A Nomogram for Predicting the Possibility of Peripheral Neuropathy in Patients with Type 2 Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Peripheral Neuropathy Assessment
2.3. Clinical and Laboratory Data Collection
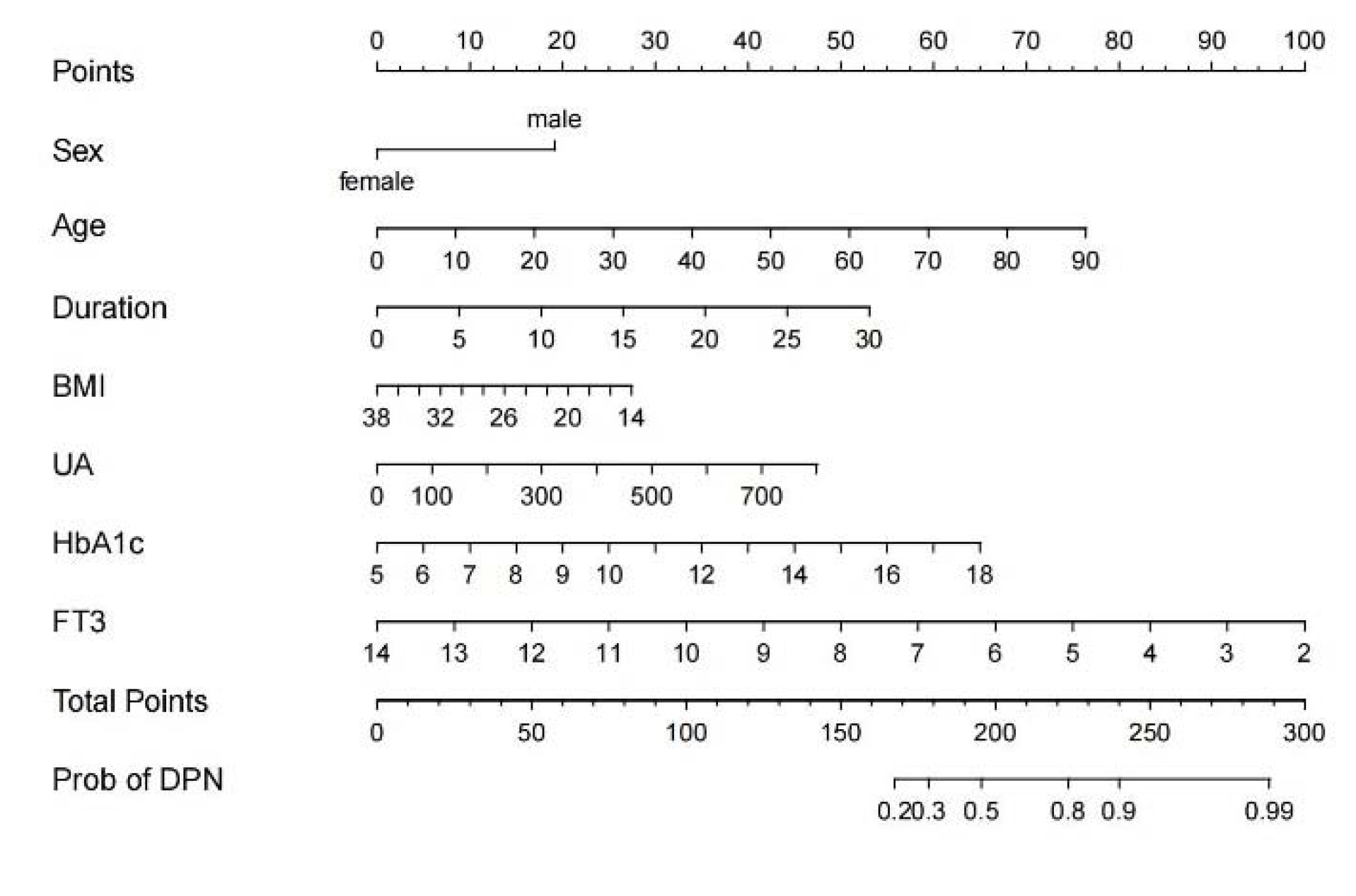
2.4. Training and Validation of the Nomogram
2.5. Statistical Analysis
3. Results
3.1. Univariate and Multivariate Analyses
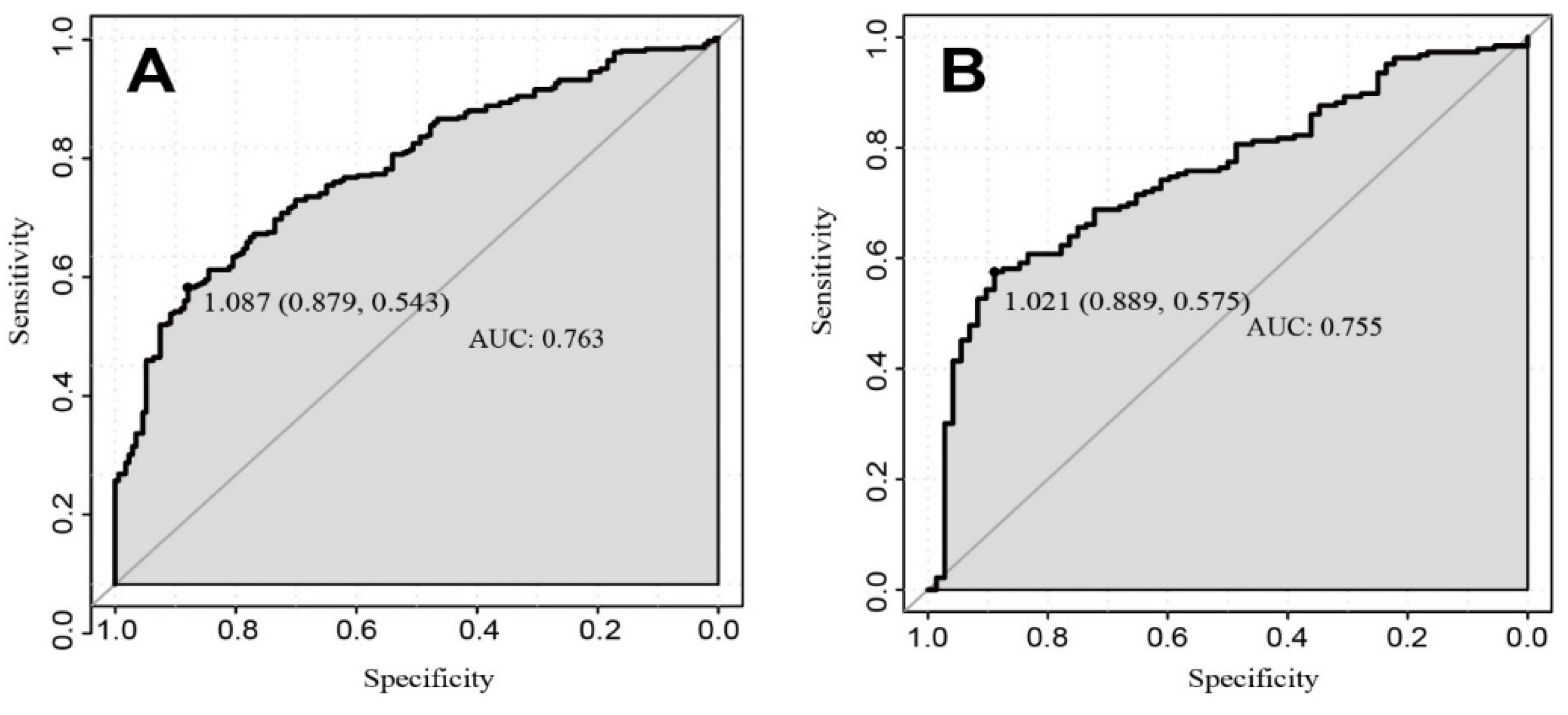
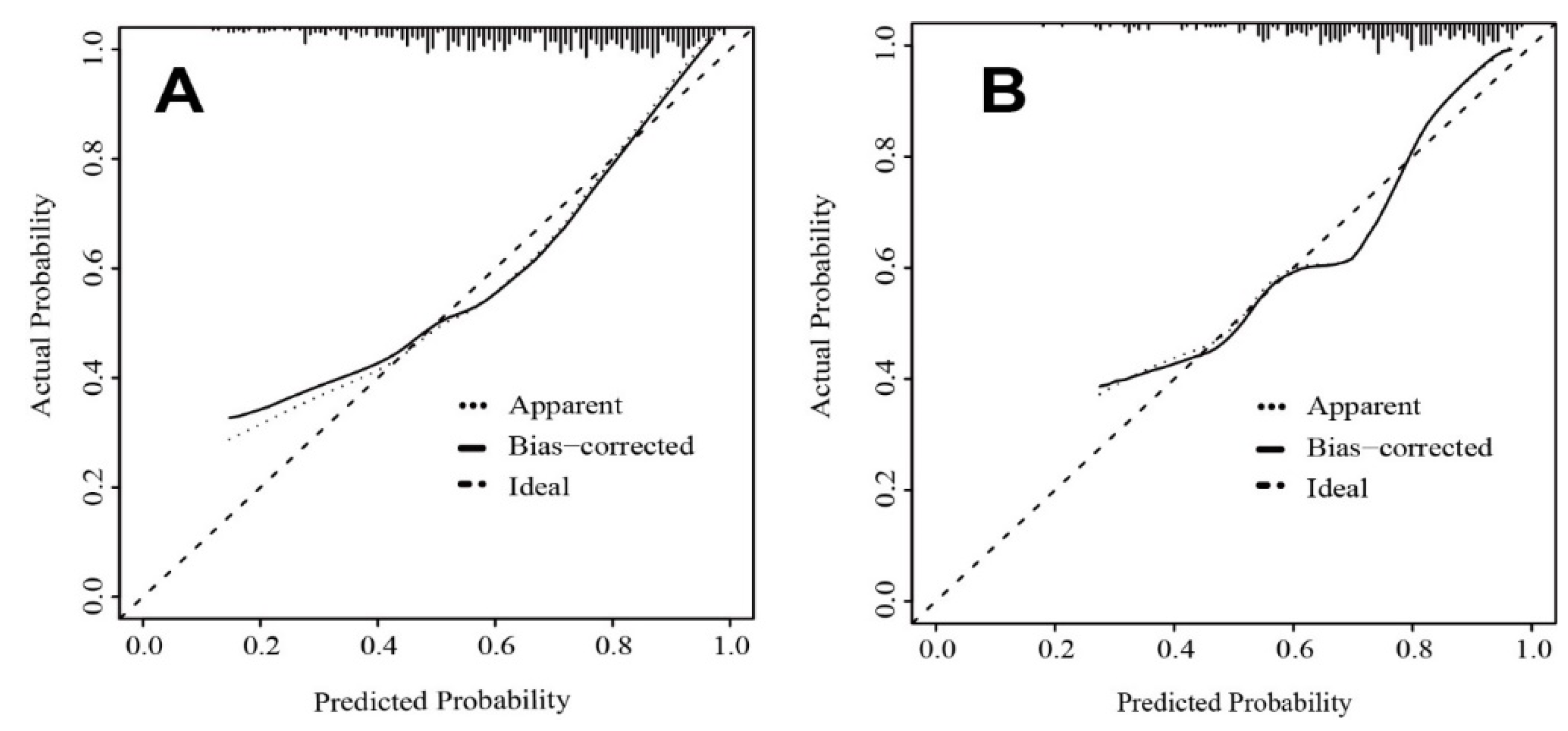
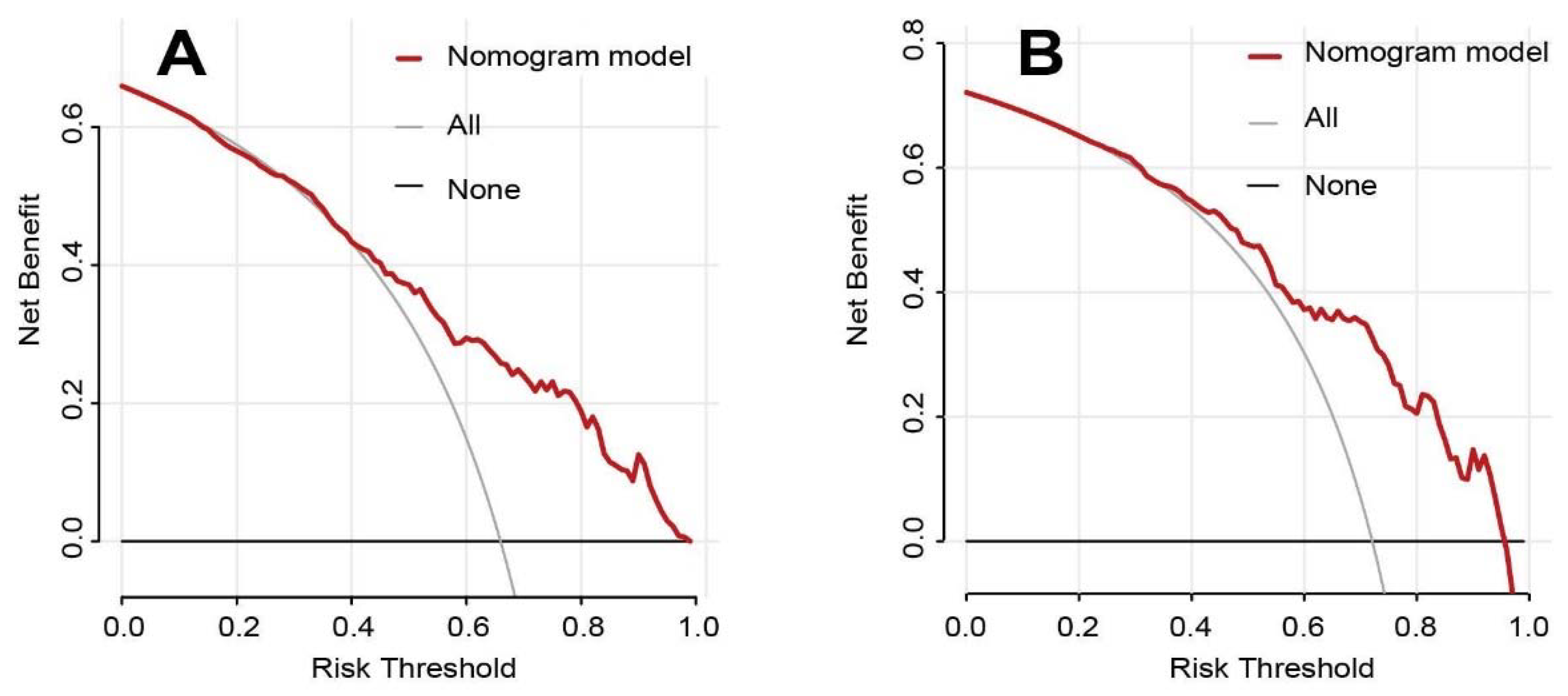
3.2. Nomogram Development and Validation
3.3. Relationship between Variables and NCSs Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Word List | Abbreviation |
| Diabetic peripheral neuropathy | DPN |
| Receiver operating characteristic | ROC |
| Areas under the curve | AUC |
| Nerve conduction studies | NCSs |
| American Diabetes Association | ADA |
| Neuropathy symptom score | NSS |
| Neuropathy disability score | NDS |
| Compound muscle action potential | CMAP |
| Conduction velocity | CV |
| Sensory nerve action potential | SNAP |
| Mean motor nerve amplitude | MNAmp |
| Mean motor nerve conduction velocity | MNCV |
| Mean sensory nerve amplitude | SNAmp |
| Mean sensory nerve conduction velocity | SNCV |
| Body mass index | BMI |
| Platelet | PLT |
| Hemoglobin A1c | HbA1c |
| Uric acid | UA |
| Fasting plasma glucose | FPG |
| Total cholesterol | TC |
| High-density lipoprotein cholesterol | HDL-C |
| Low-density lipoprotein cholesterol | LDL-C |
| Free triiodothyronine | FT3 |
| Free thyronine | FT4 |
| Thyroid stimulating hormone | TSH |
| Fibrinogen | FIB |
| Decision curve analysis | DCA |
| The ratio of neutrophil count to lymphocyte count | NLR |
References
- Lin, Y.K.; Gao, B.; Liu, L.; Ang, L.; Mizokami-Stout, K.; Pop-Busui, R.; Zhang, L. The Prevalence of Diabetic Microvascular Complications in China and the USA. Curr. Diabetes Rep. 2021, 21, 16. [Google Scholar] [CrossRef] [PubMed]
