A Scientific Approach to Conscious Experience, Introspection, and Unconscious Processing: Vision and Blindsight
Abstract
1. Introduction: The Theoretical Background
2. Psychological Terms Understood as Theoretical Concepts
3. Conscious Visual Experience and Unconscious “Vision” (Blindsight)
4. How to Distinguish between Conscious Vision, Non-Visual Experience and Blindsight?
5. Introducing the Concepts “Introspection”, “Conscious Vision” and “Blindsight”
- (1)
- p1 not significant: no visual experience;
- (2)
- p1 significant and p2 not significant: visual processing at a given level (represented by the value of p1) without conscious experience;
- (3)
- p1 significant and p2 significant: visual processing at a given level (represented by the value of p1) and conscious experience at a given level (represented by the value of p2).
6. The Neurobiological Basis of Conscious Vision and Blindsight
6.1. Levels of Activation of Impaired Cortical Networks Resulting in Blindsight or Conscious Vision
6.2. Normal and Impaired Neural Networks in the Visual Cortex That Mediate Conscious Vision and Blindsight
6.3. Is Blindsight Mediated by the Secondary Visual Pathway?
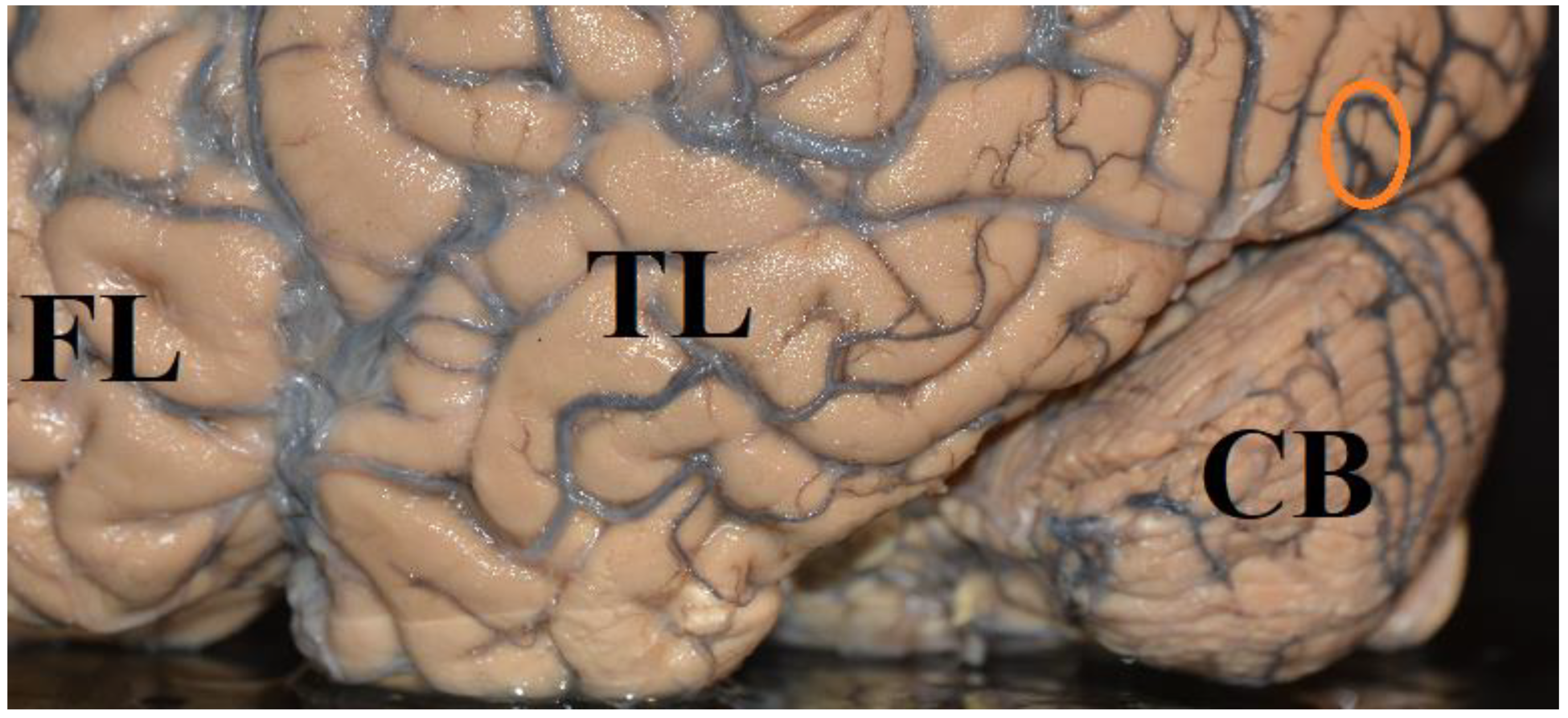
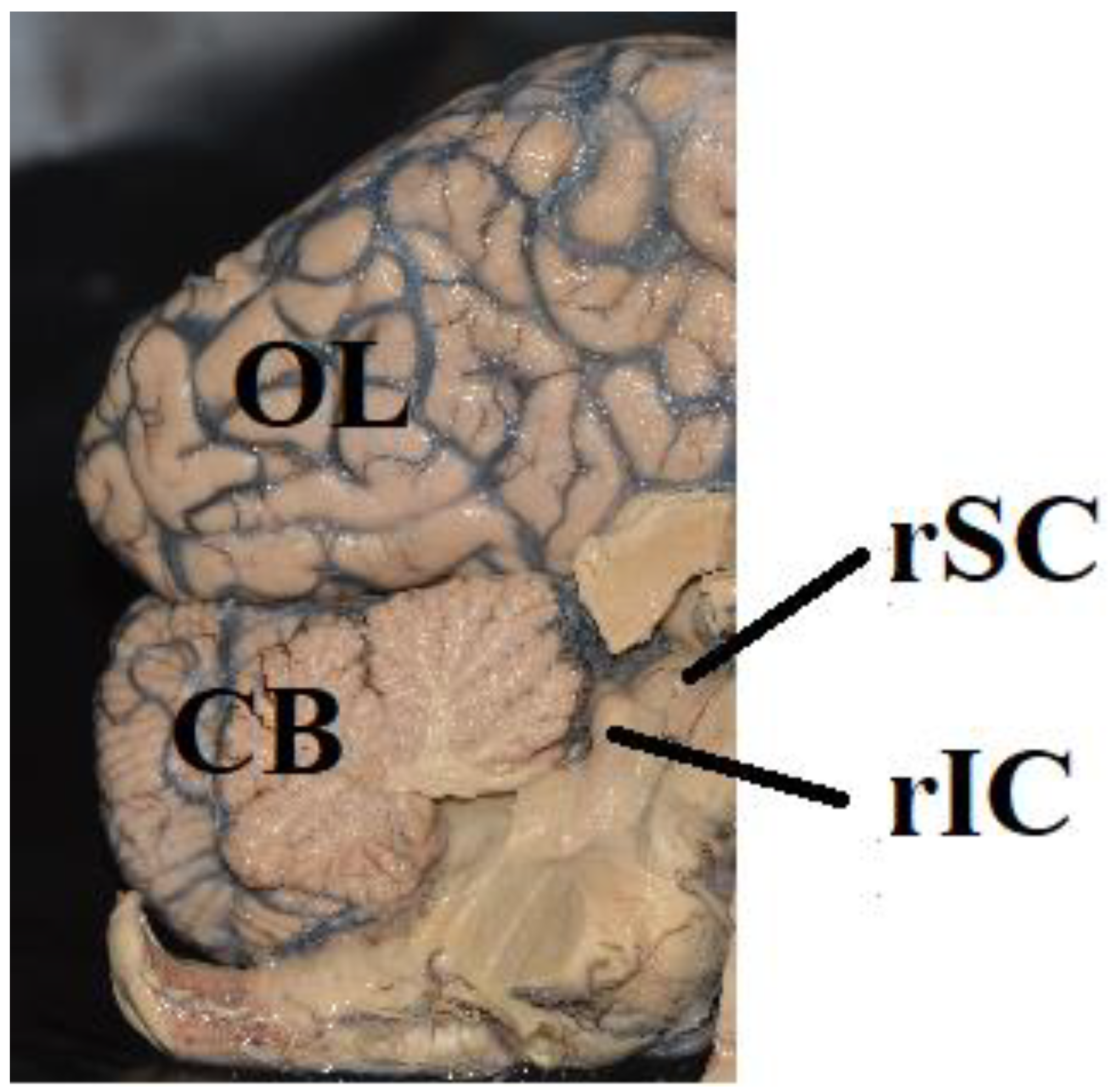
6.4. How Much Cortex Is Needed for Conscious Visual Perception?
6.5. Sensory Capacity of the Human Colliculi
6.6. A Functional Visual System Is Not Sufficient for Conscious Visual Experience
7. Summary and Conclusions
Funding
Conflicts of Interest
References
- Watson, J.B. Psychology as the behaviorist views it. Psycholol. Rev. 1913, 20, 158–177. [Google Scholar] [CrossRef]
- Wittgenstein, L. Philosophische Untersuchungen. In Wittgenstein, Schriften Volume 1; Suhrkamp: Frankfurt, Germany, 2003. [Google Scholar]
- Ryle, G. The Concept of Mind; Hutchinson: London, UK, 1949. [Google Scholar]
- Wittgenstein, L. The Blue and Brown Books: Preliminary Studies for the “Philosophical Investigations”; Harper & Row: New York, NY, USA, 1958. [Google Scholar]
- Carnap, R. Psychologie in physikalifcher Sprache. Erkenntnis 1932, 3, 107–142. [Google Scholar] [CrossRef]
- Carnap, R. The Methodological Character of Theoretical Concepts. In Minnesota Studies in the Philosophy of Science; University of Minnesota Press: Minneapolis, MI, USA, 1956; pp. 38–76. [Google Scholar]
- Skinner, B.F. Science and Human Behavior; Collier-MacMillan: New York, NY, USA; London, UK, 1953. [Google Scholar]
- Skinner, B.F. About Behaviorism; Knopf: New York, NY, USA, 1974. [Google Scholar]
- Carnap, R. “Testability and Meaning”. Philos. Sci. 1936, 3, 420–468. [Google Scholar] [CrossRef]
- Carnap, R. Beobachtungssprache und theoretische Sprache. Dialectica 1958, 12, 236–248. [Google Scholar] [CrossRef]
- American Psychiatric Association. The Diagnostic and Statistical Manual of Mental Disorders, DSM5; APA: Washington, DC, USA; London, UK, 2013; pp. 197–198. [Google Scholar]
- Carnap, R. Meaning Postulates. Philos. Stud. 1952, 3, 65–73. [Google Scholar] [CrossRef]
- Pöppel, E.; Held, R.; Frost, D. Residual visual function in patients with lesions of the central visual pathways. Nature 1973, 256, 489–490. [Google Scholar] [CrossRef]
- Weiskrantz, L.; Warrington, E.K.; Sanders, M.D.; Marshall, J. Visual capacity in the hemianopic field following a restricted occipital lesion. Brain 1974, 97, 709–728. [Google Scholar] [CrossRef]
- Perenin, M.T.; Jeannerod, M. Residual vision in cortically blind hemifields. Neuropsychologia 1975, 13, 1–7. [Google Scholar] [CrossRef]
- Perenin, M.T.; Jeannerod, M. Visual function within the hemianopic field following early hemidecortication in man: 1. Spatial localization. Neuropsychologia 1978, 16, 1–13. [Google Scholar] [CrossRef]
- Perenin, M.T. Visual function within the hemianopic field following early cerebral hemidecortication in man- II. Pattern discrimination. Neuropsychologia 1978, 16, 697–708. [Google Scholar] [CrossRef]
- Perenin, M.; Ruel, J.; Hécaen, H. Residual Visual Capacities in a Case of Cortical Blindness. Cortex 1980, 16, 605–612. [Google Scholar] [CrossRef]
- Zihl, J.; von Cramon, D. Registration of light stimuli in the cortically blind hemifield and its effect on localization. Behav. Brain Res. 1980, 1, 287–298. [Google Scholar] [CrossRef]
- Arnott, G.; Guieuj, D.; Blond, S.; Charlie, J.; Vanecloo, F.M.; Lejeune, E.; Clarisse, J.; Delandsheer, E.; Laine, E. Hémispherectomie droite totale. Étude neurophysiologique après vingt-six ans. Rev. Neurol. 1982, 138, 305–316. [Google Scholar] [PubMed]
- Zihl, J.; Werth, R. Contributions to the study of “Blindsight”—I. Can stray light account for saccadic localization in patients with postgeniculate field defects? Neuropsychologia 1984, 22, 1–11. [Google Scholar] [CrossRef]
- Zihl, J.; Werth, R. Contributions to the study of “Blindsight”—II. The role of specific practice for saccadic localization in patients with postgeniculate visual field defects. Neuropsychologia 1984, 22, 13–22. [Google Scholar] [CrossRef]
