Exosomes derived from M2 Macrophages Improve Angiogenesis and Functional Recovery after Spinal Cord Injury through HIF-1α/VEGF Axis
Abstract
1. Introduction
2. Materials and Methods
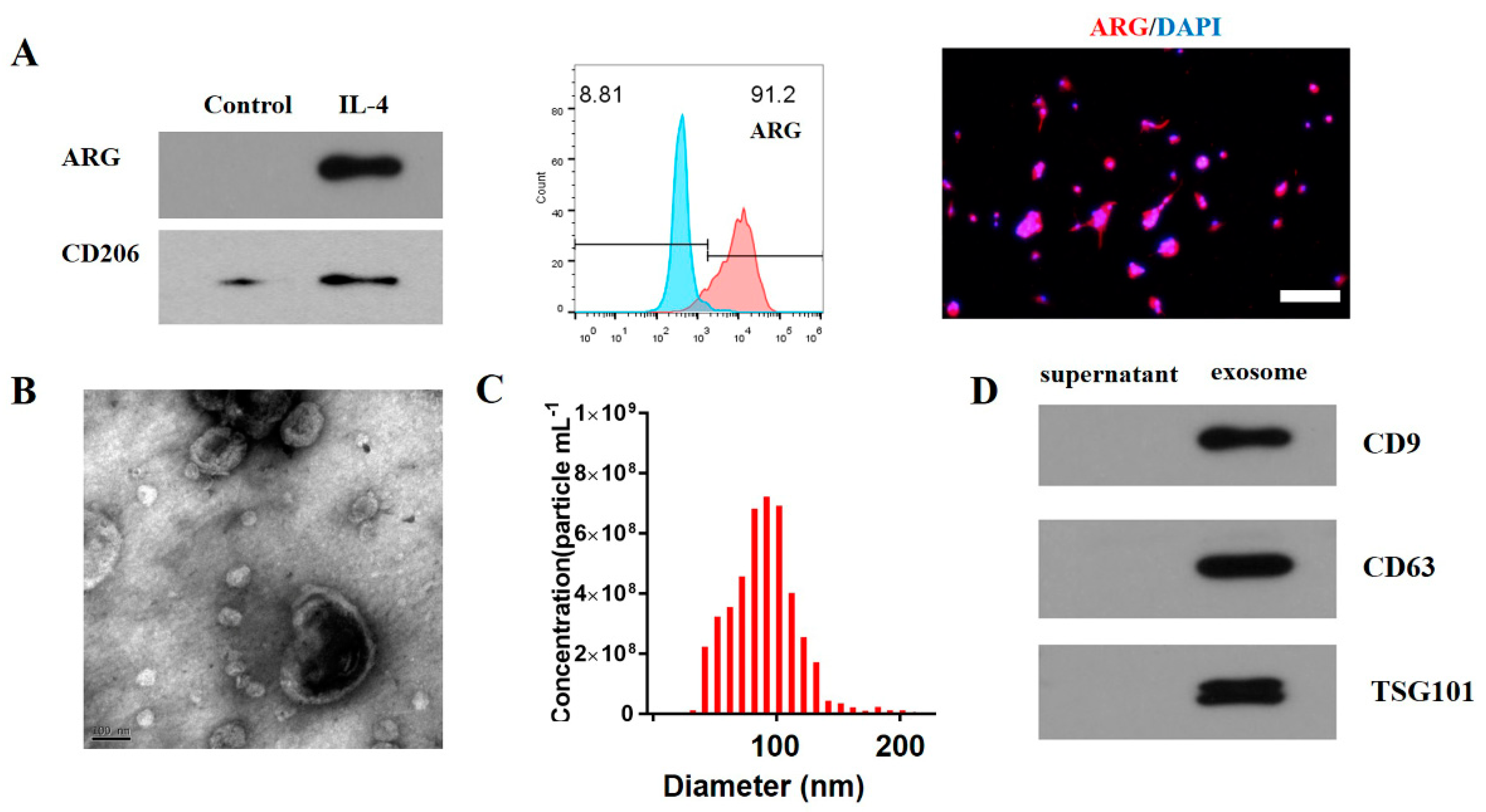
2.1. Induction and Identification of M2 Macrophages
2.2. Flow Cytometric Analysis
2.3. The Extraction and Identification of Exosomes
2.4. Construction of a Contusion SCI Rat Model
2.5. Experimental Design
2.6. Behavioral Evaluation
2.7. Identification of Lesions via Cresyl Violet Staining
2.8. Immunofluorescence Staining
2.9. Immunohistochemistry Analysis
2.10. bEnd.3 Cell Culture and Oxygen Glucose Deprivation (OGD)
2.11. Uptake of Exosomes by bEnd.3 Cells
2.12. EdU test
2.13. Capillary Network Formation (CNA) Assay
2.14. Transwell Assay
2.15. siRNAs
2.16. qRT-PCR
2.17. WB Analysis
2.18. Statistical Analysis
3. Results
3.1. Characterization of M2 Macrophage Exosomes
3.2. M2 Macrophage Exosomes Attenuate Tissue Damage (TD) and Improve Functional Recovery (FR) Post-SCI
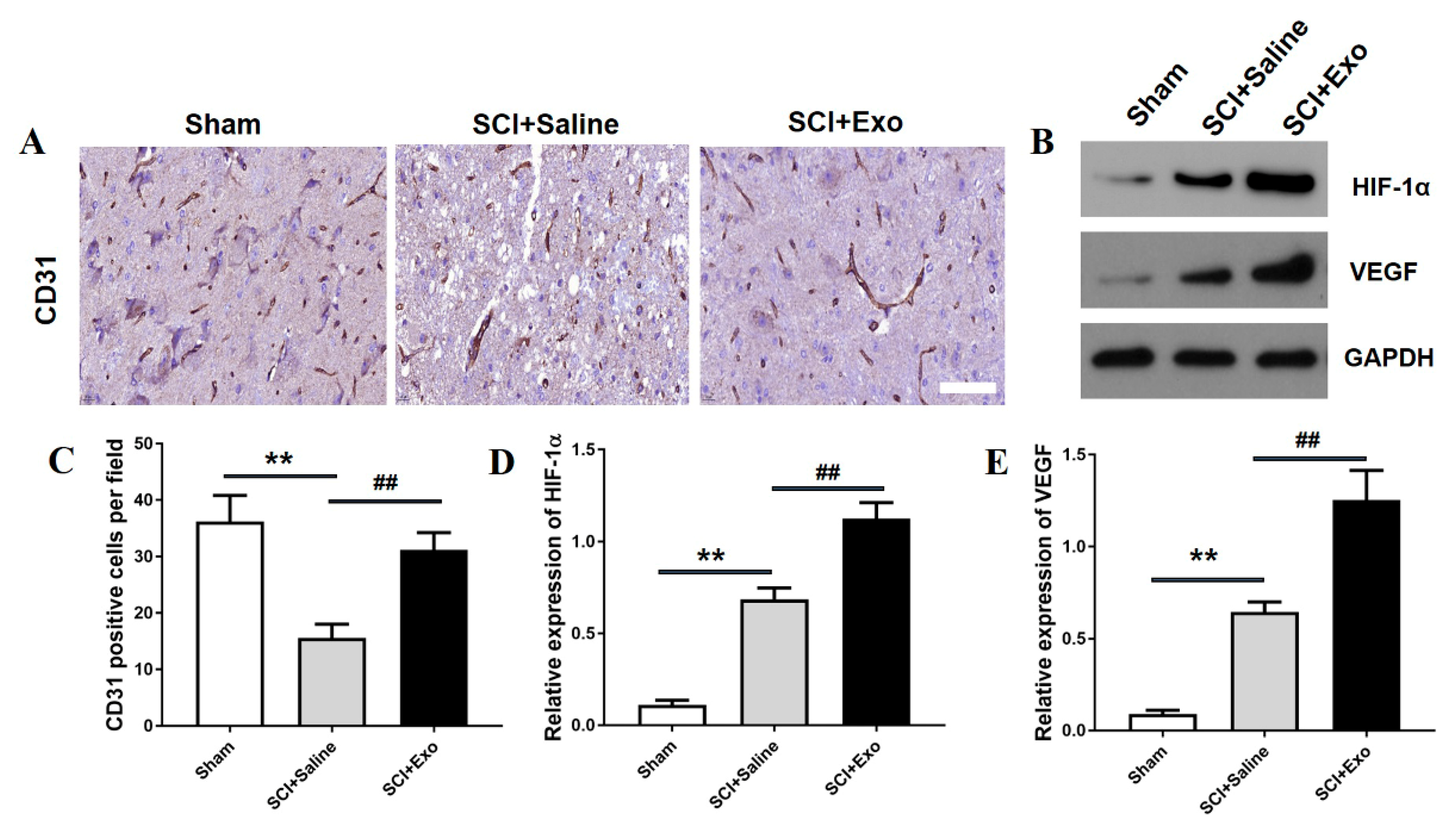
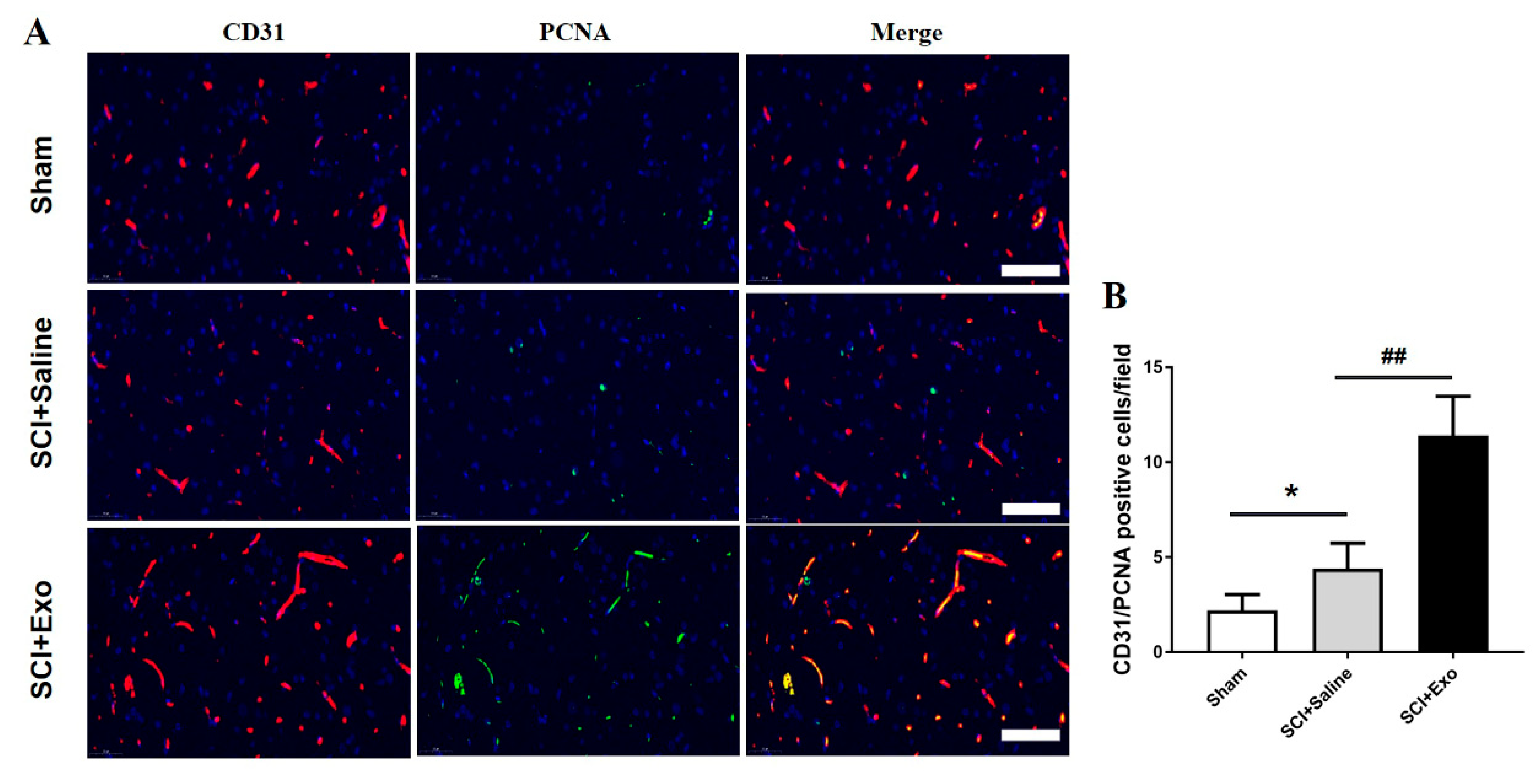
3.3. M2 Macrophage Exosomes Promote Angiogenesis after SCI
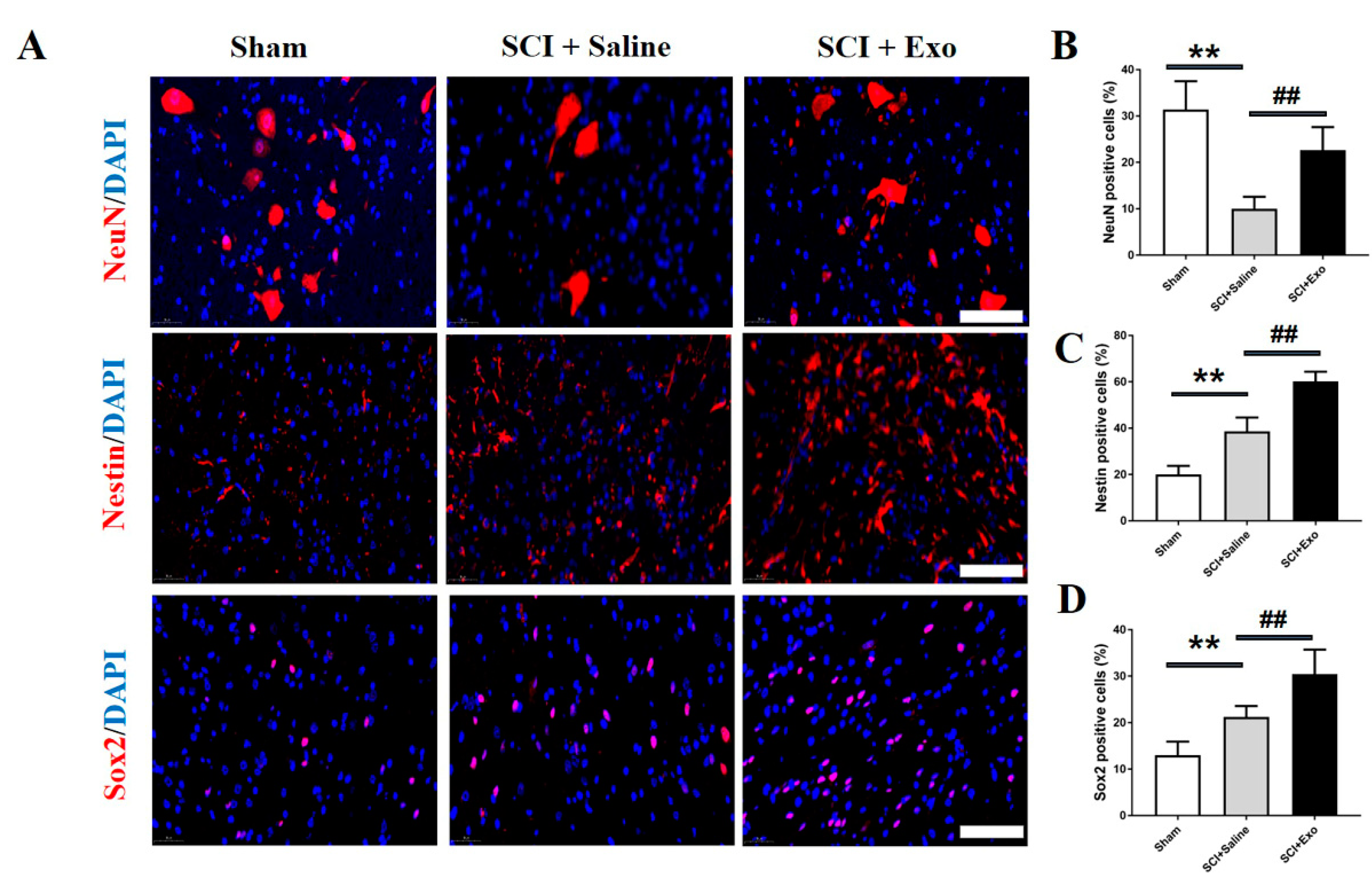
3.4. M2 Macrophage Exosomes Promote Neurogenesis after SCI
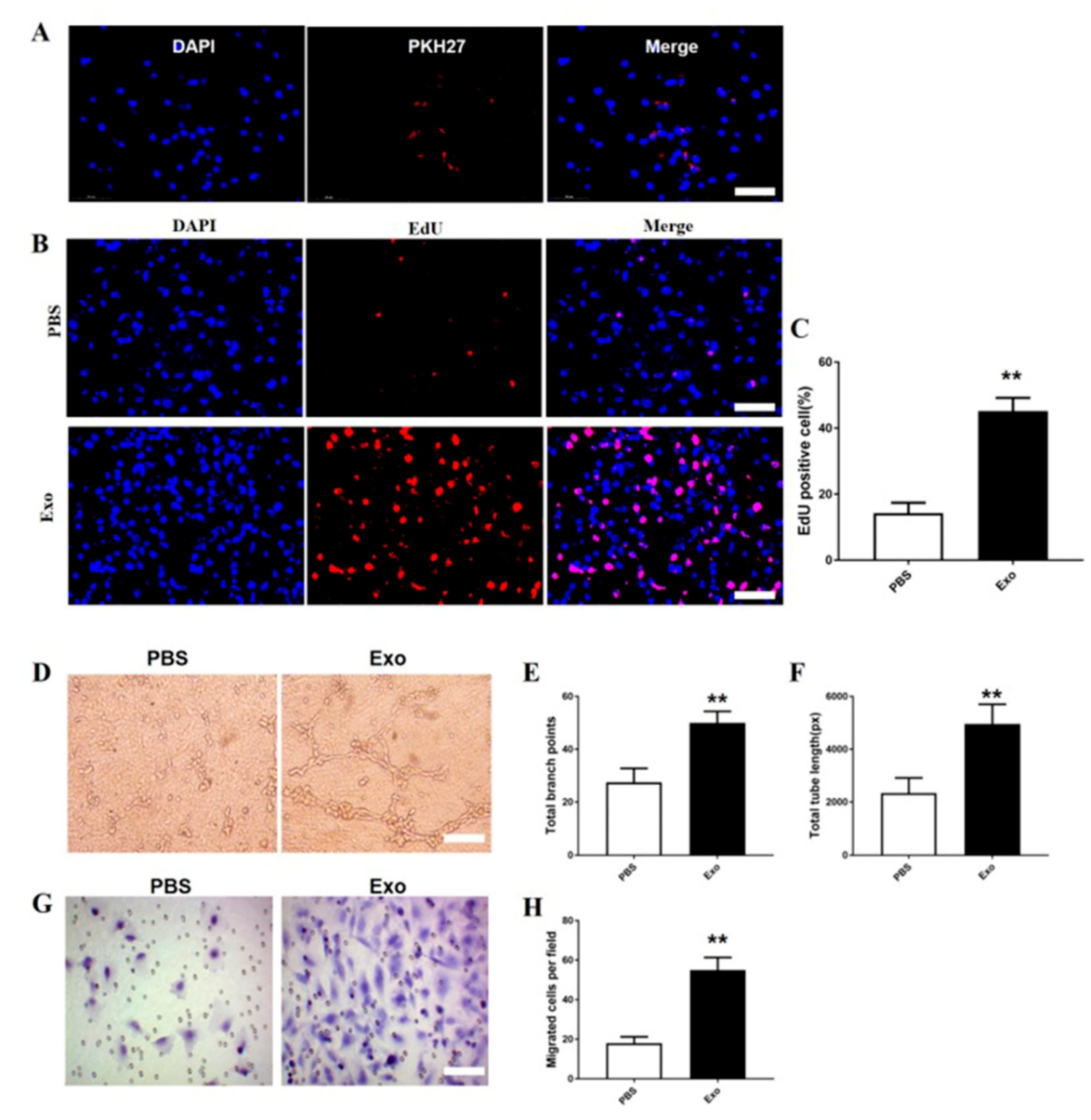
3.5. The M2 Macrophage Exosomes Exhibit Pro-Angiogenic Effects on bEnd.3 Cells
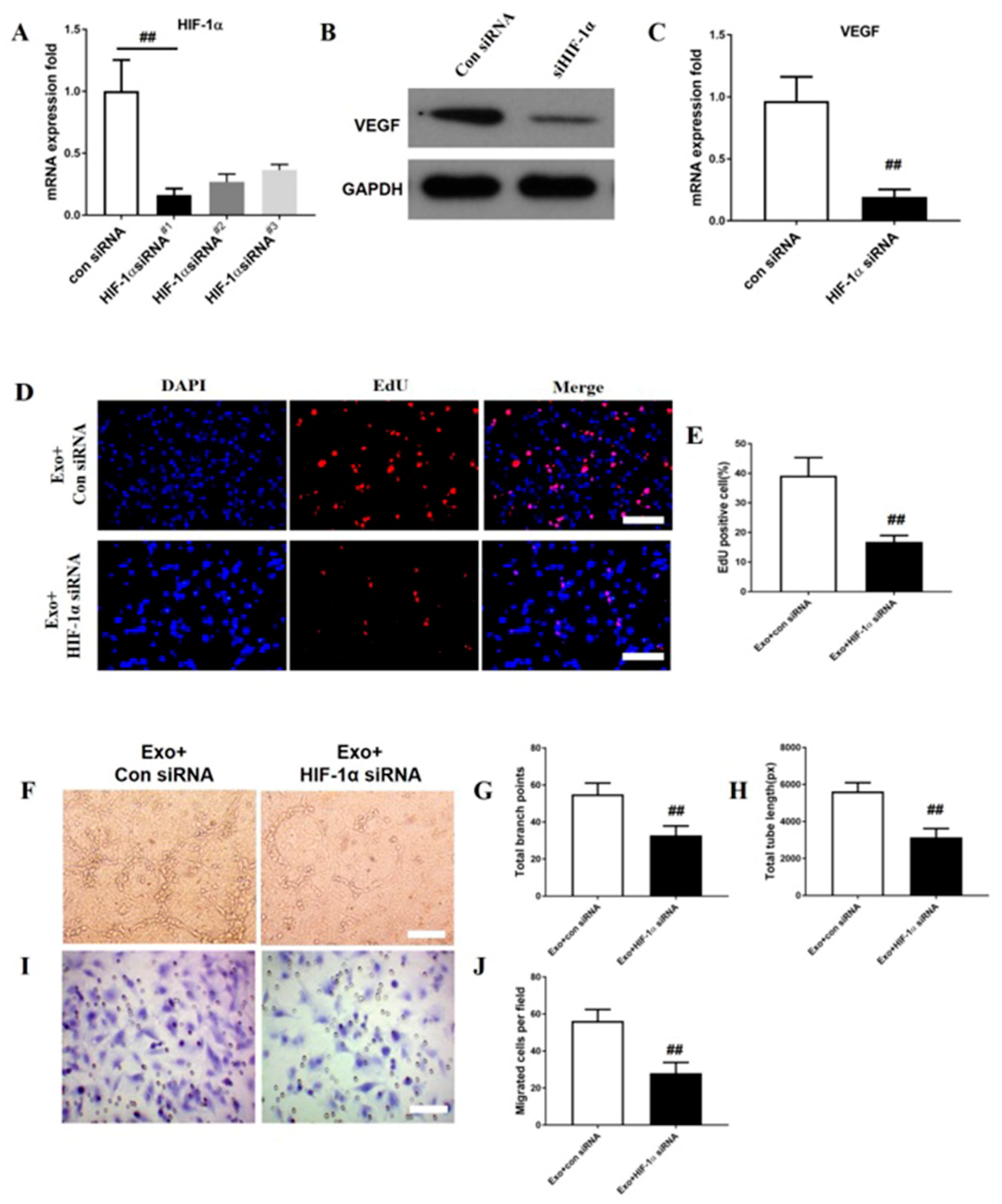
