Developing a Non-Pharmacological Intervention Programme for Wandering in People with Dementia: Recommendations for Healthcare Providers in Nursing Homes
Abstract
1. Introduction
2. Materials and Methods
2.1. Stage 1: Literature Search
2.1.1. Identification of the Research Question and Domains
2.1.2. Literature Search Strategy
2.1.3. Inclusion and Exclusion Criteria
- The participants were older adults with any form of dementia.
- The study was about specific NPIs for wandering in PwD.
- Articles meeting the following criteria were excluded:
- The study only included pharmacological interventions.
- Interventions were implemented in hospitals.
- Conference abstracts, protocols, introductions, or reviews other than systematic ones.
2.1.4. Quality Assessment of the Included Studies
2.1.5. Data Collection
2.2. Stage 2: Development of the Intervention Programme
2.3. Stage 3: Validation Process
3. Results
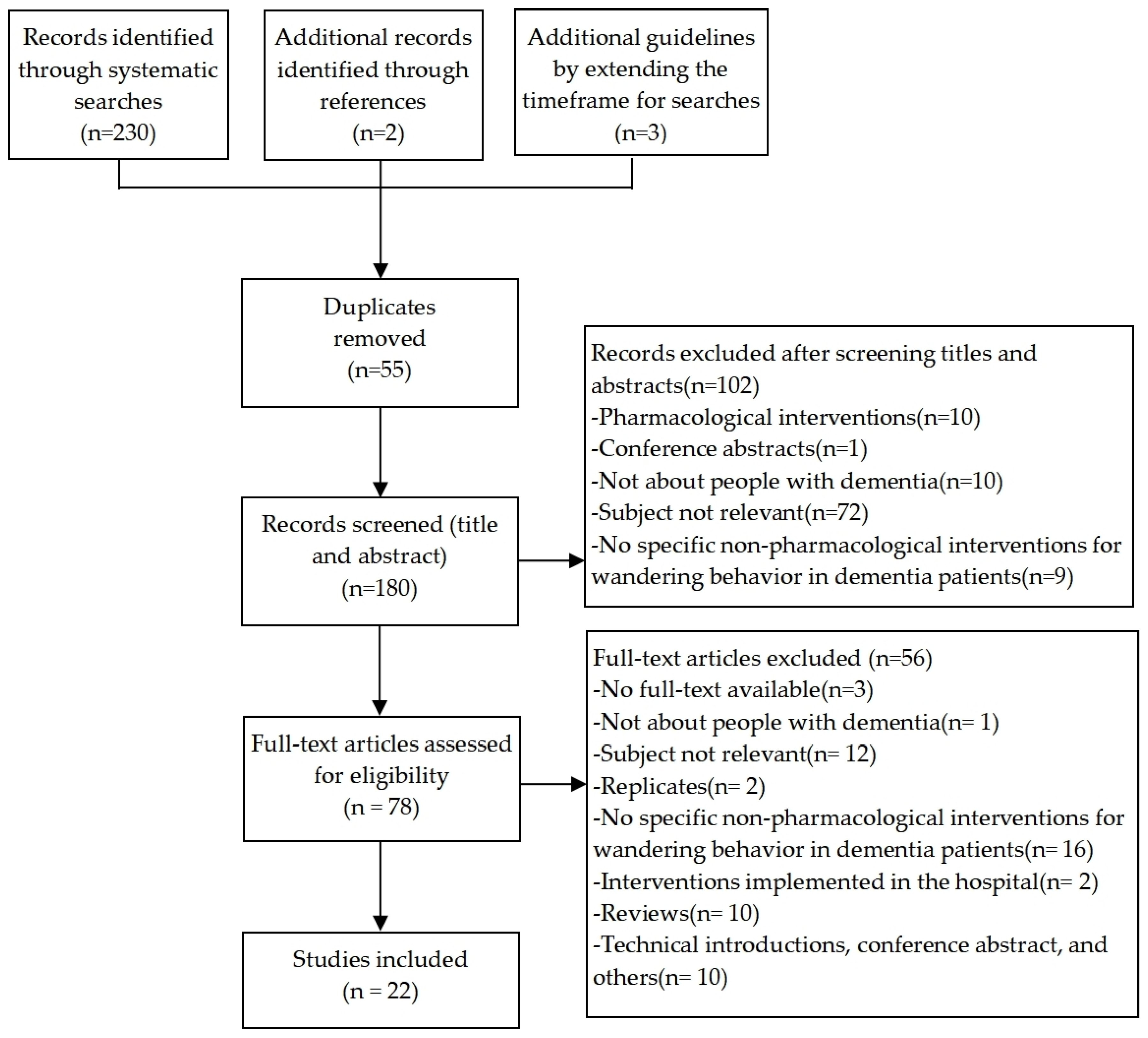
3.1. Search Results
3.2. Characteristics of the Included Studies
3.3. Quality Appraisal
3.4. Expert and User Validation
3.5. Recommendations Based on Levels of Evidence and Grades of Recommendation
3.6. Caregiver Education
- Keep calm.
- Contact the local police immediately and provide a recent colour photo of the missing person, a description of his or her clothes, and details about past walking experiences, favourite places, or anywhere the person may have gone.
- Search the house and the surrounding buildings immediately.
- The initial 6 to 12 h of the search should cover an eight-mile radius around the location where the lost person was last seen, concentrating on open, populated areas, including the inside of easily accessible buildings.