- Dal Canto, E.; Ceriello, A.; Rydén, L.; Ferrini, M.; Hansen, T.B.; Schnell, O.; Standl, E.; Beulens, J.W. Diabetes as a cardiovascular risk factor: An overview of global trends of macro and micro vascular complications. Eur. J. Prev. Cardiol. 2019, 26, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.; Papanas, N.; Schnell, O.; Nguyen, B.D.T.; Nguyen, K.T.; Kulkantrakorn, K.; Deerochanawong, C. Current concepts in the management of diabetic polyneuropathy. J. Diabetes Investig. 2021, 12, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Hicks, C.W.; Selvin, E. Epidemiology of Peripheral Neuropathy and Lower Extremity Disease in Diabetes. Curr. Diabetes Rep. 2019, 19, 86. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.; Tesfaye, S.; Spallone, V.; Gurieva, I.; Al Kaabi, J.; Mankovsky, B.; Martinka, E.; Radulian, G.; Nguyen, K.T.; O Stirban, A.; et al. Screening, diagnosis and management of diabetic sensorimotor polyneuropathy in clinical practice: International expert consensus recommendations. Diabetes Res. Clin. Pract. 2022, 186, 109063. [Google Scholar] [CrossRef] [PubMed]
- Selvarajah, D.; Kar, D.; Khunti, K.; Davies, M.J.; Scott, A.R.; Walker, J.; Tesfaye, S. Diabetic peripheral neuropathy: Advances in diagnosis and strategies for screening and early intervention. Lancet Diabetes Endocrinol. 2019, 7, 938–948. [Google Scholar] [CrossRef]
- Fujita, Y.; Murakami, T.; Nakamura, A. Recent Advances in Biomarkers and Regenerative Medicine for Diabetic Neuropathy. Int. J. Mol. Sci. 2021, 22, 2301. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Zhao, S.; Yin, Y.; Zhang, X.; Wang, K. Nomogram for Prediction of Diabetic Retinopathy Among Type 2 Diabetes Population in Xinjiang, China. Diabetes Metab. Syndr. Obes. Targets Ther. 2022, 15, 1077–1089. [Google Scholar] [CrossRef]