- Stoerig, P.; Hübner, M.; Pöppel, E. Signal detection analysis of residual vision in a field defect due to a post-geniculate lesion. Neuropsychologia 1985, 23, 589–599. [Google Scholar] [CrossRef]
- Stoerig, P.; Cowey, A. Spectral sensitivity in blindsight. Nature 1989, 342, 916–918. [Google Scholar] [CrossRef]
- Corbetta, M.; Marzi, C.A.; Tassinari, G.; Aglioti, S. Effectiveness of different task paradigms in revealing blindsight. Brain 1990, 113, 603–616. [Google Scholar] [CrossRef]
- Brent, P.J.; Kennard, C.; Ruddock, K.H. Residual colour vision in a human hemianope: Spectral responses and colour discrimination. Proc. R. Soc. B Biol. Sci. 1994, 256, 219–225. [Google Scholar] [CrossRef]
- Trevethan, C.; Sahraie, A.; Weiskrantz, L. Form discrimination in a case of blindsight. Neuropsychologia 2007, 45, 2092–2103. [Google Scholar] [CrossRef]
- Alexander, I.; Cowey, A. The cortical basis of global motion detection in blindsight. Exp. Brain Res. 2009, 192, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Celeghin, A.; Barabas, M.; Mancini, F.; Bendini, M.; Pedrotti, E.; Prior, M.; Cantagallo, A.; Savazzi, S.; Marzi, C.A. Speeded manual responses to unseen visual stimuli in hemianopic patients: What kind of blindsight? Conscious. Cogn. 2014, 32, 6–14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Smits, A.; Seijdel, N.; Scholte, H.; Heywood, C.; Kentridge, R.; de Haan, E. Action blindsight and antipointing in a hemianopic patient. Neuropsychologia 2019, 128, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Campion, J.; Latto, R.; Smith, Y.M. Is blindsight an effect of scattered-light, spared cortex, and near-threshold vision. Behav. Brain Sci. 1983, 6, 423–447. [Google Scholar] [CrossRef]
- Weiskrantz, L.; Barbur, J.L.; Sahraie, A. Parameters affecting conscious versus unconscious visual discrimination with damage to the visual cortex (V1). Proc. Natl. Acad. Sci. USA 1995, 92, 6122–6126. [Google Scholar] [CrossRef]
- Ffytche, D.H.; Zeki, S. The primary visual cortex, and feedback to it, are not necessary for conscious vision. Brain 2011, 134, 247–257. [Google Scholar] [CrossRef]
- Zeki, S.; Ffytche, D.H. The Riddoch syndrome: Insights into the neurobiology of conscious vision. Brain 1998, 121, 25–45. [Google Scholar] [CrossRef]
- Riddoch, G. On the Relative Perceptions of Movement and a Stationary Object in Certain Visual Disturbances due to Occipital Injuries. Proc. R. Soc. Med. 1917, 10, 13–34. [Google Scholar] [CrossRef]
- Werth, R. Bewusstsein: Psychologische, Neurobiologische und Wissenschaftstheoretische Aspekte; Springer: Berlin/Heidelberg, Germany; New York, NY, USA; Tokyo, Japan, 1983. [Google Scholar]
- Werth, R. Cerebral blindness and plasticity of the visual system in children. A review of visual capacities in patients with occipital lesions hemispherectomy or hydranencephaly. Restor. Neurol. Neurosci. 2008, 26, 377–389. [Google Scholar]
- Azzopardi, P.; Cowey, A. Is blindsight like normal, near-threshold vision? Proc. Natl. Acad. Sci. USA 1997, 94, 14190–14194. [Google Scholar] [CrossRef]
- Kentridge, R.W.; Heywood, C.A.; Weiskrantz, L. Residual Vision in Multiple Retinal Locations within a Scotoma: Implications for Blindsight. J. Cogn. Neurosci. 1997, 9, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Sahraie, A.; Weiskrantz, L.; Barbur, J.L.; Simmons, A.; Williams, S.C.R.; Brammer, M.J. Pattern of neuronal activity associated with conscious and unconscious processing of visual signals. Proc. Natl. Acad. Sci. USA 1997, 94, 9406–9411. [Google Scholar] [CrossRef] [PubMed]
- Lau, H.C.; Passingham, R.E. Relative blindsight in normal observers and the neural correlate of visual consciousness. Proc. Natl. Acad. Sci. USA 2006, 103, 18763–18768. [Google Scholar] [CrossRef] [PubMed]
- Sahraie, A.; Trevethan, C.T.; MacLeod, M.J.; Murray, A.D.; Olson, J.A.; Weiskrantz, L. Increased sensitivity after repeated stimulation of residual spatial channels in blindsight. Proc. Natl. Acad. Sci. USA 2006, 103, 14971–14976. [Google Scholar] [CrossRef] [PubMed]
- Overgaard, M.; Grünbaum, T. Consciousness and modality: On the possible preserved visual consciousness in blindsight subjects. Conscious. Cogn. 2011, 20, 1855–1859. [Google Scholar] [CrossRef] [PubMed]
- Balsdon, T.; Azzopardi, P. Absolute and relative blindsight. Conscious. Cogn. 2015, 32, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Foley, R. The case for Ccaracterizing type-2 blindsight as a genuinely visual phenomenon. Conscious. Cogn. 2015, 32, 56–67. [Google Scholar] [CrossRef]
- Kentridge, R.W. What is it like to have type-2 blindsight? Drawing inferences from residual function in type-1 blindsight. Conscious. Cogn. 2015, 32, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, F. The structure of experience, the nature of the visual, and type 2 blindsight. Conscious. Cogn. 2015, 32, 104–128. [Google Scholar] [CrossRef]