- If the missing person has not been found, intense foot searches should focus on natural and sparsely populated areas, beginning within a two-mile radius of the last known location and extending from there, and ponds, gardens, and tree lines should be carefully searched.
- Search strategies should not be based on personal characteristics and experiences since PwD often exhibit unpredictable behaviour when lost.
- If the missing person has not been found, searches should continue through the night.
- If PwD travelled by bus or subway, initial search efforts should focus on locating the vehicle.
3.7. Preventing Excessive Wandering
3.8. Promoting Safe Walking
3.9. Preventing PwD from Going Missing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
Appendix A
| Search | Query |
|---|---|
| #1 | Dementia.mp. [mp = text, heading word, subject area node, title] |
| #2 | Dementias.mp. [mp = text, heading word, subject area node, title] |
| #3 | Amentia.mp. [mp = text, heading word, subject area node, title] |
| #4 | Amentias.mp. [mp = text, heading word, subject area node, title] |
| #5 | Senile Paranoid Dementia.mp. [mp = text, heading word, subject area node, title] |
| #6 | Dementias, Senile Paranoid.mp. [mp = text, heading word, subject area node, title] |
| #7 | Paranoid Dementia, Senile.mp. [mp = text, heading word, subject area node, title] |
| #8 | Paranoid Dementias, Senile.mp. [mp = text, heading word, subject area node, title] |
| #9 | Senile Paranoid Dementias.mp. [mp = text, heading word, subject area node, title] |
| #10 | Familial Dementia.mp. [mp = text, heading word, subject area node, title] |
| #11 | Dementia, Familial.mp. [mp = text, heading word, subject area node, title] |
| #12 | Dementias, Familial.mp. [mp = text, heading word, subject area node, title] |
| #13 | Familial Dementias.mp. [mp = text, heading word, subject area node, title] |
| #14 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 |
| #15 | Wandering Behavior.mp. [mp = text, heading word, subject area node, title] |
| #16 | Behavior, Wandering.mp. [mp = text, heading word, subject area node, title] |
| #17 | #15 OR #16 |
| #18 | #14 AND #17 |
| #19 | limit #18 to yr = ”2016–2021“ |
| Search | Query |
|---|---|
| #1 | “Dementia” [Mesh] |
| #2 | Dementias [Title/Abstract] OR Amentia [Title/Abstract] OR Amentias [Title/Abstract] OR Senile Paranoid Dementia [Title/Abstract] OR Dementias, Senile Paranoid [Title/Abstract] OR Paranoid Dementia, Senile [Title/Abstract] OR Paranoid Dementias, Senile [Title/Abstract] OR Senile Paranoid Dementias [Title/Abstract] OR Familial Dementia [Title/Abstract] OR Dementia, Familial [Title/Abstract] OR Dementias, Familial [Title/Abstract] OR Familial Dementias [Title/Abstract] |
| #3 | “Wandering Behavior” [Mesh] |
| #4 | Behavior, Wandering [Title/Abstract] |
| #5 | #1 OR #2 |
| #6 | #3 OR #4 |
| #7 | #5 AND #6 |
| Limiters: Published Date: 20160101–20210630 | |
| Search | Query |
|---|---|
| #1 | ‘Dementia’/exp |
| #2 | ‘Dementias’:ab,ti |
| #3 | ‘Amentia’:ab,ti |
| #4 | ‘Amentias’:ab,ti |
| #5 | ‘Senile Paranoid Dementia’:ab,ti |
| #6 | ‘Dementias, Senile Paranoid’:ab,ti |
| #7 | ‘Paranoid Dementia, Senile’:ab,ti |
| #8 | ‘Paranoid Dementias, Senile’:ab,ti |
| #9 | ‘Senile Paranoid Dementias’:ab,ti |
| #10 | ‘Familial Dementia’:ab,ti |
| #11 | ‘Dementia, Familial’:ab,ti |
| #12 | ‘Dementias, Familial’:ab,ti |
| #13 | ‘Familial Dementias’:ab,ti |
| #14 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 |
| #15 | ‘Wandering Behavior’/exp |
| #16 | ‘Behavior, Wandering’:ab,ti |
| #17 | #15 OR #16 |
| #18 | #14 AND #17 |
| Limiters: Published Date: 2016–2021 | |
| Search | Query |
|---|---|
| #1 | (MM “Dementia”) |
| #2 | TI(Dementias OR Amentia OR Amentias OR Senile Paranoid Dementia OR Dementias, Senile Paranoid OR Paranoid Dementia, Senile) OR AB(Dementias OR Amentia OR Amentias OR Senile Paranoid Dementia OR Dementias, Senile Paranoid OR Paranoid Dementia, Senile) |
| #3 | TI(Paranoid Dementias, Senile OR Senile Paranoid Dementias OR Familial Dementia OR Dementia, Familial OR Dementias, Familial OR Familial Dementias) OR AB(Paranoid Dementias, Senile OR Senile Paranoid Dementias OR Familial Dementia OR Dementia, Familial OR Dementias, Familial OR Familial Dementias) |
| #4 | (MM “Wandering Behavior”) |
| #5 | TI(Behavior, Wandering) OR AB(Behavior, Wandering) |
| #6 | #1 OR #2 OR #3 |
| #7 | #4 OR #5 |
| #8 | #6 AND #7 |
| Limiters: Published Date: 201601–202106, Search modes: Boolean/Phrase, Apply related words, Full Text | |
| Search | Query |
|---|---|
| #1 | MeSH descriptor: [Dementia] explode all trees |
| #2 | (Dementias OR Amentia OR Amentias OR Senile Paranoid Dementia OR Dementias, Senile Paranoid): ti,ab,kw |