3.6. HIF-1α Is Required in M2 Macrophage Exosomes to Promote VEGF Expression and ANGIOGENESIS on bEnd.3 cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carlson, G.D.; Gorden, C. Current developments in spinal cord injury research. Spine J. 2002, 2, 116–128. [Google Scholar] [CrossRef]
- Thuret, S.; Moon, L.D.; Gage, F.H. Therapeutic interventions after spinal cord injury. Nat. Rev. Neurosci. 2006, 7, 628–643. [Google Scholar] [CrossRef] [PubMed]
- Dumont, R.J.; Okonkwo, D.O.; Verma, S.; Hurlbert, R.J.; Boulos, P.T.; Ellegala, D.B.; Dumont, A.S. Acute spinal cord injury, part I: Pathophysiologic mechanisms. Clin. Neuropharmacol. 2001, 24, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Casella, G.T.; Marcillo, A.; Bunge, M.B.; Wood, P.M. New vascular tissue rapidly replaces neural parenchyma and vessels destroyed by a contusion injury to the rat spinal cord. Exp. Neurol. 2002, 173, 63–76. [Google Scholar] [CrossRef]
- Casella, G.T.; Bunge, M.B.; Wood, P.M. Endothelial cell loss is not a major cause of neuronal and glial cell death following contusion injury of the spinal cord. Exp. Neurol. 2006, 202, 8–20. [Google Scholar] [CrossRef]
- Benton, R.L.; Maddie, M.A.; Dincman, T.A.; Hagg, T.; Whittemore, S.R. Transcriptional activation of endothelial cells by TGFβ coincides with acute microvascular plasticity following focal spinal cord ischaemia/reperfusion injury. ASN Neuro. 2009, 1, e00015. [Google Scholar] [CrossRef]
- Dray, C.; Rougon, G.; Debarbieux, F. Quantitative analysis by in vivo imaging of the dynamics of vascular and axonal networks in injured mouse spinal cord. Proc. Natl. Acad. Sci. USA 2009, 106, 9459–9464. [Google Scholar] [CrossRef]