- Bollini, A.; Sanchez-Lopez, J.; Savazzi, S.; Marzi, C.A. Lights from the Dark: Neural Responses from a Blind Visual Hemifield. Front. Neurosci. 2017, 11, 290. [Google Scholar] [CrossRef]
- Ramsøy, T.Z.; Overgaard, M. Introspection and subliminal perception. Phenomenol. Cogn. Sci. 2004, 3, 1–23. [Google Scholar] [CrossRef]
- Mazzi, C.; Bagattini, C.; Savazzi, S. Blind-Sight vs. Degraded-Sight: Different Measures Tell a Different Story. Front. Psychol. 2016, 7, 901. [Google Scholar] [CrossRef] [PubMed]
- Mazzi, C.; Tagliabue, C.; Mazzeo, G.; Savazzi, S. Reliability in reporting perceptual experience: Behaviour and electrophysiology in hemianopic patients. Neuropsychologia 2019, 128, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Barleben, M.; Stoppel, C.M.; Kaufmann, J.; Merkel, C.; Wecke, T.; Goertler, M.; Heinze, H.-J.; Hopf, J.-M.; Schoenfeld, M.A. Neural correlates of visual motion processing without awareness in patients with striate cortex and pulvinar lesions. Hum. Brain Mapp. 2015, 36, 1585–1594. [Google Scholar] [CrossRef]
- Railo, H.; Hurme, M. Is the primary visual cortex necessary for blindsight-like behavior? Review of transcranial magnetic stimulation studies in neurologically healthy individuals. Neurosci. Biobehav. Rev. 2021, 127, 353–364. [Google Scholar] [CrossRef]
- Blau, U. Abstract objects. Theoret. Linguist. 1981, 8, 131–144. [Google Scholar] [CrossRef]
- Werth, R. Visual functions without the occipital lobe or after cerebral hemispherectomy in infancy. Eur. J. Neurosci. 2006, 24, 2932–2944. [Google Scholar] [CrossRef]
- Werth, R.; Moehrenschlager, M. The development of visual functions in cerebrally blind children during a systematic visual field training. Restor. Neurol. Neurosci. 1999, 15, 229–241. [Google Scholar]
- Werth, R.; Seelos, K. Restitution of visual functions in cerebrally blind children. Neuropsychologia 2005, 43, 2011–2023. [Google Scholar] [CrossRef]
- Zihl, J.; von Cramon, D. Restitution of visual function in patients with cerebral blindness. J. Neurol. Neurosurg. Psychiatry 1979, 42, 312–322. [Google Scholar] [CrossRef]
- Zihl, J.; Von Cramon, D. Visual field recovery from scotoma in patients with postgeniculate damage. Brain 1985, 108, 335–365. [Google Scholar] [CrossRef] [PubMed]
- Kasten, E.; Wüst, S.; Behrens-Baumann, W.; Sabel, B.A. Computer-based training for the treatment of partial blindness. Nat. Med. 1998, 4, 1083–1087. [Google Scholar] [CrossRef] [PubMed]
- Werth, R. Residual Visual Function after Loss of Both Cerebral Hemispheres in Infancy. Investig. Opthalmology Vis. Sci. 2007, 48, 3098–3106. [Google Scholar] [CrossRef]
- Fendrich, R.; Wessinger, C.M.; Gazzaniga, M.S. Residual Vision in a Scotoma: Implications for Blindsight. Science 1992, 258, 1489–1491. [Google Scholar] [CrossRef]
- Morland, A.B.; Lê, S.; Carroll, E.; Hoffmann, M.B.; Pambakian, A. The Role of Spared Calcarine Cortex and Lateral Occipital Cortex in the Responses of Human Hemianopes to Visual Motion. J. Cogn. Neurosci. 2004, 16, 204–218. [Google Scholar] [CrossRef] [PubMed]
- Radoeva, P.D.; Prasad, S.; Brainard, D.H.; Aguirre, G.K. Neural Activity within Area V1 Reflects Unconscious Visual Performance in a Case of Blindsight. J. Cogn. Neurosci. 2008, 20, 1927–1939. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, A.; Keliris, G.A.; Papageorgiou, T.D.; Shao, Y.; Krapp, E.; Papageorgiou, E.; Stingl, K.; Bruckmann, A.; Schiefer, U.; Logothetis, N.K.; et al. Population receptive field analysis of the primary visual cortex complements perimetry in patients with homonymous visual field defects. Proc. Natl. Acad. Sci. USA 2014, 111, E1656–E1665. [Google Scholar] [CrossRef]
- Papanikolaou, A.; Keliris, G.A.; Papageorgiou, T.D.; Schiefer, U.; Logothetis, N.K.; Smirnakis, S.M. Organization of area hV5/MT+ in subjects with homonymous visual field defects. NeuroImage 2018, 190, 254–268. [Google Scholar] [CrossRef]
- DeFelipe, J. The Evolution of the Brain, the Human Nature of Cortical Circuits, and Intellectual Creativity. Front. Neuroanat. 2011, 5, 29. [Google Scholar] [CrossRef]
- Gilman, J.P.; Medalla, M.; Luebke, J.I. Area-Specific Features of Pyramidal Neurons—A Comparative Study in Mouse and Rhesus Monkey. Cereb. Cortex 2016, 27, 2078–2094. [Google Scholar] [CrossRef]
- Wildenberg, G.A.; Rosen, M.R.; Lundell, J.; Paukner, D.; Freedman, D.J.; Kasthuri, N. Primate neuronal connections are sparse in cortex as compared to mouse. Cell Rep. 2021, 36, 109709. [Google Scholar] [CrossRef] [PubMed]
- Bryant, K.L.; Suwyn, C.; Reding, K.M.; Smiley, J.F.; Hackett, T.A.; Preuss, T.M. Evidence for Ape and Human Specializations in Geniculostriate Projections from VGLUT2 Immunohistochemistry. Brain Behav. Evol. 2012, 80, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Balaram, P.; Kaas, J.H. Towards a unified scheme of cortical lamination for primary visual cortex across primates: Insights from NeuN and VGLUT2 immunoreactivity. Front. Neuroanat. 2014, 8, 81. [Google Scholar] [CrossRef]