| #3 | (Paranoid Dementia, Senile OR Paranoid Dementias, Senile OR Senile Paranoid Dementias OR Familial Dementia OR Dementia, Familial): ti,ab,kw |
| #4 | (Dementias, Familial OR Familial Dementias): ti,ab,kw |
| #5 | #1 OR #2 OR #3 OR #4 |
| #6 | MeSH descriptor: [Wandering Behavior] explode all trees |
| #7 | (Behavior, Wandering): ti,ab,kw |
| #8 | #6 OR #7 |
| #9 | #5 AND #8 |
| Limiters: Published Date: 20160101–20210630(Cochrane Reviews), 2016–2021(Trials) | |
Appendix B
| Study | Domain 1 (%) | Domain 2 (%) | Domain 3 (%) | Domain 4 (%) | Domain 5 (%) | Domain 6 (%) | Total Score Mean (SD) (%) | Overall Quality | Overall Recommendation | Quality |
|---|---|---|---|---|---|---|---|---|---|---|
| Futrell et al., 2014 [28] | 91.7 | 61.1 | 29.2 | 80.6 | 41.7 | 91.7 | 66.0 (26.5) | 4 | Recommended with modifications | Average |
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Overall Appraisal |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Husebo et al., 2020 [46] | Y | Y | Y | Y | U | U | U | U | U | Y | Y | Include |
| Jensen et al., 2017 [27] | Y | U | Y | Y | U | Y | Y | Y | Y | Y | Y | Include |
| Howes et al., 2021 [23] | Y | Y | Y | Y | Y | Y | Y | U | U | Y | Y | Include |
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Overall Appraisal |
|---|---|---|---|---|---|---|---|---|---|---|
| Bautrant et al., 2019 [25] | Y | Y | U | N | U | Y | Y | U | Y | Include |
| Lau et al., 2019 [26] | Y | Y | U | N | U | Y | Y | U | U | Include |
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Overall Appraisal |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sato et al., 2018 [47] | Y | Y | U | Y | Y | Y | Y | Y | Y | NA | Y | Include |
| Bowen et al., 2018 [48] | Y | Y | Y | Y | Y | U | Y | Y | Y | U | Y | Include |
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Overall Appraisal |
|---|---|---|---|---|---|---|---|---|---|
| Shih et al., 2017 [49] | Y | Y | Y | Y | Y | Y | Y | Y | Include |
| Leung et al., 2020 [30] | Y | Y | U | U | U | U | U | Y | Include |
References
- World Alzheimer Report 2019: Attitudes to Dementia. Available online: https://www.alzint.org/u/WorldAlzheimerReport2019.pdf (accessed on 21 November 2021).
- Xu, J.; Wang, J.; Wimo, A.; Fratiglioni, L.; Qiu, C. The economic burden of dementia in China, 1990–2030: Implications for health policy. Bull. World Health Organ. 2017, 95, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 5 September 2022).
- Jia, J.; Wei, C.; Chen, S.; Li, F.; Tang, Y.; Qin, W.; Zhao, L.; Jin, H.; Xu, H.; Wang, F.; et al. The cost of Alzheimer’s disease in China and re-estimation of costs worldwide. Alzheimers Dement. 2018, 14, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Bessey, L.J.; Walaszek, A. Management of Behavioral and Psychological Symptoms of Dementia. Curr. Psychiatry Rep. 2019, 21, 66. [Google Scholar] [CrossRef] [PubMed]
- Backhouse, T.; Camino, J.; Mioshi, E. What Do We Know about Behavioral Crises in Dementia? A Systematic Review. J. Alzheimers Dis. 2018, 62, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, G.; Lucetti, C.; Nuti, A.; Danti, S. Wandering and dementia. Psychogeriatrics 2014, 14, 135–142. [Google Scholar] [CrossRef]
- MacAndrew, M.; Beattie, E.; O’Reilly, M.; Kolanowski, A.; Windsor, C. The Trajectory of Tolerance for Wandering-related Boundary Transgression: An Exploration of Care Staff and Family Perceptions. Gerontologist 2017, 57, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Mangini, L.; Wick, J.Y. Wandering: Unearthing New Tracking Devices. Consult. Pharm. 2017, 32, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Barrett, B.; Bulat, T.; Schultz, S.K.; Luther, S.L. Factors Associated with Wandering Behaviors in Veterans with Mild Dementia: A Prospective Longitudinal Community-Based Study. Am. J. Alzheimers Dis. Other Demen. 2018, 33, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Adekoya, A.A.; Guse, L. Wandering Behavior from the Perspectives of Older Adults with Mild to Moderate Dementia in Long-Term Care. Res. Gerontol. Nurs. 2019, 12, 239–247. [Google Scholar] [CrossRef]
- Bowen, M.E.; Rowe, M. Wandering Behaviors and Activities of Daily Living Among Older Adults with Cognitive Impairment. Rehabil. Nurs. 2019, 44, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Luther, S.L.; Volicer, L.; Algase, D.; Beattie, E.; Brown, L.M.; Molinari, V.; Moore, H.; Joseph, I. Risk assessment of wandering behavior in mild dementia. Int. J. Geriatr. Psychiatry 2016, 31, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Kales, H.C.; Gitlin, L.N.; Lyketsos, C.G. Assessment and management of behavioral and psychological symptoms of dementia. BMJ 2015, 350, h369. [Google Scholar] [CrossRef] [PubMed]