- De Laporte, L.; des Rieux, A.; Tuinstra, H.M.; Zelivyanskaya, M.L.; De Clerck, N.M.; Postnov, A.A.; Préat, V.; Shea, L.D. Vascular endothelial growth factor and fibroblast growth factor 2 delivery from spinal cord bridges to enhance angiogenesis following injury. J. Biomed. Mater. Res. A. 2011, 98, 372–382. [Google Scholar] [CrossRef]
- Samantaray, S.; Das, A.; Matzelle, D.C.; Yu, S.P.; Wei, L.; Varma, A.; Ray, S.K.; Banik, N.L. Administration of low dose estrogen attenuates persistent inflammation, promotes angiogenesis, and improves locomotor function following chronic spinal cord injury in rats. J. Neurochem. 2016, 137, 604–617. [Google Scholar] [CrossRef]
- Yahata, K.; Kanno, H.; Ozawa, H.; Yamaya, S.; Tateda, S.; Ito, K.; Shimokawa, H.; Itoi, E. Low-energy extracorporeal shock wave therapy for promotion of vascular endothelial growth factor expression and angiogenesis and improvement of locomotor and sensory functions after spinal cord injury. J. Neurosurg. Spine 2016, 25, 745–755. [Google Scholar] [CrossRef]
- Tykocki, T.; Poniatowski, Ł.A.; Czyz, M.; Wynne-Jones, G. Oblique corpectomy in the cervical spine. Spinal Cord 2018, 56, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Kubaszewski, Ł.; Wojdasiewicz, P.; Rożek, M.; Słowińska, I.E.; Romanowska-Próchnicka, K.; Słowiński, R.; Poniatowski, Ł.A.; Gasik, R. Syndromes with chronic non-bacterial osteomyelitis in the spine. Reumatologia 