- Bryant, K.L.; Glasser, M.F.; Li, L.; Bae, J.J.-C.; Jacquez, N.J.; Alarcón, L.; Fields, A., 3rd; Preuss, T.M. Organization of extrastriate and temporal cortex in chimpanzees compared to humans and macaques. Cortex 2019, 118, 223–243. [Google Scholar] [CrossRef]
- Isa, T.; Yoshida, M. Neural Mechanism of Blindsight in a Macaque Model. Neuroscience 2021, 469, 138–161. [Google Scholar] [CrossRef] [PubMed]
- Balaram, P.; Kaas, J.H.; Young, N.A. Histological features of layers and sublayers in cortical visual areas V1 and V2 of chimpanzees, macaque monkeys, and humans. Eye Brain 2014, 6, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Schiller, P.H.; Malpeli, J.G. Functional specificity of lateral geniculate nucleus laminae of the rhesus monkey. J. Neurophysiol. 1978, 41, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Michael, C.R. Retinal afferent arborization patterns, dendritic field orientations, and the segregation of function in the lateral geniculate nucleus of the monkey. Proc. Natl. Acad. Sci. USA 1988, 85, 4914–4918. [Google Scholar] [CrossRef]
- Conley, M.; Fitzpatrick, D. Morphology of retinogeniculate axons in the macaque. Vis. Neurosci. 1989, 2, 287–296. [Google Scholar] [CrossRef]
- Rodieck, R.W.; Binmoeller, K.F.; Dineen, J. Parasol and midget ganglion cells of the human retina. J. Comp. Neurol. 1985, 233, 115–132. [Google Scholar] [CrossRef]
- Dacey, D. The mosaic of midget ganglion cells in the human retina. J. Neurosci. 1993, 13, 5334–5355. [Google Scholar] [CrossRef] [PubMed]
- Masri, R.A.; Grünert, U.; Martin, P.R. Analysis of Parvocellular and Magnocellular Visual Pathways in Human Retina. J. Neurosci. 2020, 40, 8132–8148. [Google Scholar] [CrossRef] [PubMed]
- Kaas, J.H.; Huerta, M.F.; Weber, J.T.; Harting, J.K. Patterns of retinal terminations and laminar organization of the lateral geniculate nucleus of primates. J. Comp. Neurol. 1978, 182, 517–553. [Google Scholar] [CrossRef] [PubMed]
- Fries, W. The projection from the lateral geniculate nucleus to the prestriate cortex of the macaque monkey. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1981, 213, 73–80. [Google Scholar] [CrossRef]
- Yukie, M.; Iwai, E. Direct projection from the dorsal lateral geniculate nucleus to the prestriate cortex in macaque monkeys. J. Comp. Neurol. 1981, 201, 81–97. [Google Scholar] [CrossRef]
- Benevento, L.A.; Yoshida, K. The afferent and efferent organization of the lateral geniculo-prestriate pathways in the macaque monkey. J. Comp. Neurol. 1981, 203, 455–474. [Google Scholar] [CrossRef]
- Lyon, D.C.; Kaas, J.H. Connectional Evidence for Dorsal and Ventral V3, and Other Extrastriate Areas in the Prosimian Primate, Galago garnetti. Brain, Behav. Evol. 2002, 59, 114–129. [Google Scholar] [CrossRef]
- Lysakowski, A.; Standage, G.P.; Benevento, L.A. An investigation of collateral projections of the dorsal lateral geniculate nucleus and other subcortical structures to cortical areas V1 and V4 in the macaque monkey: A double label retrograde tracer study. Exp. Brain Res. 1988, 69, 651–661. [Google Scholar] [CrossRef]
- Ferrera, V.P.; Nealey, T.A.; Maunsell, J.H.R. Mixed parvocellular and magnocellular geniculate signals in visual area V4. Nature 1992, 358, 756–758. [Google Scholar] [CrossRef]
- Ferrera, V.; Nealey, T.; Maunsell, J. Responses in macaque visual area V4 following inactivation of the parvocellular and magnocellular LGN pathways. J. Neurosci. 1994, 14, 2080–2088. [Google Scholar] [CrossRef]
- Lyon, D.C.; Rabideau, C. Lack of robust LGN label following transneuronal rabies virus injections into macaque area V4. J. Comp. Neurol. 2012, 520, 2500–2511. [Google Scholar] [CrossRef] [PubMed]
- Maunsell, J.H.; Nealey, T.A.; De Priest, D.D. Magnocellular and parvocellular contributions to responses in the middle temporal visual area (MT) of the macaque monkey. J. Neurosci. 1990, 10, 3323–3334. [Google Scholar] [CrossRef] [PubMed]
- Ffytche, D.; Guy, C.N.; Zeki, S. The parallel visual motion inputs into areas V1 and V5 of human cerebral cortex. Brain 1995, 118, 1375–1394. [Google Scholar] [CrossRef] [PubMed]
- Sincich, L.C.; Park, K.F.; Wohlgemuth, M.; Horton, J.C. Bypassing V1: A direct geniculate input to area MT. Nat. Neurosci. 2004, 7, 1123–1128. [Google Scholar] [CrossRef]
- Hernández-González, A.; Cavada, C.; Reinoso-Suárez, F. The lateral geniculate nucleus projects to the inferior temporal cortex in the macaque monkey. NeuroReport 1994, 5, 2693–2696. [Google Scholar] [CrossRef]
- Hubel, D.H.; Wiesel, T.N. Laminar and columnar distribution of geniculo-cortical fibers in the macaque monkey. J. Comp. Neurol. 1972, 146, 421–450. [Google Scholar] [CrossRef]
- Livingstone, M.S.; Hubel, D.H. Thalamic inputs to cytochrome oxidase-rich regions in monkey visual cortex. Proc. Natl. Acad. Sci. USA 1982, 79, 6098–6101. [Google Scholar] [CrossRef] [PubMed]
- Blasdel, G.; Lund, J. Termination of afferent axons in macaque striate cortex. J. Neurosci. 1983, 3, 1389–1413. [Google Scholar] [CrossRef]