- Cheung, G.; Su, A.Y.; Wu, K.; Yue, B.; Yates, S.; Martinez Ruiz, A.; Krishnamurthi, R.; Cullum, S. The Understanding and Experiences of Living with Dementia in Chinese New Zealanders. Int. J. Environ. Res. Public Health 2022, 19, 1280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Clarke, C.L.; Rhynas, S.J. Tensions in dementia care in China: An interpretative phenomenological study from Shandong province. Int. J. Older People Nurs. 2020, 15, e12291. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, L.; Ding, Y.; Chan, H.Y.L. Understanding dementia care in care home setting in China: An exploratory qualitative study. Health Soc. Care Community 2021, 29, 1511–1521. [Google Scholar] [CrossRef]
- Kong, E.H.; Kim, H.; Kim, H. Nursing home staff’s perceptions of barriers and needs in implementing person-centred care for people living with dementia: A qualitative study. J. Clin. Nurs. 2022, 31, 1896–1906. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, L.; Ding, Y.; Shan, Y.; Chan, H.Y.L. Translation and validation of Chinese version of sense of competence in dementia care staff scale in healthcare providers: A cross-sectional study. BMC Nurs. 2022, 21, 35. [Google Scholar] [CrossRef]
- Wang, S.; Cheung, D.S.K.; Leung, A.Y.M. Overview of dementia care under the three-tier long-term care system of China. Public Health Nurs. 2019, 36, 199–206. [Google Scholar] [CrossRef]
- Wu, C.; Gao, L.; Chen, S.; Dong, H. Care services for elderly people with dementia in rural China: A case study. Bull. World Health Organ. 2016, 94, 167–173. [Google Scholar] [CrossRef]
- Neubauer, N.A.; Azad-Khaneghah, P.; Miguel-Cruz, A.; Liu, L. What do we know about strategies to manage dementia-related wandering? A scoping review. Alzheimers Dement. 2018, 10, 615–628. [Google Scholar]
- Howes, J.; Gastmans, C. Electronic tracking devices in dementia care: A systematic review of argument-based ethics literature. Arch. Gerontol. Geriatr. 2021, 95, 104419. [Google Scholar] [CrossRef] [PubMed]
- Kamil, R.J.; Bakar, D.; Ehrenburg, M.; Wei, E.X.; Pletnikova, A.; Xiao, G.; Oh, E.S.; Mancini, M.; Agrawal, Y. Detection of Wandering Behaviors Using a Body-Worn Inertial Sensor in Patients With Cognitive Impairment: A Feasibility Study. Front. Neurol. 2021, 12, 529661. [Google Scholar] [CrossRef] [PubMed]
- Bautrant, T.; Grino, M.; Peloso, C.; Schiettecatte, F.; Planelles, M.; Oliver, C.; Franqui, C. Impact of Environmental Modifications to Enhance Day-Night Orientation on Behavior of Nursing Home Residents with Dementia. J. Am. Med. Dir. Assoc. 2019, 20, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.M.; Chan, T.Y.; Szeto, S.L. Effectiveness of a home-based missing incident prevention program for community-dwelling elderly patients with dementia. Int. Psychogeriatr. 2019, 31, 91–99. [Google Scholar] [CrossRef]
- Jensen, L.; Padilla, R. Effectiveness of Environment-Based Interventions That Address Behavior, Perception, and Falls in People With Alzheimer’s Disease and Related Major Neurocognitive Disorders: A Systematic Review. Am. J. Occup. Ther. 2017, 71, 7105180030p1–7105180030p10. [Google Scholar] [CrossRef]
- Futrell, M.; Melillo, K.D.; Remington, R.; Butcher, H.K. Evidence-based practice guideline: Wandering. J. Gerontol. Nurs. 2014, 40, 16–23. [Google Scholar] [CrossRef]
- Gu, L. Nursing interventions in managing wandering behavior in patients with dementia: A literature review. Arch. Psychiatr. Nurs. 2015, 29, 454–457. [Google Scholar] [CrossRef]
- Leung, M.; Wang, C.; Kwok, T.C. Effects of supporting facilities on memory loss among older people with dementia in care and attention homes. Indoor Built Environ. 2020, 29, 438–448. [Google Scholar] [CrossRef]
- Wandering and Getting Lost: Who’s at Risk and How to Be Prepared. Available online: https://alz.org/media/Documents/alzheimers-dementia-wandering-behavior-ts.pdf (accessed on 21 November 2021).
- Falls: Prevention in Community-Dwelling Older Persons. Available online: https://www.uptodate.com/contents/falls-prevention-in-community-dwelling-older-persons?search=Falls:%20Prevention%20in%20community-dwelling%20older%20persons&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1 (accessed on 5 September 2022).
- Adriaenssens, J.; Eyssen, M.; Jonckheer, P.; Vriesacker, K.; Sonnaert, M. The Belgian Evidence-Based Practice Program: Network governance to improve efficiency and effectiveness of evidence-based practice uptake. Int. J. Evid. Based Healthc. 2019, 17, S68–S71. [Google Scholar] [CrossRef]
- Alper, B.S.; Haynes, R.B. EBHC pyramid 5.0 for accessing preappraised evidence and guidance. Evid. Based Med. 2016, 21, 123–125. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Member Associations. Available online: https://www.alzint.org/our-members/member-associations/ (accessed on 21 November 2021).