2015, 53, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Gensel, J.C.; Zhang, B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 2015, 1619, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Gao, J. Macrophage polarization: A key event in the secondary phase of acute spinal cord injury. J. Cell. Mol. Med. 2017, 21, 941–954. [Google Scholar] [CrossRef]
- Zajac, E.; Schweighofer, B.; Kupriyanova, T.A.; Juncker-Jensen, A.; Minder, P.; Quigley, J.P.; Deryugina, E.I. Angiogenic capacity of M1- and M2-polarized macrophages is determined by the levels of TIMP-1 complexed with their secreted proMMP-9. Blood 2013, 122, 4054–4067. [Google Scholar] [CrossRef]
- Jetten, N.; Verbruggen, S.; Gijbels, M.J.; Post, M.J.; De Winther, M.P.; Donners, M.M. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis 2014, 17, 109–118. [Google Scholar] [CrossRef]
- Stoorvogel, W.; Kleijmeer, M.J.; Geuze, H.J.; Raposo, G. The biogenesis and functions of exosomes. Traffic 2002, 3, 321–330. [Google Scholar] [CrossRef]
- Record, M.; Subra, C.; Silvente-Poirot, S.; Poirot, M. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem. Pharmacol. 2011, 81, 1171–1182. [Google Scholar] [CrossRef]
- Dreyer, F.; Baur, A. Biogenesis and Functions of Exosomes and Extracellular Vesicles. Methods Mol. Biol. 2016, 1448, 201–216. [Google Scholar]
- Goetzl, E.J.; Boxer, A.; Schwartz, J.B.; Abner, E.L.; Petersen, R.C.; Miller, B.L.; Kapogiannis, D. Altered lysosomal proteins in neural-derived plasma exosomes in preclinical Alzheimer disease. Neurology 2015, 85, 40–47. [Google Scholar] [CrossRef]