- Wu, B.; Niu, Z.; Hu, F. Study on Risk Factors of Peripheral Neuropathy in Type 2 Diabetes Mellitus and Establishment of Prediction Model. Diabetes Metab. J. 2021, 45, 526–538. [Google Scholar] [CrossRef]
- Tesfaye, S.; Boulton, A.J.M.; Dyck, P.J.; Freeman, R.; Horowitz, M.; Kempler, P.; Lauria, G.; Malik, R.A.; Spallone, V.; Vinik, A.; et al. Diabetic neuropathies: Update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010, 33, 2285–2293. [Google Scholar] [CrossRef]
- Sloan, G.; Selvarajah, D.; Tesfaye, S. Pathogenesis, diagnosis and clinical management of diabetic sensorimotor peripheral neuropathy. Nat. Rev. Endocrinol. 2021, 17, 400–420. [Google Scholar] [CrossRef] [PubMed]
- Cefula, W. Standards of Medical Care in Diabetes-2017: Summary of Revisions. Diabetes Care 2017, 40, S4–S5. [Google Scholar]
- Abbott, C.A.; Malik, R.A.; van Ross, E.R.; Kulkarni, J.; Boulton, A.J. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care 2011, 34, 2220–2224. [Google Scholar] [CrossRef]
- Ishibashi, F.; Taniguchi, M.; Kosaka, A.; Uetake, H.; Tavakoli, M. Improvement in Neuropathy Outcomes With Normalizing HbA1c in Patients With Type 2 Diabetes. Diabetes Care 2019, 42, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Young, M.J.; Boulton, A.J.; MacLeod, A.F.; Williams, D.R.; Sonksen, P.H. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia 1993, 36, 150–154. [Google Scholar] [CrossRef]
- Mao, F.; Zhu, X.; Liu, S.; Qiao, X.; Zheng, H.; Lu, B.; Li, Y. Age as an Independent Risk Factor for Diabetic Peripheral Neuropathy in Chinese Patients with Type 2 Diabetes. Aging Dis. 2019, 10, 592–600. [Google Scholar] [CrossRef]
- Dyck, P.J.; Carter, R.E.; Litchy, W.J. Modeling nerve conduction criteria for diagnosis of diabetic polyneuropathy. Muscle Nerve 2011, 44, 340–345. [Google Scholar] [CrossRef]
