seasonal variation in mood and behavior on cognitive test performance among young adults. Nord. J. Psychiatry 2012, 66, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Drake, C.L.; Schwartz, P.J.; Turner, E.H.; Rosenthal, N.E. Cognitive performance in seasonal affective disorder: Pattern recognition and the Stroop task. J. Nerv. Ment. Dis. 1996, 184, 56–59. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.T.; Sahakian, B.J.; Checkley, S.A. Cognitive disabilities in patients with seasonal affective disorder. Br. J. Psychiatry 1993, 163, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, B.; Payne, T.W. Affective disorders and cognitive failures: A comparison of seasonal and nonseasonal depression. Am. J. Psychiatry 2007, 164, 1663–1667. [Google Scholar] [CrossRef] [PubMed]
- La Bar, K.S.; Cabeza, R. Cognitive neuroscience of emotional memory. Nat. Rev. Neurosci. 2006, 7, 54–64. [Google Scholar] [CrossRef]
- Nielsen, S.E.; Ahmed, I.; Cahill, L. Sex and menstrual cycle phase at encoding influence emotional memory for gist and detail. Neurobiol. Learn. Mem. 2013, 106, 56–65. [Google Scholar] [CrossRef]
- Gasbarri, A.; Pompili, A.; Arnone, B.; D’Onofrio, A.; Marchetti, A.; Tavares, M.C.; Tomaz, C. Declarative memory retention and emotional stimuli. A study of an italian sample. Funct. Neurol. 2005, 20, 157–162. [Google Scholar]
- Pompili, A.; Arnone, B.; D’Amico, M.; Federico, P.; Gasbarri, A. Evidence of estrogen modulation on memory processes for emotional content in healthy young women. Psychoneuroendocrinology 2016, 65, 94–101. [Google Scholar] [CrossRef]
- Arnone, B.; Pompili, A.; Tavares, M.C.; Gasbarri, A. Sex-related memory recall and talkativeness for emotional stimuli. Frontiers Behav. Neurosci. 2011, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Gasbarri, A.; Pompili, A.; D’Onofrio, A.; Tostes Abreu, C.; Tomaz, C.; Tavares, M.C. Working memory for emotional facial expressions: Role of the estrogen in human and non-human primates. Rev. Neurosci. 2008, 200819, 129–148. [Google Scholar] [CrossRef]
- Cahill, L.; McGaugh, J.L. A novel demonstration of enhanced memory associated with emotional arousal. Conscious Cogn. 1995, 4, 410–421. [Google Scholar] [CrossRef]
- Nielsen, S.E.; Ertman, N.; Lakhani, Y.S.; Cahill, L. Hormonal contraception usage is associated with altered memory for an emotional story. Neurobiol. Learn. Mem. 2011, 96, 378–384. [Google Scholar] [CrossRef]
- Frank, J.E.; Tomaz, C. Enhancement of declarative memory associated with emotional content in a Brazilian sample. Braz. J. Med. Biol. Res. 2000, 33, 1483–1489. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Steinhausen, H.; Gundelfinger, R.; Winkler Metzke, C. Prevalence of self-reported seasonal affective disorders and the validity of the seasonal pattern assessment questionnaire in young adults: Findings from a Swiss community study. J. Affect. Disord. 2008, 115, 347–354. [Google Scholar] [CrossRef]
- Mersch, P.P.A.; Vastenburg, N.C.; Meestersetal, Y. The reliability and validity of the seasonal pattern assessment questionnaire: A comparison between patient groups. J. Affect. Disord. 2004, 80, 209–219. [Google Scholar] [CrossRef]
- Magnusson, A.; Friis, S.; Opjordsmoen, S. Internal consistency of the seasonal pattern assessment questionnaire (SPAQ). J. Affect. Disord. 1997, 42, 113–116. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Brown, G.K. Beck Depression Inventory: Second Edition Manual; The Psychological Corporation: San Antonio, TX, USA, 1996. [Google Scholar]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 1961, 4, 562–571. [Google Scholar] [CrossRef]