- Lee, M.; Ban, J.J.; Kim, K.Y.; Jeon, G.S.; Im, W.; Sung, J.J.; Kim, M. Adipose-derived stem cell exosomes alleviate pathology of amyotrophic lateral sclerosis in vitro. Biochem. Biophys. Res. Commun. 2016, 479, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chopp, M.; Liu, X.S.; Katakowski, M.; Wang, X.; Tian, X.; Wu, D.; Zhang, Z.G. Exosomes Derived from Mesenchymal Stromal Cells Promote Axonal Growth of Cortical Neurons. Mol. Neurobiol. 2017, 54, 2659–2673. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Huang, M.; Ma, X.; Chen, H.; Gao, X. Harnessing Exosomes for the Development of Brain Drug Delivery Systems. Bioconjug. Chem. 2019, 30, 994–1005. [Google Scholar] [CrossRef]
- Zheng, Y.; He, R.; Wang, P.; Shi, Y.; Zhao, L.; Liang, J. Exosomes from LPS-stimulated macrophages induce neuroprotection and functional improvement after ischemic stroke by modulating microglial polarization. Biomater. Sci. 2019, 7, 2037–2049. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.H.; Yin, X.M.; Xu, Y.; Xu, C.C.; Lin, X.; Ye, F.B.; Cao, Y.; Lin, F.Y. Systemic Administration of Exosomes Released from Mesenchymal Stromal Cells Attenuates Apoptosis, Inflammation, and Promotes Angiogenesis after Spinal Cord Injury in Rats. J. Neurotrauma. 2017, 34, 3388–3396. [Google Scholar] [CrossRef]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma. 1995, 12, 1–21. [Google Scholar] [CrossRef]