- Lin, X.; Xu, L.; Zhao, D.; Luo, Z.; Pan, S. Correlation between serum uric acid and diabetic peripheral neuropathy in T2DM patients. J. Neurol. Sci. 2018, 385, 78–82. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Huang, L.; Shen, X.; Zhao, F.; Wu, C.; Yan, S. Relationship between Hyponatremia and Peripheral Neuropathy in Patients with Diabetes. J. Diabetes Res. 2021, 2021, 9012887. [Google Scholar] [CrossRef]
- Jiang, A.J.; Gu, H.; Feng, Z.R.; Ding, Y.; Xu, X.H.; Yin, G.P.; Zhang, W.L.; Shen, Z.Y.; Li, Q. Heart rate-corrected QT interval: A novel diagnostic biomarker for diabetic peripheral neuropathy. J. Diabetes Investig. 2022, 13, 850–857. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, H.; Zhu, X.; Mao, F.; Zhang, S.; Shi, H.; Li, Y.; Lu, B. Neutrophil-to-lymphocyte ratio is associated with diabetic peripheral neuropathy in type 2 diabetes patients. Diabetes Res. Clin. Pract. 2017, 130, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.E.; Sloane, L.B.; Fischer, K.E.; Austad, S.N.; Richardson, A.; Van Remmen, H. Use of Nerve Conduction Velocity to Assess Peripheral Nerve Health in Aging Mice. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2015, 70, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Goss, J.R.; Stolz, D.B.; Robinson, A.R.; Zhang, M.; Arbujas, N.; Robbins, P.D.; Glorioso, J.C.; Niedernhofer, L.J. Premature aging-related peripheral neuropathy in a mouse model of progeria. Mech. Ageing Dev. 2011, 132, 437–442. [Google Scholar] [CrossRef]
- Wang, K.; Gong, M.; Xie, S.; Zhang, M.; Zheng, H.; Zhao, X.; Liu, C. Nomogram prediction for the 3-year risk of type 2 diabetes in healthy mainland China residents. EPMA J. 2019, 10, 227–237. [Google Scholar] [CrossRef] [PubMed]
- TODAY Study Group. Risk Factors for Diabetic Peripheral Neuropathy in Adolescents and Young Adults With Type 2 Diabetes: Results From the TODAY Study. Diabetes Care 2021, 45, 1065–1072. [Google Scholar]