- Fitzpatrick, D.; Lund, J.S.; Blasdel, G.G. Intrinsic connections of macaque striate cortex: Afferent and efferent connections of lamrna 4C. J. Neurosci. 1985, 5, 3329–3349. [Google Scholar] [CrossRef]
- Fitzpatrick, D.; Itoh, K.; Diamond, I. The laminar organization of the lateral geniculate body and the striate cortex in the squirrel monkey (Saimiri sciureus). J. Neurosci. 1983, 3, 673–702. [Google Scholar] [CrossRef]
- Garcia-Marin, V.; Kelly, J.G.; Hawken, M.J. Major Feedforward Thalamic Input Into Layer 4C of Primary Visual Cortex in Primate. Cereb. Cortex 2017, 29, 134–149. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.C.; Hubel, D.H. Regular patchy distribution of cytochrome oxidase staining in primary visual cortex of macaque monkey. Nature 1981, 292, 762–764. [Google Scholar] [CrossRef] [PubMed]
- Irvin, G.E.; Norton, T.T.; Sesma, M.A.; Casagrande, V.A. W-like response properties of interlaminar zone cells in the lateral geniculate nucleus of a primate (Galago crassicaudatus). Brain Res. 1986, 362, 254–270. [Google Scholar] [CrossRef]
- Hendry, S.H.G.; Yoshioka, T.A. neurochemically distinct third channel in the macaque lateral gniculate nucleus. Science 1994, 264, 575–577. [Google Scholar] [CrossRef]
- Jayakumar, J.; Dreher, B.; Vidyasagar, T. Tracking blue cone signals in the primate brain. Clin. Exp. Optom. 2013, 96, 259–266. [Google Scholar] [CrossRef]
- Hendry, S.H.C.; Reid, R.C. The Koniocellular Pathway in Primate Vision. Annu. Rev. Neurosci. 2000, 23, 127–153. [Google Scholar] [CrossRef]
- Casagrande, V.; Yazar, F.; Jones, K.; Ding, Y. The Morphology of the Koniocellular Axon Pathway in the Macaque Monkey. Cereb. Cortex 2007, 17, 2334–2345. [Google Scholar] [CrossRef]
- Rockland, K.S. Cytochrome oxidase “blobs”: A call for more anatomy. Brain Struct. Funct. 2021, 226, 2793–2806. [Google Scholar] [CrossRef]
- Szentágothai, J. Synaptology of the visual cortex. In Handbook of Sensory Physiology; Jung, R., Ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1973; Volume VII 3B, pp. 269–324. [Google Scholar]
- Douglas, R.J.; Martin, K.A. Neuronal circuits of the neocortex. Annu. Rev. Neurosci. 2004, 27, 419–451. [Google Scholar] [CrossRef]
- Vanni, S.; Hokkanen, H.; Werner, F.; Angelucci, A. Anatomy and Physiology of Macaque Visual Cortical Areas V1, V2, and V5/MT: Bases for Biologically Realistic Models. Cereb. Cortex 2020, 30, 3483–3517. [Google Scholar] [CrossRef]
- DeFelipe, J.; López-Cruz, P.L.; Benavides-Piccione, R.; Bielza, C.; Larrañaga, P.; Anderson, S.; Burkhalter, A.; Cauli, B.; Fairén, A.; Feldmeyer, D.; et al. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat. Rev. Neurosci. 2013, 14, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Nassi, J.; Callaway, E.M. Specialized Circuits from Primary Visual Cortex to V2 and Area MT. Neuron 2007, 55, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Blasdel, G.G.; Lund, J.S.; Fitzpatrick, D. Intrinsic connections of macaque striate cortex: Axonal projections of cells outside lamina 4C. J. Neurosci. 1985, 5, 3350–3369. [Google Scholar] [CrossRef] [PubMed]
- Mangia, S.; Tkáč, I.; Gruetter, R.; Van de Moortele, P.-F.; Maraviglia, B.; Uğurbil, K. Sustained Neuronal Activation Raises Oxidative Metabolism to a New Steady-State Level: Evidence from 1H NMR Spectroscopy in the Human Visual Cortex. J. Cereb. Blood Flow Metab. 2007, 27, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.M.; Weinberg, R.J. Ultrastructure of Synapses in the Mammalian Brain. Cold Spring Harb. Perspect. Biol. 2012, 4, a005587. [Google Scholar] [CrossRef]
- Dutertre, S.; Becker, C.-M.; Betz, H. Inhibitory Glycine Receptors: An Update. J. Biol. Chem. 2012, 287, 40216–40223. [Google Scholar] [CrossRef]
- Lim, L.; Mi, D.; Llorca, A.; Marín, O. Development and functional diversification of cortical interneurons. Neuron 2018, 100, 294–313. [Google Scholar] [CrossRef] [PubMed]
- Kurcyus, K.; Annac, E.; Hanning, N.M.; Harris, A.D.; Oeltzschner, G.; Edden, R.; Riedl, V. Opposite Dynamics of GABA and Glutamate Levels in the Occipital Cortex during Visual Processing. J. Neurosci. 2018, 38, 9967–9976. [Google Scholar] [CrossRef]
- Zuber, B.; Nikonenko, I.; Klauser, P.; Muller, D.; Dubochet, J. The mammalian central nervous synaptic cleft contains a high density of periodically organized complexes. Proc. Natl. Acad. Sci. USA 2005, 102, 19192–19197. [Google Scholar] [CrossRef]
- He, Z.; Jin, Y. Intrinsic Control of Axon Regeneration. Neuron 2016, 90, 437–451. [Google Scholar] [CrossRef]
- Yang, J.-L.; Mukda, S.; Chen, S.-D. Diverse roles of mitochondria in ischemic stroke. Redox Biol. 2018, 16, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Chen, S.; Wu, Y.; Murphy, T.H. Prolonged Deficits in Parvalbumin Neuron Stimulation-Evoked Network Activity Despite Recovery of Dendritic Structure and Excitability in the Somatosensory Cortex following Global Ischemia in Mice. J. Neurosci. 2014, 34, 14890–14900. [Google Scholar] [CrossRef] [PubMed]