- Van Balkom, B.W.; de Jong, O.G.; Smits, M.; Brummelman, J.; den Ouden, K.; de Bree, P.M.; van Eijndhoven, M.A.; Pegtel, D.M.; Stoorvogel, W.; Würdinger, T.; et al. Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood 2013, 121, 3997–4006. [Google Scholar] [CrossRef]
- Kimura, H.; Weisz, A.; Ogura, T.; Hitomi, Y.; Kurashima, Y.; Hashimoto, K.; D’Acquisto, F.; Makuuchi, M.; Esumi, H. Identification of hypoxia-inducible factor 1 ancillary sequence and its function in vascular endothelial growth factor gene induction by hypoxia and nitric oxide. J. Biol. Chem. 2001, 276, 2292–2298. [Google Scholar] [CrossRef]
- Sun, G.; Li, G.; Li, D.; Huang, W.; Zhang, R.; Zhang, H.; Duan, Y.; Wang, B. hucMSC derived exosomes promote functional recovery in spinal cord injury mice via attenuating inflammation. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 89, 194–204. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Gong, F.; Rong, Y.; Luo, Y.; Tang, P.; Zhou, Z.; Zhou, Z.; Xu, T.; Jiang, T.; et al. Exosomes Derived from Bone Mesenchymal Stem Cells Repair Traumatic Spinal Cord Injury by Suppressing the Activation of A1 Neurotoxic Reactive Astrocytes. J. Neurotrauma. 2019, 36, 469–484. [Google Scholar] [CrossRef]
- Barrette, B.; Hébert, M.A.; Filali, M.; Lafortune, K.; Vallières, N.; Gowing, G.; Julien, J.P.; Lacroix, S. Requirement of myeloid cells for axon regeneration. J. Neurosci. 2008, 28, 9363–9376. [Google Scholar] [CrossRef] [PubMed]