- Fayazi, H.S.; Yaseri, M.; Mortazavi, S.S.; Sharifhassan, Z.; Assadinia, A.S. The relation between serum uric acid levels and diabetic peripheral neuropathy in type 2 diabetes in Guilan, north of Iran. BMC Endocr. Disord. 2022, 22, 39. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, S.; Chaturvedi, N.; Eaton, S.E.; Ward, J.D.; Manes, C.; Ionescu-Tirgoviste, C.; Witte, D.R.; Fuller, J.H. Vascular risk factors and diabetic neuropathy. N. Engl. J. Med. 2005, 352, 341–350. [Google Scholar] [CrossRef]
- Khawaja, N.; Abu-Shennar, J.; Saleh, M.; Dahbour, S.S.; Khader, Y.S.; Ajlouni, K.M. The prevalence and risk factors of peripheral neuropathy among patients with type 2 diabetes mellitus; the case of Jordan. Diabetol. Metab. Syndr. 2018, 10, 8. [Google Scholar] [CrossRef]
- Xu, F.; Zhao, L.-H.; Su, J.-B.; Chen, T.; Wang, X.-Q.; Chen, J.-F.; Wu, G.; Jin, Y.; Wang, X.-H. The relationship between glycemic variability and diabetic peripheral neuropathy in type 2 diabetes with well-controlled HbA1c. Diabetol. Metab. Syndr. 2014, 6, 139. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Y.; Shen, X.; Zhao, F.; Yan, S. Lower body mass index is not of more benefit for diabetic complications. J. Diabetes Investig. 2019, 10, 1307–1317. [Google Scholar] [CrossRef]
- Hong, Q.; Wang, L.; Huang, Z.; Feng, Z.; Cui, S.; Fu, B.; Cai, G.; Chen, X.; Wu, D. High Concentrations of Uric Acid and Angiotensin II Act Additively to Produce Endothelial Injury. Mediat. Inflamm. 2020, 2020, 8387654. [Google Scholar] [CrossRef] [PubMed]
- Maruhashi, T.; Hisatome, I.; Kihara, Y.; Higashi, Y. Hyperuricemia and endothelial function: From molecular background to clinical perspectives. Atherosclerosis 2018, 278, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Chen, Y.; Hou, X.; Xu, N.; Che, K.; Li, C.; Yan, S.; Wang, Y.; Wang, B. Serum Uric Acid Levels and Diabetic Peripheral Neuropathy in Type 2 Diabetes: A Systematic Review and Meta-analysis. Mol. Neurobiol. 