- Lang, P.J.; Bradley, M.M.; Cuthbert, B.N. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual; Technical Report A-8; University of Florid: Gainesville, FL, USA, 2008. [Google Scholar]
- Dillon, D.G.; Pizzagalli, D.A. Mechanisms of Memory Disruption in Depression. Trends Neurosci. 2018, 41, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Semkovska, M.; Quinlivan, L.; O’grady, T.; Johnson, R.; Collins, A.; O’Connor, J.; Knittle, H.; Ahern, E.; Gload, T. Cognitive function following a major depressive episode: A systematic review and meta-analysis. Lancet Psychiatry 2019, 6, 851–861. [Google Scholar] [CrossRef]
- Pan, Z.; Park, C.; Brietzke, E.; Zuckerman, H.; Rong, C.; Mansur, R.B.; Fus, D.; Subramaniapillai, M.; Lee, Y.; McIntyre, R.S. Cognitive impairment in major depressive disorder. CNS Spectr. 2019, 24, 22–29. [Google Scholar] [CrossRef]
- Clark, L.; Chamberlain, S.R.; Sahakian, B.J. Neurocognitive mechanisms in depression: Implications for treatment. Ann. Rev. Neurosci. 2009, 32, 57–74. [Google Scholar] [CrossRef]
- Burt, D.B.; Zembar, M.J.; Niederehe, G. Depression and memory impairment: A meta-analysis of the association, its pattern, and specificity. Psychol. Bull. 1995, 117, 285–305. [Google Scholar] [CrossRef]
- Gorwood, P.; Corruble, E.; Falissard, B.; Goodwin, G.M. Toxic effects of depression on brain function: Impairment of delayed recall and the cumulative length of depressive disorder in a large sample of depressed outpatients. Am. J. Psychiatry 2008, 165, 731–739. [Google Scholar] [CrossRef]
- Deuschle, M.; Kniest, A.; Niemann, H.; Erb-Bies, M.; Colla, N.; Hamann, B.; Heuser, I. Impaired declarative memory in depressed patients is slow to recover: Clinical experience. Pharmacopsychiatry 2004, 37, 147–151. [Google Scholar] [CrossRef]
- Ge, R.; Torres, I.; Brown, J.J.; Gregory, E.; McLellan, E.; Downar, J.H.; Blumberger, D.M.; Daskalakis, Z.J.; Lam, R.W.; Vila-Rodriguez, F. Functional disconnectivity of the hippocampal network and neural correlates of memory impairment in treatment-resistant depression. J. Affect. Disord. 2019, 253, 248–256. [Google Scholar] [CrossRef]
- Li, L.; Li, R.; Shen, F.; Wang, X.; Zou, T.; Deng, C.; Wang, C.; Li, J.; Wang, H.; Huang, X.; et al. Negative bias effects during audiovisual emotional processing in major depression disorder. Hum. Brain Mapp. 2022, 43, 1449–1462. [Google Scholar] [CrossRef] [PubMed]
- Lukmanji, A.; Williams, J.V.A.; Bulloch, A.G.M.; Patten, S.B. Seasonal variation in symptoms of depression: A Canadian population based study. J. Affect. Disord. 2019, 255, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Alvaro Guzman, A.; Rohan, K.J.; Yousufi, S.M.; Nguyen, M.; Jackson, M.A.; Soriano, J.J.; Postolache, T.T. Mood sensitivity to seasonal changes in African college students living in the greater Washington D.C. metropolitan area. Sci. World J. 2007, 7, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wang, K.; Cheng, Y.; Du, Z.; Rosenthal, N.E.; Primeau, F. Summer and winter patterns of seasonality in Chinese college students: A replication. Compr. Psychiatry 2000, 41, 57–62. [Google Scholar] [CrossRef]
- Rohan, K.J.; Sigmon, S.T. Seasonal mood patterns in a northeastern college sample. J. Affect. Disord. 2000, 59, 85–96. [Google Scholar] [CrossRef]