- Povysheva, N.; Nigam, A.; Brisbin, A.K.; Johnson, J.W.; Barrionuevo, G. Oxygen–Glucose Deprivation Differentially Affects Neocortical Pyramidal Neurons and Parvalbumin-Positive Interneurons. Neuroscience 2019, 412, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.H.; Li, P.; Betts, K.; Liu, R. Two-Photon Imaging of Stroke Onset In Vivo Reveals That NMDA-Receptor Independent Ischemic Depolarization Is the Major Cause of Rapid Reversible Damage to Dendrites and Spines. J. Neurosci. 2008, 28, 1756–1772. [Google Scholar] [CrossRef] [PubMed]
- Risher, W.C.; Ard, D.; Yuan, J.; Kirov, S.A. Recurrent Spontaneous Spreading Depolarizations Facilitate Acute Dendritic Injury in the Ischemic Penumbra. J. Neurosci. 2010, 30, 9859–9868. [Google Scholar] [CrossRef] [PubMed]
- Mostany, R.; Portera-Cailliau, C. Absence of Large-Scale Dendritic Plasticity of Layer 5 Pyramidal Neurons in Peri-Infarct Cortex. J. Neurosci. 2011, 31, 1734–1738. [Google Scholar] [CrossRef]
- Alia, C.; Cangi, D.; Massa, V.; Salluzzo, M.; Vignozzi, L.; Caleo, M.; Spalletti, C. Cell-to-Cell Interactions Mediating Functional Recovery after Stroke. Cells 2021, 10, 3050. [Google Scholar] [CrossRef]
- Stoerig, P.; Cowey, A. Blindsight in man and monkey. Brain 1997, 120, 535–559. [Google Scholar] [CrossRef]
- Stoerig, P. Spatial summation in blindsight. Vis. Neurosci. 1993, 10, 1141–1149. [Google Scholar] [CrossRef]
- Stoerig, P.; Kleinschmidt, A.; Frahm, J. No visual responses in denervated V1. NeuroReport 1998, 9, 21–25. [Google Scholar] [CrossRef]
- Barbur, J.L.; Watson, J.; Frackowiak, R.; Zeki, S. Conscious visual perception without VI. Brain 1993, 116, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Blythe, I.M.; Bromley, J.M.; Kennard, C.; Ruddock, K.H. Visual discrimination of target displacement remains after damage to the striate cortex in humans. Nature 1986, 320, 619–621. [Google Scholar] [CrossRef] [PubMed]
- Stoerig, P.; Cowey, A. Wavelength discrimination in blindsight. Brain 1992, 115, 425–444. [Google Scholar] [CrossRef] [PubMed]
- Morland, A.B.; Ogilvie, J.A.; Ruddock, K.H.; Wright, J.R. Orientation discrimination is impaired in the absence of the striate cortical contribution to human vision. Proc. R. Soc. B Biol. Sci. 1996, 263, 633–640. [Google Scholar] [CrossRef]
- Finlay, A.L.; Jones, S.R.; Morland, A.B.; Ogilvie, J.A.; Ruddock, K.H. Movement in the normal visual hemifield induces a percept in the ’blind’ hemifield of a human hemianope. Proc. R. Soc. B Biol. Sci. 1997, 264, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Benson, P.J.; Guo, K.; Blakemore, C. Direction discrimination of moving gratings and plaids and coherence in dot displays without primary visual cortex (V1). Eur. J. Neurosci. 1998, 10, 3767–3772. [Google Scholar] [CrossRef] [PubMed]
- Kentridge, R.; Heywood, C.; Weiskrantz, L. Effects of temporal cueing on residual visual discrimination in blindsight. Neuropsychologia 1999, 37, 479–483. [Google Scholar] [CrossRef]
- Goebel, R.; Muckli, L.; Zanella, F.E.; Singer, W.; Stoerig, P. Sustained extrastriate cortical activation without visual awareness revealed by fMRI studies of hemianopic patients. Vis. Res. 2001, 41, 1459–1474. [Google Scholar] [CrossRef]
- Aronowski, J.; Zhao, X. Molecular Pathophysiology of Cerebral Hemorrhage: Secondary brain injury. Stroke 2011, 42, 1781–1786. [Google Scholar] [CrossRef]
- Zhang, Y.; Khan, S.; Liu, Y.; Wu, G.; Yong, V.W.; Xue, M. Oxidative Stress Following Intracerebral Hemorrhage: From Molecular Mechanisms to Therapeutic Targets. Front. Immunol. 2022, 13, 847246. [Google Scholar] [CrossRef]
- Cooke, P.; Janowitz, H.; Dougherty, S.E. Neuronal Redevelopment and the Regeneration of Neuromodulatory Axons in the Adult Mammalian Central Nervous System. Front. Cell. Neurosci. 2022, 16, 872501. [Google Scholar] [CrossRef] [PubMed]
- Kajstura, T.J.; Dougherty, S.E.; Linden, D.J. Serotonin axons in the neocortex of the adult female mouse regrow after traumatic brain injury. J. Neurosci. Res. 2018, 96, 512–526. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, S.E.; Kajstura, T.J.; Jin, Y.; Chan-Cortés, M.H.; Kota, A.; Linden, D.J. Catecholaminergic axons in the neocortex of adult mice regrow following brain injury. Exp. Neurol. 2020, 323, 113089. [Google Scholar] [CrossRef] [PubMed]
- Coggan, J.S.; Prescott, S.A.; Bartol, T.M.; Sejnowski, T.J. Imbalance of ionic conductances contributes to diverse symptoms of demyelination. Proc. Natl. Acad. Sci. USA 2010, 107, 20602–20609. [Google Scholar] [CrossRef] [PubMed]
- Love, S. Demyelinating diseases. J. Clin. Pathol. 2006, 59, 1151–1159. [Google Scholar] [CrossRef]
- Garg, N.; Smith, T.W. An update on immunopathogenesis, diagnosis, and treatment of multiple sclerosis. Brain Behav. 2015, 5, e00362. [Google Scholar] [CrossRef]
- Steward, O.; Worley, P. Local Synthesis of Proteins at Synaptic Sites on Dendrites: Role in Synaptic Plasticity and Memory Consolidation? Neurobiol. Learn. Mem. 2002, 78, 508–527. [Google Scholar] [CrossRef]