2016, 53, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.N.; Li, Y.F.; Huo, L.L.; Zhang, Q.; Wang, L.Y.; Zhao, C.L.; Liu, L.G. Association between serum uric acid and large-nerve fiber dysfunction in type 2 diabetes: A cross-sectional study. Chin. Med. J. 2019, 132, 1015–1022. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, Z.; Qin, H.; Yao, Z. Thyroid Hormone Potentially Benefits Multiple Sclerosis via Facilitating Remyelination. Mol. Neurobiol. 2016, 53, 4406–4416. [Google Scholar] [CrossRef]
- Biondi, B.; Kahaly, G.J.; Robertson, R.P. Thyroid Dysfunction and Diabetes Mellitus: Two Closely Associated Disorders. Endocr. Rev. 2019, 40, 789–824. [Google Scholar] [CrossRef]
- Zhu, F.F.; Yang, L.Z. The Association Between the Levels of Thyroid Hormones and Peripheral Nerve Conduction in Patients with Type 2 Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2018, 126, 493–504. [Google Scholar] [CrossRef]
| Variables | Training Cohort (n = 519) | Validation Cohort (n = 259) | p Value |
|---|---|---|---|
| DPN, no. (%) | 341 (65.7%) | 187 (72.2%) | 0.081 |
| Age (years) | 57.76 ± 12.95 | 58.97 ± 12.49 | 0.265 |
| Male, no. (%) | 334 (64.4%) | 159 (61.4%) | 0.465 |
| Smoking, no. (%) | 180 (34.7%) | 77 (29.7%) | 0.193 |
| Hypertension, no. (%) | 266 (51.3%) | 143 (55.4%) | 0.307 |
| Dyslipidemia, no. (%) | 168 (32.4%) | 78 (30.1%) | 0.579 |
| Duration (years) | 10 (4–12) | 10 (5–15) | 0.061 |
| BMI (kg/m2) | 24.21 ± 3.49 | 24.23 ± 3.60 | 0.803 |
| FPG (mmol/L) | 7.95 ± 2.90 | 8.10 ± 3.69 | 0.813 |
| UA (umol/L) | 329.04 ± 101.10 | 326.47 ± 98.71 | 0.748 |
| TC (mmol/L) | 4.79 ± 1.35 | 4.81 ± 1.48 | 0.835 |
| TG (mmol/L) | 1.91 ± 1.74 | 1.89 ± 1.47 | 0.481 |
| HDL-C (mmol/L) | 1.02 ± 0.29 | 1.05 ± 0.43 | 0.753 |
| LDL-C (mmol/L) | 2.61 ± 0.98 | 2.58 ± 1.03 | 0.629 |
| FIB (g/L) | 3.79 ± 1.24 | 3.71 ± 1.05 | 0.950 |
| NLR | 2.36 ± 1.41 | 2.45 ± 1.62 | 0.438 |
| PLT (×109/L) | 221.10 ± 64.93 | 222.26 ± 61.99 | 0.809 |