- The AGREE II Instrument. Available online: http://www.agreetrust.org (accessed on 21 November 2021).
- Chiappini, E.; Bortone, B.; Galli, L.; de Martino, M. Guidelines for the symptomatic management of fever in children: Systematic review of the literature and quality appraisal with AGREE II. BMJ Open 2017, 7, e015404. [Google Scholar] [CrossRef] [PubMed]
- Yeganeh, L.; Boyle, J.A.; Wood, A.; Teede, H.; Vincent, A.J. Menopause guideline appraisal and algorithm development for premature ovarian insufficiency. Maturitas 2019, 130, 21–31. [Google Scholar] [CrossRef]
- Messina, C.; Bignotti, B.; Tagliafico, A.; Orlandi, D.; Corazza, A.; Sardanelli, F.; Sconfienza, L.M. A critical appraisal of the quality of adult musculoskeletal ultrasound guidelines using the AGREE II tool: An EuroAIM initiative. Insights Imaging 2017, 8, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Aromataris, E.; Fernandez, R.; Godfrey, C.; Holly, C.; Kahlil, H.; Tungpunkom, P. Summarizing systematic reviews: Methodological development, conduct and reporting of an Umbrella review approach. Int. J. Evid. Based Healthc. 2015, 13, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K.; et al. Chapter 7: Systematic reviews of etiology and risk. In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; JBI: Adelaide, Australia, 2020. [Google Scholar]
- Tufanaru, C.; Munn, Z.; Aromataris, E.; Campbell, J.; Hopp, L. Chapter 3: Systematic reviews of effectiveness. In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; JBI: Adelaide, Australia, 2020. [Google Scholar]
- Husebo, B.S.; Heintz, H.L.; Berge, L.I.; Owoyemi, P.; Rahman, A.T.; Vahia, I.V. Sensing Technology to Monitor Behavioral and Psychological Symptoms and to Assess Treatment Response in People with Dementia. A Systematic Review. Front. Pharmacol. 2020, 10, 1699. [Google Scholar] [CrossRef]
- Shih, Y.H.; Pai, M.C.; Huang, Y.C.; Wang, J.J. Sundown Syndrome, Sleep Quality, and Walking Among Community-Dwelling People with Alzheimer Disease. J. Am. Med. Dir. Assoc. 2017, 18, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Kakamu, T.; Hayakawa, T.; Kumagai, T.; Hidaka, T.; Masuishi, Y.; Endo, S.; Fukushima, T. Predicting falls from behavioral and psychological symptoms of dementia in older people residing in facilities. Geriatr. Gerontol. Int. 2018, 18, 1573–1577. [Google Scholar] [CrossRef] [PubMed]
- Bowen, M.E.; Crenshaw, J.; Stanhope, S.J. Balance ability and cognitive impairment influence sustained walking in an assisted living facility. Arch. Gerontol. Geriatr. 2018, 77, 133–141. [Google Scholar] [CrossRef]
- JBI. Recommended Practice. Wandering: Management. JBI EBP Database 2021, JBI-RP-4256-1. [Google Scholar]
- Sleep-Wake Disturbances and Sleep Disorders in Patients with Dementia. Available online: https://www.uptodate.com/contents/sleep-wake-disturbances-and-sleep-disorders-in-patients-with-dementia?search=Sleep-wake%20disturbances%20and%20sleep%20disorders%20in%20patients%20with%20dementia.&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1 (accessed on 5 September 2022).
- Management of the Patient with Dementia. Available online: https://www.uptodate.com/contents/management-of-the-patient-with-dementia (accessed on 5 September 2022).
- Koh, G. Evidence Summary. Dementia: Management of Wandering. JBI EBP Database 2021, JBI-ES-2040-1. [Google Scholar]
- Wandering. Available online: https://www.dementia.org.au/national/support-and-services/carers/behaviour-changes/wandering (accessed on 21 November 2021).
- Home Safety. Available online: https://www.alz.org/help-support/caregiving/safety/home-safety (accessed on 21 November 2021).
- Keeping the Home Safe. Available online: https://alzheimers.org.za/keeping-the-home-safe/ (accessed on 21 November 2021).
- Wandering. Available online: https://www.eng.hkada.org.hk/wandering (accessed on 21 November 2021).
- Safer Walking. Available online: https://cdn.alzheimers.org.nz/wp-content/uploads/2021/04/Info_Sheet_Safe-walking-2.pdf (accessed on 21 November 2021).
- How Technology Can Help. Available online: https://www.alzheimers.org.uk/get-support/staying-independent/how-technology-can-help (accessed on 21 November 2021).
- JBI Levels of Evidence. Available online: https://jbi.global/sites/default/files/2019-05/JBI-Levels-of-evidence_2014_0.pdf (accessed on 21 November 2021).
- JBI Grades of Recommendation. Available online: https://jbi.global/sites/default/files/2019-05/JBI-grades-of-recommendation_2014.pdf (accessed on 21 November 2021).
- Byard, R.W.; Langlois, N.E.I. Wandering Dementia-A Syndrome with Forensic Implications. J. Forensic. Sci. 2019, 64, 443–445. [Google Scholar] [CrossRef] [PubMed]
- Sleep Issues and Sundowning. Available online: https://www.alz.org/help-support/caregiving/stages-behaviors/sleep-issues-sundowning (accessed on 21 November 2021).