- Südhof, T.C.; Malenka, R.C. Understanding Synapses: Past, Present, and Future. Neuron 2008, 60, 469–476. [Google Scholar] [CrossRef]
- Large, I.; Bridge, H.; Ahmed, B.; Clare, S.; Kolasinski, J.; Lam, W.W.; Miller, K.L.; Dyrby, T.B.; Parker, A.J.; Smith, J.E.T.; et al. Individual Differences in the Alignment of Structural and Functional Markers of the V5/MT Complex in Primates. Cereb. Cortex 2016, 26, 3928–3944. [Google Scholar] [CrossRef]
- Bridge, H.; Hicks, S.L.; Xie, J.; Okell, T.; Mannan, S.; Alexander, I.; Cowey, A.; Kennard, C. Visual activation of extra-striate cortex in the absence of V1 activation. Neuropsychologia 2010, 48, 4148–4154. [Google Scholar] [CrossRef]
- Ajina, S.; Kennard, C.; Rees, G.; Bridge, H. Motion area V5/MT+ response to global motion in the absence of V1 resembles early visual cortex. Brain 2015, 138, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Ajina, S.; Bridge, H. Subcortical pathways to extrastriate visual cortex underlie residual vision following bilateral damage to V1. Neuropsychologia 2019, 128, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Arcaro, M.; Thaler, L.; Quinlan, D.J.; Monaco, S.; Khan, S.; Valyear, K.; Goebel, R.; Dutton, G.N.; Goodale, M.A.; Kastner, S.; et al. Psychophysical and neuroimaging responses to moving stimuli in a patient with the Riddoch phenomenon due to bilateral visual cortex lesions. Neuropsychologia 2019, 128, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Danckert, J.; Rossetti, Y. Blindsight in action: What can the different sub-types of blindsight tell us about the control of visually guided actions? Neurosci. Biobehav. Rev. 2005, 29, 1035–1046. [Google Scholar] [CrossRef]
- Mazzi, C.; Savazzi, S.; Silvanto, J. On the “blindness” of blindsight: What is the evidence for phenomenal awareness in the absence of primary visual cortex (V1)? Neuropsychologia 2019, 128, 103–108. [Google Scholar] [CrossRef]
- Pedersini, C.A.; Lingnau, A.; Cardobi, N.; Sanchez-Lopez, J.; Savazzi, S.; Marzi, C.A. Neural bases of visual processing of moving and stationary stimuli presented to the blind hemifield of hemianopic patients. Neuropsychologia 2020, 141, 107430. [Google Scholar] [CrossRef]
- Downing, P.E.; Jiang, Y.; Shuman, M.; Kanwisher, N. A Cortical Area Selective for Visual Processing of the Human Body. Science 2001, 293, 2470–2473. [Google Scholar] [CrossRef]
- Weiner, K.S.; Grill-Spector, K. Not one extrastriate body area: Using anatomical landmarks, hMT+, and visual field maps to parcellate limb-selective activations in human lateral occipitotemporal cortex. NeuroImage 2011, 56, 2183–2199. [Google Scholar] [CrossRef]
- Stock, J.V.D.; Tamietto, M.; Zhan, M.; Heinecke, A.; Hervais-Adelman, A.G.; Legrand, L.B.; Pegna, A.J.; De Gelder, B. Neural correlates of body and face perception following bilateral destruction of the primary visual cortices. Front. Behav. Neurosci. 2014, 8, 30. [Google Scholar] [CrossRef]
- Wilson, M.E.; Toyne, M.J. Retino-tectal and cortico-tectal projections inMacaca mulatta. Brain Res. 1970, 24, 395–406. [Google Scholar] [CrossRef]
- Schiller, P.H.; Stryker, M.; Cynader, M.; Berman, N. Response characteristics of single cells in the monkey superior colliculus following ablation or cooling of visual cortex. J. Neurophysiol. 1974, 37, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Schiller, P.H.; Malpeli, J.G. Properties and tectal projections of monkey retinal ganglion cells. J. Neurophysiol. 1977, 40, 428–445. [Google Scholar] [CrossRef]
- Pollack, L.J.G.; Hickey, T.L. The distribution of retino-collicular axon terminals in rhesus monkey. J. Comp. Neurol. 1979, 185, 587–602. [Google Scholar] [CrossRef] [PubMed]
- May, P.J. The mammalian superior colliculus: Laminar structure and connections. Prog. Brain Res. 2006, 151, 321–378. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Hoffmann, K.-P.; Distler, C.; Hafed, Z.M. The Foveal Visual Representation of the Primate Superior Colliculus. Curr. Biol. 2019, 29, 2109–2119.e7. [Google Scholar] [CrossRef] [PubMed]
- Dilbeck, M.D.; Spahr, Z.R.; Nanjappa, R.; Economides, J.R.; Horton, J.C. Columnar and Laminar Segregation of Retinal Input to the Primate Superior Colliculus Revealed by Anterograde Tracer Injection Into Each Eye. Investig. Opthalmology Vis. Sci. 2022, 63, 9. [Google Scholar] [CrossRef]
- Perry, V.; Cowey, A. Retinal ganglion cells that project to the superior colliculus and pretectum in the macaque monkey. Neuroscience 1984, 12, 1125–1137. [Google Scholar] [CrossRef]
- Schiller, P.H.; Koerner, F. Discharge characteristics of single units in superior colliculus of the alert rhesus monkey. J. Neurophysiol. 1971, 34, 920–936. [Google Scholar] [CrossRef]
- Cynader, M.; Berman, N. Receptive-field organization of monkey superior colliculus. J. Neurophysiol. 1972, 35, 187–201. [Google Scholar] [CrossRef]