| HbA1c (%) | 9.32 ± 2.38 | 9.42 ± 2.82 | 0.940 |
| TSH (mIU/L) | 1.34 (0.91–2.03) | 1.31 (0.86–1.92) | 0.124 |
| FT4 (pmol/L) | 11.24 ± 2.33 | 11.34 ± 2.11 | 0.423 |
| FT3 (pmol/L) | 4.64 ± 0.79 | 4.67 ± 0.84 | 0.568 |
| Variables | Training Cohort | Univariate Logistic Regression Analysis | |||
|---|---|---|---|---|---|
| DPN Group (n =341) | Non-DPN Groups (n =178) | p Value | OR (95% CI) | p Value | |
| Male, no. (%) | 235 (68.9%) | 99 (55.6%) | 0.004 | 1.769 (1.217–2.572) | 0.003 |
| Age (years) | 59.57 ± 12.36 | 54.31 ± 13.40 | <0.001 | 1.032 (1.017–1.047) | <0.001 |
| Duration (years) | 10.00 (5.00–15.00) | 6.50 (2.00–10.00) | <0.001 | 1.089 (1.056–1.124) | <0.001 |
| Smoking, no. (%) | 130 (38.1%) | 50 (28.1%) | 0.029 | 1.577 (1.064–2.337) | 0.023 |
| BMI (kg/m2) | 24.03 ± 3.22 | 24.56 ± 3.93 | 0.128 | 0.958 (0.909–1.009) | 0.105 |
| Hypertension, no. (%) | 189 (55.4%) | 77 (43.3%) | 0.011 | 1.631 (1.132–2.350) | 0.009 |
| Dyslipidemia, no. (%) | 114 (33.4%) | 54 (30.3%) | 0.538 | 1.153 (0.783–1.712) | 0.475 |
| FPG (mmol/L) | 8.08 ± 3.01 | 7.68 ± 2.67 | 0.123 | 1.050 (0.984–1.121) | 0.137 |
| UA (umol/L) | 337.95 ± 106.03 | 311.97 ± 88.71 | 0.003 | 1.003 (1.001–1.005) | 0.006 |
| TC (mmol/L) | 4.74 ± 1.37 | 4.88 ± 1.30 | 0.266 | 0.928 (0.812–1.061) | 0.274 |
| TG (mmol/L) | 1.87 ± 1.33 | 1.99 ± 1.43 | 0.523 | 0.962 (0.869–1.065) | 0.461 |
| HDL-C (mmol/L) | 1.03 ± 0.29 | 1.01 ± 0.29 | 0.491 | 1.253 (0.660–2.378) | 0.490 |
| LDL-C (mmol/L) | 2.58 ± 1.03 | 2.67 ± 0.88 | 0.280 | 0.904 (0.753–1.086) | 0.280 |
| FIB (g/L) | 3.90 ± 1.32 | 3.58 ± 1.06 | 0.003 | 1.260 (1.068–1.487) | 0.006 |
| NLR | 2.41 ± 1.41 | 2.27 ± 1.40 | 0.259 | 1.082 (0.942–1.243) | 0.262 |
| PLT (×109/L) | 220.54 ± 65.16 | 222.17 ± 64.64 | 0.786 | 1.000 (0.997–1.002) | 0.786 |
| HbA1c (%) | 9.53 ± 2.36 | 8.91 ± 2.36 | 0.004 | 1.122 (1.036–1.215) | 0.005 |