- Andrews, J. “Wandering” and dementia. Br. J. Community Nurs. 2017, 22, 322–323. [Google Scholar] [CrossRef] [PubMed]
- Keall, M.D.; Tupara, H.; Pierse, N.; Wilkie, M.; Baker, M.G.; Howden-Chapman, P.; Cunningham, C. Home modifications to prevent home fall injuries in houses with Māori occupants (MHIPI): A randomised controlled trial. Lancet Public Health 2021, 6, e631–e640. [Google Scholar] [CrossRef]
- Green, Y.S. Safety Implications for the Homebound Patient with Dementia. Home Healthc. Now 2018, 36, 386–391. [Google Scholar] [CrossRef]
- Tricco, A.C.; Thomas, S.M.; Veroniki, A.A.; Hamid, J.S.; Cogo, E.; Strifler, L.; Khan, P.A.; Robson, R.; Sibley, K.M.; MacDonald, H.; et al. Comparisons of Interventions for Preventing Falls in Older Adults: A Systematic Review and Meta-analysis. JAMA 2017, 318, 1687–1699. [Google Scholar] [CrossRef]
- Lomas-Vega, R.; Obrero-Gaitán, E.; Molina-Ortega, F.J.; Del-Pino-Casado, R. Tai Chi for Risk of Falls. A Meta-analysis. J. Am. Geriatr. Soc. 2017, 65, 2037–2043. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Song, G.G. Interventions to Prevent Falls in Older Adults. JAMA 2018, 319, 1382. [Google Scholar] [CrossRef] [PubMed]
- Gulka, H.J.; Patel, V.; Arora, T.; McArthur, C.; Iaboni, A. Efficacy and Generalizability of Falls Prevention Interventions in Nursing Homes: A Systematic Review and Meta-analysis. J. Am. Med. Dir. Assoc. 2020, 21, 1024–1035.e4. [Google Scholar] [CrossRef]
- Sherrington, C.; Michaleff, Z.A.; Fairhall, N.; Paul, S.S.; Tiedemann, A.; Whitney, J.; Cumming, R.G.; Herbert, R.D.; Close, J.C.T.; Lord, S.R. Exercise to prevent falls in older adults: An updated systematic review and meta-analysis. Br. J. Sports Med. 2017, 51, 1750–1758. [Google Scholar] [CrossRef] [PubMed]
- Harvey, L.; Mitchell, R.; Brodaty, H.; Draper, B.; Close, J. Differing trends in fall-related fracture and non-fracture injuries in older people with and without dementia. Arch. Gerontol. Geriatr. 2016, 67, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Mishler, A.D.; Neider, M.B. Improving Wayfinding for Older Users with Selective Attention Deficits. Ergon. Des. 2017, 25, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Pulido Herrera, E. Location-based technologies for supporting elderly pedestrian in ‘‘getting lost’’ events. Disabil. Rehabil. Assist. Technol. 2017, 12, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.; Burrow, S.; Pusey, H. What are the perceptions of people living with dementia, family carers, professionals and other potential stakeholders to the use of global positioning systems to promote safer outdoor walking?: A qualitative literature review. Disabil. Rehabil. Assist. Technol. 2021, 16, 614–623. [Google Scholar] [CrossRef]
- Eibling, D.; Fried, M.; Blitzer, A.; Postma, G. Commentary on the role of expert opinion in developing evidence-based guidelines. Laryngoscope 2014, 124, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Piers, R.; Albers, G.; Gilissen, J.; De Lepeleire, J.; Steyaert, J.; Van Mechelen, W.; Steeman, E.; Dillen, L.; Vanden Berghe, P.; Van den Block, L. Advance care planning in dementia: Recommendations for healthcare professionals. BMC Palliat. Care 2018, 17, 88. [Google Scholar] [CrossRef]
| The Alzheimer’s Society | Website | Location |
|---|---|---|
| Alzheimer’s Disease International | https://www.alz.co.uk | The global |
| China Association for Alzheimer’s Disease | http://caad.org.cn | China |
| Dementia Australia | http://www.dementia.org.au | Australia |
| Alzheimer’s Association | http://alz.org | USA |
| Alzheimer’s Society | http://www.alheimers.org.uk | UK |
| Hong Kong Alzheimer’s Disease Association | http://www.hkada.org.hk | Hong Kong, China |
| Alzheimer’s New Zealand | http://www.alzheimers.org.nz | New Zealand |
| Alzheimer’s South Africa | http://www.alzheimers.org.za | South Africa |
| Dementia Singapore | http://alz.org.sg | Singapore |
| Systematic Reviews (n = 3) | ||||
| Study (Author, Year) | Study Type | Number of Publications Included (n) | Overall Appraisal a | |
| 1 | Husebo et al., 2020 [44] | Systematic Review | 34 | Include |
| 2 | Jensen et al., 2017 [27] | Systematic Review | 42 | Include |
| 3 | Howes et al., 2021 [23] | Systematic Review | 22 | Include |
| Quantitative and Experimental Research (n = 6) | ||||
| Study (Author, Year) | Study Type | Setting (Sample, n) | Overall Appraisal a | |
| 1 | Shih et al., 2017 [45] | Analytical cross-sectional study | Participants from dementia outpatient clinics of several hospitals and long-term care resource management centres in southern Taiwan (n = 184) | Include |
| 2 | Leung et al., 2020 [30] | Analytical cross-sectional study | Elders with dementia living in care and attention homes in Hong Kong (n = 65) | Include |