- Goldberg, M.E.; Wurtz, R.H. Activity of superior colliculus in behaving monkey. I. Visual receptive fields of single neurons. J. Neurophysiol. 1972, 35, 542–559. [Google Scholar] [CrossRef]
- Marrocco, R.T.; Li, R.H. Monkey superior colliculus: Properties of single cells and their afferent inputs. J. Neurophysiol. 1977, 40, 844–860. [Google Scholar] [CrossRef] [PubMed]
- Rizzolatti, G.; Buchtel, H.A.; Camarda, R.; Scandolara, C. Neurons with complex visual properties in the superior colliculus of the macaque monkey. Exp. Brain Res. 1980, 38, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.A.; Kastner, S.; Roska, B.; Molnar, A.; Werblin, F.S. Visual Responses of the Human Superior Colliculus: A High-Resolution Functional Magnetic Resonance Imaging Study. J. Neurophysiol. 2005, 94, 2491–2503. [Google Scholar] [CrossRef] [PubMed]
- Kaas, J.H.; Lyon, D.C. Pulvinar contributions to the dorsal and ventral streams of visual processing in primates. Brain Res. Rev. 2007, 55, 285–296. [Google Scholar] [CrossRef]
- Gattass, R.; Galkin, T.W.; Desimone, R.; Ungerleider, L.G. Subcortical connections of area V4 in the macaque. J. Comp. Neurol. 2014, 522, 1941–1965. [Google Scholar] [CrossRef]
- Stepniewska, I.; Qi, H.-X.; Kaas, J.H. Do superior colliculus projection zones in the inferior pulvinar project to MT in primates? Eur. J. Neurosci. 1999, 11, 469–480. [Google Scholar] [CrossRef]
- Stepniewska, I.; Qi, H.-X.; Kaas, J.H. Projections of the superior colliculus to subdivisions of the inferior pulvinar in New World and Old World monkeys. Vis. Neurosci. 2000, 17, 529–549. [Google Scholar] [CrossRef]
- Adams, M.M.; Hof, P.R.; Gattass, R.; Webster, M.J.; Ungerleider, L.G. Visual cortical projections and chemoarchitecture of macaque monkey pulvinar. J. Comp. Neurol. 2000, 419, 377–393. [Google Scholar] [CrossRef]
- Rockland, K.S. Distinctive Spatial and Laminar Organization of Single Axons from Lateral Pulvinar in the Macaque. Vision 2019, 4, 1. [Google Scholar] [CrossRef]
- Kaas, J.H.; Baldwin, M.K.L. The Evolution of the Pulvinar Complex in Primates and Its Role in the Dorsal and Ventral Streams of Cortical Processing. Vision 2019, 4, 3. [Google Scholar] [CrossRef]
- Baldwin, M.K.; Wong, P.; Reed, J.L.; Kaas, J.H. Superior colliculus connections with visual thalamus in gray squirrels (Sciurus carolinensis): Evidence for four subdivisions within the pulvinar complex. J. Comp. Neurol. 2011, 519, 1071–1094. [Google Scholar] [CrossRef] [PubMed]
- Arcaro, M.J.; Pinsk, M.A.; Kastner, S. The Anatomical and Functional Organization of the Human Visual Pulvinar. J. Neurosci. 2015, 35, 9848–9871. [Google Scholar] [CrossRef] [PubMed]
- Bender, D.B. Retinotopic organization of macaque pulvinar. J. Neurophysiol. 1981, 46, 672–693. [Google Scholar] [CrossRef] [PubMed]
- Georgy, L.; Celeghin, A.; Marzi, C.A.; Tamietto, M.; Ptito, A. The superior colliculus is sensitive to gestalt-like stimulus configuration in hemispherectomy patients. Cortex 2016, 81, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Vallar, G. Spatial hemineglect in humans. Trends Cogn. Sci. 1998, 2, 87–97. [Google Scholar] [CrossRef]
- Werth, R.; von Cramon, D.; Zihl, J. Neglect: Phänomene halbseitiger Vernachlässigung. Fortschr. Neurol. Psychiat. 1986, 54, 1–34. [Google Scholar] [CrossRef]
- Mesulam, M.-M. Spatial attention and neglect: Parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1999, 354, 1325–1346. [Google Scholar] [CrossRef]
- Mort, D.J.; Malhotra, P.; Mannan, S.K.; Rorden, C.; Pambakian, A.; Kennard, C.; Husain, M. The anatomy of visual neglect. Brain 2003, 126, 1986–1997. [Google Scholar] [CrossRef]
- Hillis, A.E.; Newhart, M.; Heidler, J.; Barker, P.B.; Herskovits, E.H.; Degaonkar, M. Anatomy of Spatial Attention: Insights from Perfusion Imaging and Hemispatial Neglect in Acute Stroke. J. Neurosci. 2005, 25, 3161–3167. [Google Scholar] [CrossRef]
- Saxena, S.; Keser, Z.; Rorden, C.; Bonilha, L.; Fridriksson, J.; Walker, A.; Hillis, A.E. Disruptions of the Human Connectome Associated With Hemispatial Neglect. Neurology 2022, 98, e107–e114. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Werth, R. A Scientific Approach to Conscious Experience, Introspection, and Unconscious Processing: Vision and Blindsight. Brain Sci. 2022, 12, 1305. https://doi.org/10.3390/brainsci12101305
Werth R. A Scientific Approach to Conscious Experience, Introspection, and Unconscious Processing: Vision and Blindsight. Brain Sciences. 2022; 12(10):1305. https://doi.org/10.3390/brainsci12101305
Chicago/Turabian StyleWerth, Reinhard. 2022. "A Scientific Approach to Conscious Experience, Introspection, and Unconscious Processing: Vision and Blindsight" Brain Sciences 12, no. 10: 1305. https://doi.org/10.3390/brainsci12101305
APA StyleWerth, R. (2022). A Scientific Approach to Conscious Experience, Introspection, and Unconscious Processing: Vision and Blindsight. Brain Sciences, 12(10), 1305. https://doi.org/10.3390/brainsci12101305