- Lucas, T.; Waisman, A.; Ranjan, R.; Roes, J.; Krieg, T.; Müller, W.; Roers, A.; Eming, S.A. Differential roles of macrophages in diverse phases of skin repair. J. Immunol. 2010, 184, 3964–3977. [Google Scholar] [CrossRef]
- Okuno, Y.; Nakamura-Ishizu, A.; Kishi, K.; Suda, T.; Kubota, Y. Bone marrow-derived cells serve as proangiogenic macrophages but not endothelial cells in wound healing. Blood 2011, 117, 5264–5272. [Google Scholar] [CrossRef]
- Chen, H.; Li, J.; Liang, S.; Lin, B.; Peng, Q.; Zhao, P.; Cui, J.; Rao, Y. Effect of hypoxia-inducible factor-1/vascular endothelial growth factor signaling pathway on spinal cord injury in rats. Exp. Ther. Med. 2017, 13, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Long, H.Q.; Li, G.S.; Cheng, X.; Xu, J.H.; Li, F.B. Role of hypoxia-induced VEGF in blood-spinal cord barrier disruption in chronic spinal cord injury. Chin. J. Traumatol. 2015, 18, 293–295. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Ren, Q.X.; Yang, W.F.; Chen, X.L.; Lu, C.; Sun, J. Influences of HIF-lα on Bax/Bcl-2 and VEGF expressions in rats with spinal cord injury. Int. J. Clin. Exp. Pathol. 2013, 6, 2312–2322. [Google Scholar] [PubMed]
- Johansson, C.B.; Momma, S.; Clarke, D.L.; Risling, M.; Lendahl, U.; Frisén, J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell 1999, 96, 25–34. [Google Scholar] [CrossRef]
- Azari, M.F.; Profyris, C.; Zang, D.W.; Petratos, S.; Cheema, S.S. Induction of endogenous neural precursors in mouse models of spinal cord injury and disease. Eur. J. Neurol. 2005, 12, 638–648. [Google Scholar] [CrossRef]