| 3 | Lau et al., 2019 [26] | Quasi-experimental study | Patients from a hospital-based geriatric memory clinic (n = 54) | Include |
| 4 | Bautrant et al., 2019 [25] | Quasi-experimental study | Patients aged 65 years or older (n = 19) | Include |
| 5 | Sato et al., 2018 [46] | Cohort study | People from three geriatric health service facilities (n = 242) | Include |
| 6 | Bowen et al., 2018 [47] | Cohort study | Older adults from a residential care facility (n = 26) | Include |
| Guideline (n = 1) | ||||
| Study (Author, Year) | Study Type | Overall Assessment b | ||
| 1 | Futrell et al., 2014 [28] | Guideline | Recommended with modifications | |
| Recommended Practice, Evidence Summary, and Clinical Decision-Making (n = 5) | ||||
| Study (Author, Year) | Study Type | |||
| 1 | JBI, 2021 [48] | Recommended practice | ||
| 2 | Koh, 2021 [49] | Evidence summary | ||
| 3 | Daniel Press, 2021 [50] | Clinical decision-making | ||
| 4 | Ariel B Neikrug et al., 2022 [51] | Clinical decision-making | ||
| 5 | Douglas P Kiel, 2022 [32] | Clinical decision-making | ||
| Articles from the Alzheimer’s Society (n = 7) | ||||
| Title | Date of Last Update | The Alzheimer’s Society | ||
| 1 | Wandering [52] | Dementia Australia | ||
| 2 | Wandering and getting lost: Who is at risk and how to be prepared [31] | 2020 | Alzheimer’s Association | |
| 3 | Home Safety [53] | Alzheimer’s Association | ||
| 4 | Keeping the home safe [54] | 2017 | Alzheimer’s South Africa | |
| 5 | Wandering [55] | Hong Kong Alzheimer’s disease association | ||
| 6 | Safer Walking [56] | 2019 | Alzheimer’s New Zealand | |
| 7 | How technology can help [57] | Alzheimer’s Society | ||
| Feasibility | Degree of Completion | The Difference between Feasibility and Degree of Completion | ||
|---|---|---|---|---|
| M ± SD | M ± SD | t | p | |
| Domain 2 Preventing excessive wandering | ||||
| 6 | 7.47 ± 2.625 | 7.47 ± 2.495 | 0.000 | 1.000 |
| 7 | 7.18 ± 2.622 | 7.59 ± 2.228 | –1.509 | 0.135 |
| 8 | 7.24 ± 2.993 | 7.41 ± 2.694 | –0.568 | 0.572 |
| 9 | 8.41 ± 2.258 | 8.58 ± 1.982 | –0.819 | 0.416 |
| 10 | 8.46 ± 2.119 | 8.30 ± 2.091 | 0.807 | 0.422 |
| Domain 3 Promoting safe walking | ||||
| 11 | 8.64 ± 2.171 | 8.70 ± 1.973 | –0.231 | 0.818 |
| 12 | 8.63 ± 1.986 | 8.45 ± 2.036 | 0.935 | 0.353 |
| 13 | 7.80 ± 2.697 | 7.94 ± 2.228 | –0.577 | 0.566 |
| 14 | 8.41 ± 2.264 | 8.50 ± 2.036 | –0.357 | 0.722 |
| 15 | 7.21 ± 2.968 | 7.43 ± 2.754 | –0.776 | 0.440 |
| Domain 4 Preventing PwD from going missing | ||||
| 16 | 7.37 ± 2.627 | 7.49 ± 2.615 | –0.386 | 0.700 |
| 17 | 7.38 ± 2.771 | 7.29 ± 2.627 | 0.288 | 0.774 |
| 18 | 7.36 ± 2.595 | 7.11 ± 2.721 | 0.800 | 0.426 |
| 19 | 7.47 ± 2.089 | 7.16 ± 2.649 | 1.168 | 0.246 |
| 20 | 7.21 ± 2.968 | 7.43 ± 2.754 | −0.776 | 0.440 |
| 21 | 8.36 ± 2.089 | 8.22 ± 2.114 | 0.756 | 0.452 |
| Experts (n = 7) | |||
| Professional Background | Working Unit | Seniority (Year) | |
| 1 | Clinical nurse assistant in dementia care | Institution | 30 |
| 2 | Social worker in geriatric and dementia care | College (Japan) | 38 |
| 3 | Geriatric psychiatrist | Hospital | 27 |
| 4 | Clinical nurse assistant in dementia care | Institution | 20 |
| 5 | Neurologist | Hospital | 17 |
| 6 | Specialist nurse in dementia care | Hospital | 23 |
| 7 | Specialist nurse in dementia care | Hospital | 10 |
| End Users (n = 76) | |||
| Participants | Number of Participants (n) | ||
| 1 | Health care professionals in hospitals | 16 | |
| 2 | Health care professionals in institutions | 8 | |
| 3 | Family members | 52 | |
| Recommendations | Levels of Evidence | Grades of Recommendations | |
|---|---|---|---|
| Domain 1 | Caregiver education | ||
| 1 | Wandering may provide physical exercise and social contact and improve appetite, but it can make PwD experience adverse outcomes such as physical injuries from falls and getting lost [46,47] | 3c | A |
| 2 | Ensure continuous supervision to prevent risky situations, as all PwD are at risk of becoming lost, including those who have never wandered before [26,28,48,50] | 2d | A |
| 3 | Physical restraint is an inappropriate intervention to prevent wandering, as it is considered to be ethically problematic [23,49] | / | B |
| 4 | Healthcare providers can choose high-tech strategies, including boundary alarm systems, monitoring systems, and electronic tracking devices for PwD, but the user’s privacy and autonomy should be respected [23,28,44,49,50,57] | 1c | B |
| 5 | When PwD become lost, a response plan including the following steps should be taken [31,50,52,56] | 4d | B |
| - Contact local police immediately, provide information about PwD who got lost, and extend the search through social networks such as WeChat platforms and TikTok short video platforms | |||