- Low, K.G.; Feissner, J.M. Seasonal affective disorder in college students: Prevalence and latitude. J. Am. Coll. Health 1998, 47, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Lukmanji, A.; Williams, J.V.A.; Bulloch, A.G.M.; Patten, S.B. Seasonal variation in specific depressive symptoms: A population based study. J. Affect. Disord. 2020, 261, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Young, M.A.; Watel, L.G.; Lahmeyer, H.W.; Eastman, C.I. The temporal onset of individual symptoms in winter depression: Differentiating underlying mechanisms. J. Affect. Disord. 1991, 22, 191–197. [Google Scholar] [CrossRef]
- Lam, R.W.; Tam, E.M.; Yatham, L.N.; Shiah, I.S.; Zis, A.P. Seasonal depression: The dual vulnerability hypothesis revisited. J. Affect. Disord 2001, 63, 123–132. [Google Scholar] [CrossRef]
| Group | Age (Years) | Years of Residence in Central/Southern Italy | GSS Score | BDI Score |
|---|---|---|---|---|
| Total (n = 120) | 22.50 ± 2.676 | 18.19 ± 5.204 | 11.55 ± 4.470 | 7.44 ± 3.609 |
| SAD-AS (n = 30) | 22.20 ± 2.618 | 18.30 ± 6.193 | 14.37 ± 2.566 | 8.77 ± 3.431 |
| SAD-NS (n = 30) | 23.53 ± 3.298 | 17.77 ± 5.494 | 14.83 ± 2.902 | 8.83 ± 3.715 |
| Contr-AS (n = 30) | 21.90 ± 2.057 | 18.47 ± 3.037 | 8.67 ± 3.800 | 6.57 ± 3.390 |
| Contr-NS (n = 30) | 22.37 ± 2.414 | 18.23 ± 5.752 | 8.33 ± 3.726 | 5.60 ± 2.872 |
| SAD-AS vs. Contr-AS | p = 0.647 (F1,58 = 0.244) | p = 0.895 (F1,58 = 0.018) | p < 0.001 (F1,58 = 46.366) | p < 0.01 (F1,58 = 6.241) |
| SAD-NS vs. Contr-NS | p = 0.123 (F1,58 = 2.440) | p = 0.749 (F1,58 = 0.103) | p < 0.001 (F1,58 = 56.827) | p < 0.001 (F1,58 = 14.137) |
| Gruppo | r (Free-Recall) | r (Questionnaire) |
|---|---|---|
| SAD-NS (n = 30) | r (30) = −0.822, p ˂ 0.001 | r (30) = −0.799, p ˂ 0.001 |
| SAD-AS (n = 30) | r (30) = −0.844, p ˂ 0.001 | r (30) = −0.743, p ˂ 0.001 |
| Contr-NS (n = 30) | r (30) = −0.163, p = 0.438 | r (30) = −0.355, p = 0.081 |
| Contr-AS (n = 30) | r (30) = −0.231, p = 0.220 | r (30) = −0.260, p = 0.165 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iorio, C.; Pacitti, F.; Rossi, A.; Iorio, P.; Pompili, A. Declarative Memory Impairment and Emotional Bias in Recurrent Depression with a Seasonal Pattern: The Interplay between Emotion and Cognition in Seasonal Affective Disorder. Brain Sci. 2022, 12, 1352. https://doi.org/10.3390/brainsci12101352
Iorio C, Pacitti F, Rossi A, Iorio P, Pompili A. Declarative Memory Impairment and Emotional Bias in Recurrent Depression with a Seasonal Pattern: The Interplay between Emotion and Cognition in Seasonal Affective Disorder. Brain Sciences. 2022; 12(10):1352. https://doi.org/10.3390/brainsci12101352
Chicago/Turabian StyleIorio, Carla, Francesca Pacitti, Alessandro Rossi, Paola Iorio, and Assunta Pompili. 2022. "Declarative Memory Impairment and Emotional Bias in Recurrent Depression with a Seasonal Pattern: The Interplay between Emotion and Cognition in Seasonal Affective Disorder" Brain Sciences 12, no. 10: 1352. https://doi.org/10.3390/brainsci12101352
APA StyleIorio, C., Pacitti, F., Rossi, A., Iorio, P., & Pompili, A. (2022). Declarative Memory Impairment and Emotional Bias in Recurrent Depression with a Seasonal Pattern: The Interplay between Emotion and Cognition in Seasonal Affective Disorder. Brain Sciences, 12(10), 1352. https://doi.org/10.3390/brainsci12101352