- Pineau, I.; Lacroix, S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: Multiphasic expression pattern and identification of the cell types involved. J. Comp. Neurol. 2007, 500, 267–285. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.-H.; He, H.; Chen, Y.-N.; Liu, Z.; Romani, M.D.; Xu, Z.-Y.; Xu, Y.; Lin, F.-Y. Exosomes derived from M2 Macrophages Improve Angiogenesis and Functional Recovery after Spinal Cord Injury through HIF-1α/VEGF Axis. Brain Sci. 2022, 12, 1322. https://doi.org/10.3390/brainsci12101322
Huang J-H, He H, Chen Y-N, Liu Z, Romani MD, Xu Z-Y, Xu Y, Lin F-Y. Exosomes derived from M2 Macrophages Improve Angiogenesis and Functional Recovery after Spinal Cord Injury through HIF-1α/VEGF Axis. Brain Sciences. 2022; 12(10):1322. https://doi.org/10.3390/brainsci12101322
Chicago/Turabian StyleHuang, Jiang-Hu, Hang He, Yong-Neng Chen, Zhen Liu, Manini Daudi Romani, Zhao-Yi Xu, Yang Xu, and Fei-Yue Lin. 2022. "Exosomes derived from M2 Macrophages Improve Angiogenesis and Functional Recovery after Spinal Cord Injury through HIF-1α/VEGF Axis" Brain Sciences 12, no. 10: 1322. https://doi.org/10.3390/brainsci12101322
APA StyleHuang, J.-H., He, H., Chen, Y.-N., Liu, Z., Romani, M. D., Xu, Z.-Y., Xu, Y., & Lin, F.-Y. (2022). Exosomes derived from M2 Macrophages Improve Angiogenesis and Functional Recovery after Spinal Cord Injury through HIF-1α/VEGF Axis. Brain Sciences, 12(10), 1322. https://doi.org/10.3390/brainsci12101322






