Different Music Training Modulates Theta Brain Oscillations Associated with Executive Function
Abstract
1. Introduction
2. Method and Materials
2.1. Participants
- Basic Information Tests: This test is mainly aimed at gathering basic information about the subjects such as gender, age, occupation, and education level. It includes 10 questions. Among them, the age and education level have been analyzed to guarantee the subject data standardization, of which the specific p-value, mean value, and standard deviation are listed in the article.
- Self-rating Anxiety Scale (SAS) & Self-rating Depression Scale (SDS): The SAS was developed by Zung in 1971 to measure anxiety and the SDS was developed by Zung in 1965 to measure depression. Both of them have proven sufficient reliability and have constructed validity to justify further application in scientific research. In our article, we have to use these tests to assure that all the subjects were in a mentally healthy status. Both of them include 20 questions. The results for different groups show no discrepancy.
- The Big five: This test is meant to measure the personality of each subject. It includes 48 questions and defines one personality as Neuroticism (whether susceptible to pressure), Extraversion (whether outgoing and optimistic), Openness (whether creative and innovative), Agreeableness (whether cooperative and friendly), Conscientiousness (whether responsible and magnanimous). The results show no discrepancy in different groups, implying that they have similar backgrounds and life experiences. In our study, this study could have further use in future research.