| - Search the house and the surrounding buildings immediately | |||
| - The initial 6 to 12 hours of the search should cover an eight-mile radius around the location where he/she disappeared, concentrating on open, populated areas | |||
| - If initial search efforts fail, intense foot searches should focus on natural and sparsely populated areas, beginning within a two-mile radius of the location where he/she disappeared and extending from there | |||
| - Search strategies should not be based on personal characteristics and experiences | |||
| - Searches should continue throughout the night if necessary | |||
| - If PwD travelled by automobile or subway, initial search efforts should focus on locating his/her vehicle | |||
| Domain 2 | Preventing excessive wandering | ||
| 6 | Listen to music chosen according to the patient’s preferences [27,28] | 2d | B |
| 7 | Provide opportunities to engage in social interactions or meaningful activities when PwD are most likely to wander, such as folding laundry, preparing dinner, receiving visitors, or participating in live violin recitals, depending on their ability [28,31,48] | 2d | B |
| 8 | Choose oversized clocks to hang in a prominent position in corridors [25] | 2d | B |
| 9 | Ensuring adequate light during the day (e.g., keeping the environment bright during the day and providing regular supervised exercise, such as walking after meals) helps to reduce wandering at night [25,28,45,48,50,51,55] | 1c | A |
| 10 | Keep the environment dark during the night, and eliminate unnecessary night-time awakenings (e.g., noise) [25,51] | 1c | B |
| Domain 3 | Promoting safe walking | ||
| Provide an environment as safe as possible | |||
| 11 | - Keep the floor clean and remove tripping hazards to promote safe walking, such as excessive clutter, loose mats, and extension cords [28,48,53,54] | / | A |
| 12 | - Minimise stressors from the environment, such as changes in daily routines and furniture arrangements [28,30] | 4b | B |
| 13 | Provide a secure place for PwD to exercise to reduce the risk of falls and fall-related injuries [28,31,47,52] | 3c | A |
| Prepare for a walk | |||
| 14 | - Wear appropriate footwear and walk in the company of healthcare providers [26,50,54] | 2d | A |
| 15 | - Monitoring devices should be used to prevent injuries, such as alarm systems or automatic lights [27] | 1c | B |
| Domain 4 | Preventing PwD from going missing | ||
| Wayfinding cues may reduce disorientation | |||
| 16 | - Provide environmental cues to help PwD find their way, including photographs, posters and murals on walls, and extra-large signage, which should be salient and simple [27,28,30,48] | 4b | B |
| 17 | - Handrails in hallways installed throughout the house should be oriented, continuous, and conspicuous to support dementia patients’ mobility [30] | 4b | B |
| Reduce attempts at exiting | |||
| 18 | - Take advantage of visual stop barriers to reduce attempts at exiting, including camouflaged doors, horizontal grids of black tape in front of exits, safety covers, and cloth of the same colour as the door in front of exit doors [27,28,31] | 2d | B |
| 19 | - Divert attention by using tactile boards, interactive walls, and 3D wall art [28] | 2d | B |
| 20 | - Use high-tech strategies, such as warning bells above doors, monitoring systems, and tracking devices with GPS [26,44,48,50,52] | 1c | B |
| 21 | Don’t leave PwD unsupervised [26] | 2d | A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhang, G.; Min, M.; Xing, Y.; Chen, H.; Li, C.; Li, C.; Zhou, H.; Li, X. Developing a Non-Pharmacological Intervention Programme for Wandering in People with Dementia: Recommendations for Healthcare Providers in Nursing Homes. Brain Sci. 2022, 12, 1321. https://doi.org/10.3390/brainsci12101321
Wang J, Zhang G, Min M, Xing Y, Chen H, Li C, Li C, Zhou H, Li X. Developing a Non-Pharmacological Intervention Programme for Wandering in People with Dementia: Recommendations for Healthcare Providers in Nursing Homes. Brain Sciences. 2022; 12(10):1321. https://doi.org/10.3390/brainsci12101321
Chicago/Turabian StyleWang, Jing, Ge Zhang, Min Min, Ying Xing, Hongli Chen, Cheng Li, Caifu Li, Hanhan Zhou, and Xianwen Li. 2022. "Developing a Non-Pharmacological Intervention Programme for Wandering in People with Dementia: Recommendations for Healthcare Providers in Nursing Homes" Brain Sciences 12, no. 10: 1321. https://doi.org/10.3390/brainsci12101321
APA StyleWang, J., Zhang, G., Min, M., Xing, Y., Chen, H., Li, C., Li, C., Zhou, H., & Li, X. (2022). Developing a Non-Pharmacological Intervention Programme for Wandering in People with Dementia: Recommendations for Healthcare Providers in Nursing Homes. Brain Sciences, 12(10), 1321. https://doi.org/10.3390/brainsci12101321