| TSH (mIU/L) | 1.35 (0.91–2.02) | 1.33 (0.94–2.22) | 0.941 | 1.048 (0.927–1.185) | 0.457 |
| FT4 (pmol/L) | 11.36 ± 2.41 | 11.03 ± 2.16 | 0.112 | 1.069 (0.981–1.165) | 0.127 |
| FT3 (pmol/L) | 4.55 ± 0.82 | 4.81 ± 0.69 | <0.001 | 0.642 (0.495–0.834) | 0.001 |
| Variables | Multivariable Analysis | p Value | |
|---|---|---|---|
| OR | (95% CI) | ||
| Gender | 2.607 | 1.658–4.138 | <0.001 |
| Age (year) | 1.040 | 1.022–1.060 | <0.001 |
| Duration (year) | 1.091 | 1.053–1.132 | <0.001 |
| BMI (kg/m2) | 0.939 | 0.884–0.996 | 0.037 |
| UA (umol/L) | 1.003 | 1.001–1.005 | 0.012 |
| HbA1c (%) | 1.267 | 1.151–1.402 | <0.001 |
| FT3 (pmol/L) | 0.674 | 0.505–0.876 | 0.005 |
| Subgroups | AUC |
|---|---|
| Gender | |
| Male | 0.769 |
| Female | 0.715 |
| Age (year) | |
| ≥65 (years) | 0.762 |
| <65 (years) | 0.736 |
| Duration (year) | |
| >10 years | 0.802 |
| ≤10 years | 0.681 |
| BMI | |
| ≥24 (kg/m2) | 0.763 |
| <24 (kg/m2) | 0.723 |
| Hypertension | |
| Hypertension | 0.788 |
| No hypertension | 0.694 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Chen, L. A Nomogram for Predicting the Possibility of Peripheral Neuropathy in Patients with Type 2 Diabetes Mellitus. Brain Sci. 2022, 12, 1328. https://doi.org/10.3390/brainsci12101328
Zhang W, Chen L. A Nomogram for Predicting the Possibility of Peripheral Neuropathy in Patients with Type 2 Diabetes Mellitus. Brain Sciences. 2022; 12(10):1328. https://doi.org/10.3390/brainsci12101328
Chicago/Turabian StyleZhang, Wanli, and Lingli Chen. 2022. "A Nomogram for Predicting the Possibility of Peripheral Neuropathy in Patients with Type 2 Diabetes Mellitus" Brain Sciences 12, no. 10: 1328. https://doi.org/10.3390/brainsci12101328
APA StyleZhang, W., & Chen, L. (2022). A Nomogram for Predicting the Possibility of Peripheral Neuropathy in Patients with Type 2 Diabetes Mellitus. Brain Sciences, 12(10), 1328. https://doi.org/10.3390/brainsci12101328