- Edinburgh Handedness Inventory: In our article, we used the short form Edinburgh Handedness Inventory which was developed in 2014 by Veale. This is an improved version based on confirmatory factor analysis. Furthermore, we utilized this test to make sure that all the subjects were right-handed.
- Barcelona Music Reward Questionnaire (BMRQ): This questionnaire is intended to gather the music preferences of the subjects. It includes 20 questions and defines four types of music preferences: Sensory Motor (inclined to listen to the music along with humming, clapping, or dancing), Mood Regulation (inclined to get emotional, sentimental, or affectionate when listening to music), Musical Seeking (inclined to constantly seek for new music), and Social Reward (inclined to build connections with others through playing, listening or talking about music). This scale could be applied in future research.
- Montreal Battery of Evaluation of Musical Abilities (MBEMA): This questionnaire is intended to gather the musical abilities of the subjects. It includes 59 questions. It includes questions about musical abilities such as absolute pitch and relative pitch, music theory, composing, and improvisation. It also includes questions about musical information such as the onset age, the years of formal musical training and different stages of learning music, and how many hours of practice per day and week. The results for String and Piano show no discrepancy either.
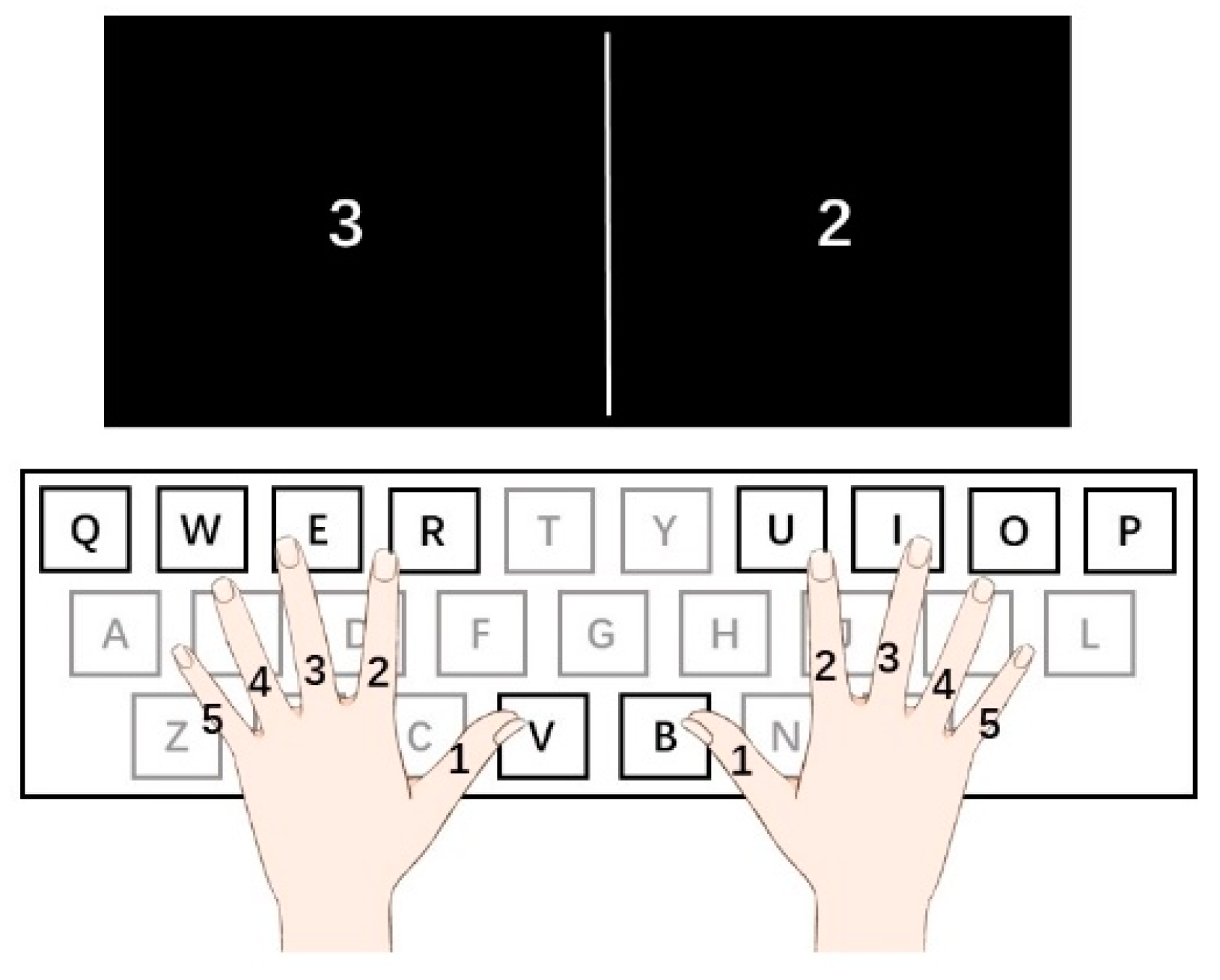
2.2. Procedure
2.3. EEG Recording and Data Preprocessing
2.4. Time-Frequency Analysis
2.5. Functional Connectivity
2.6. Statistics
3. Results
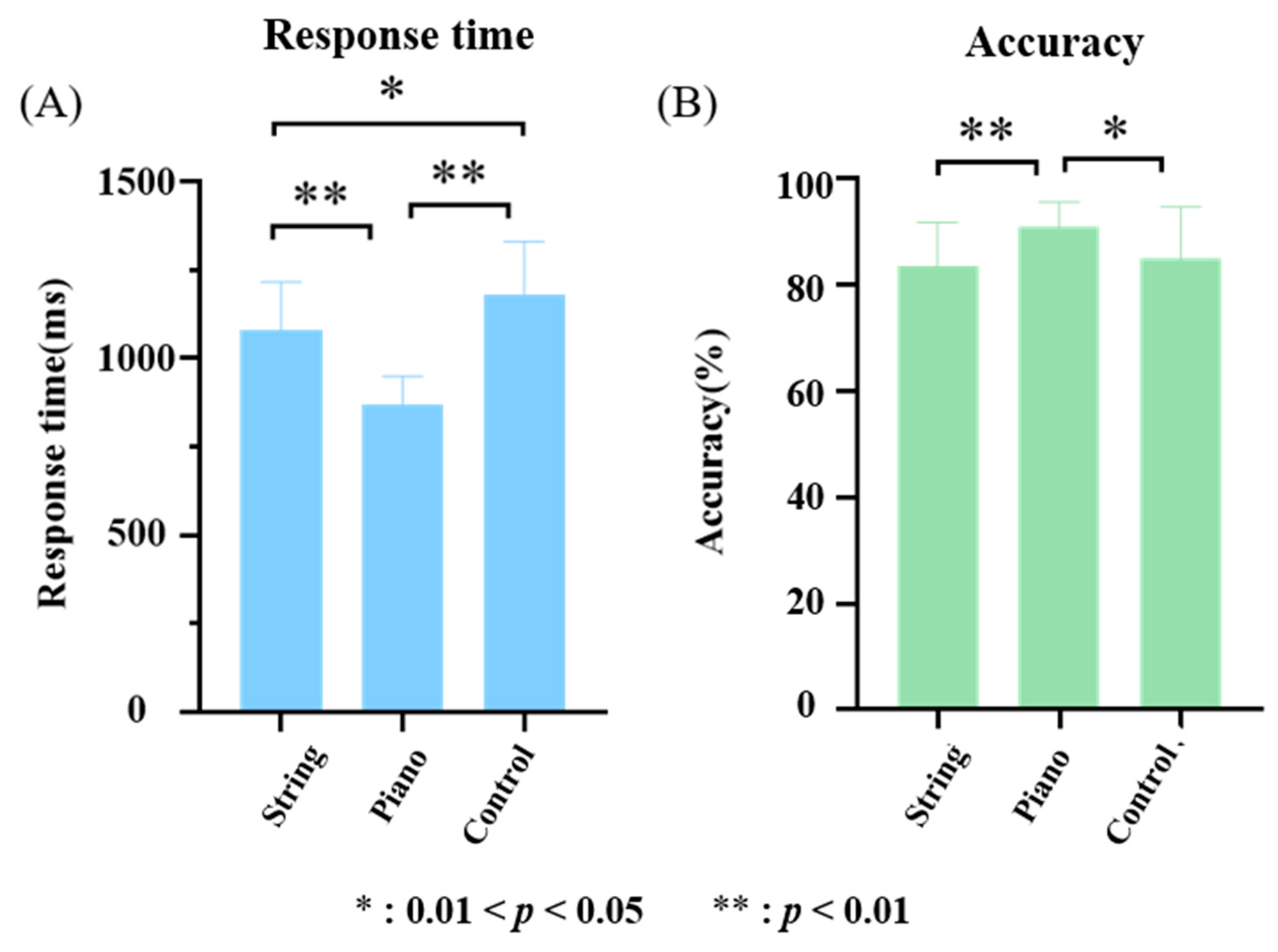
3.1. Response Time and Accuracy
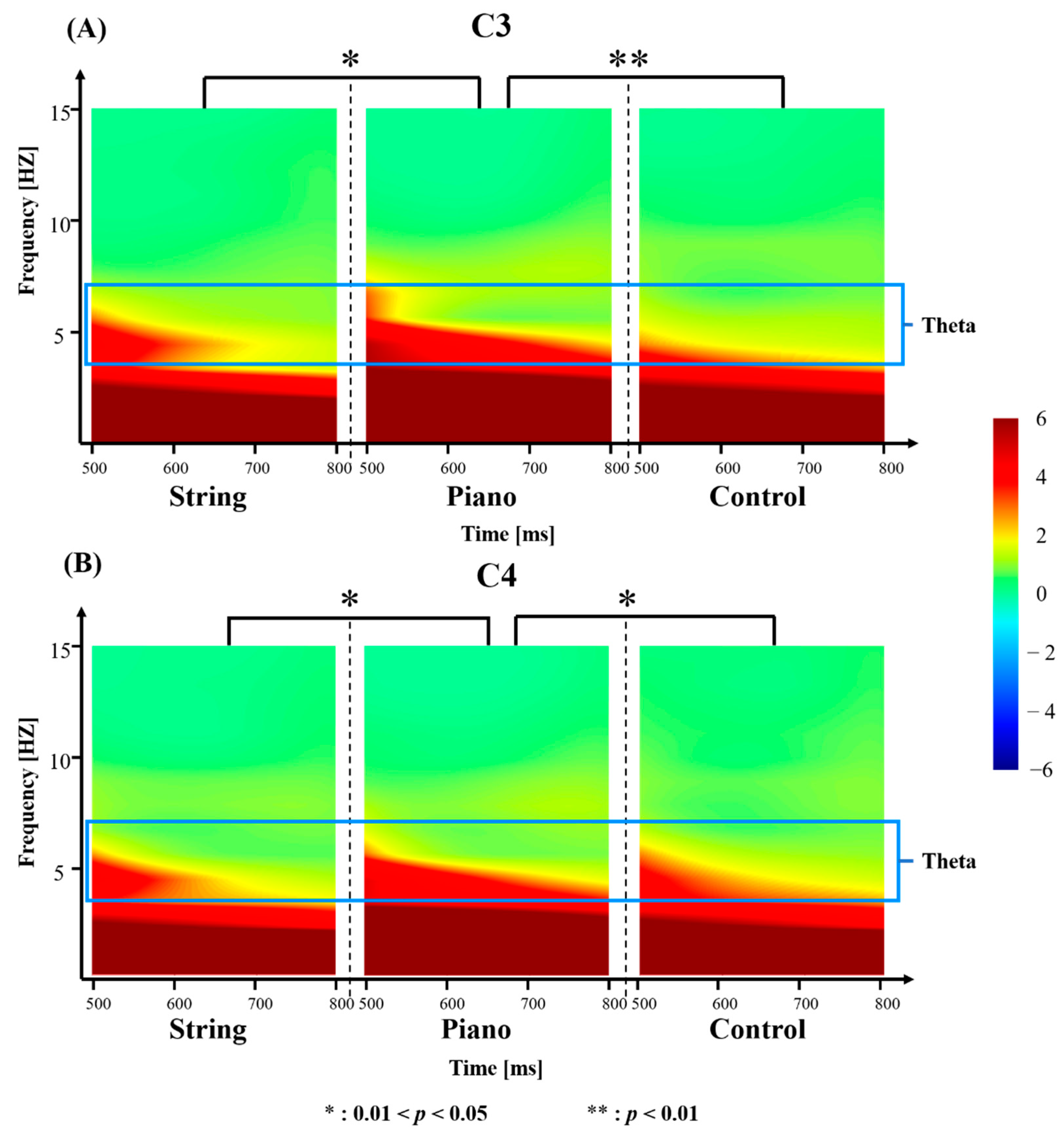
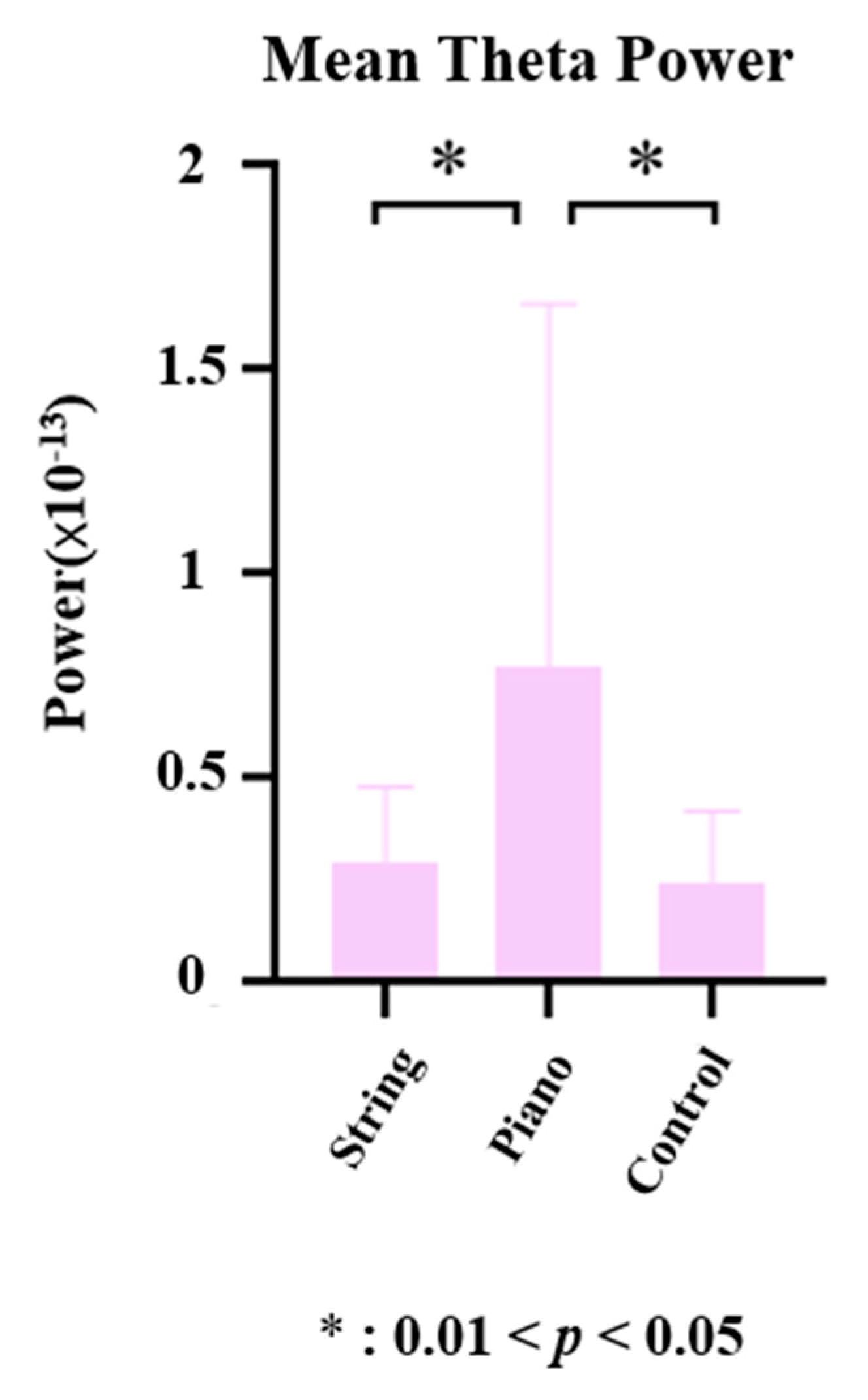
3.2. Effects of Different Music Training on Theta Power
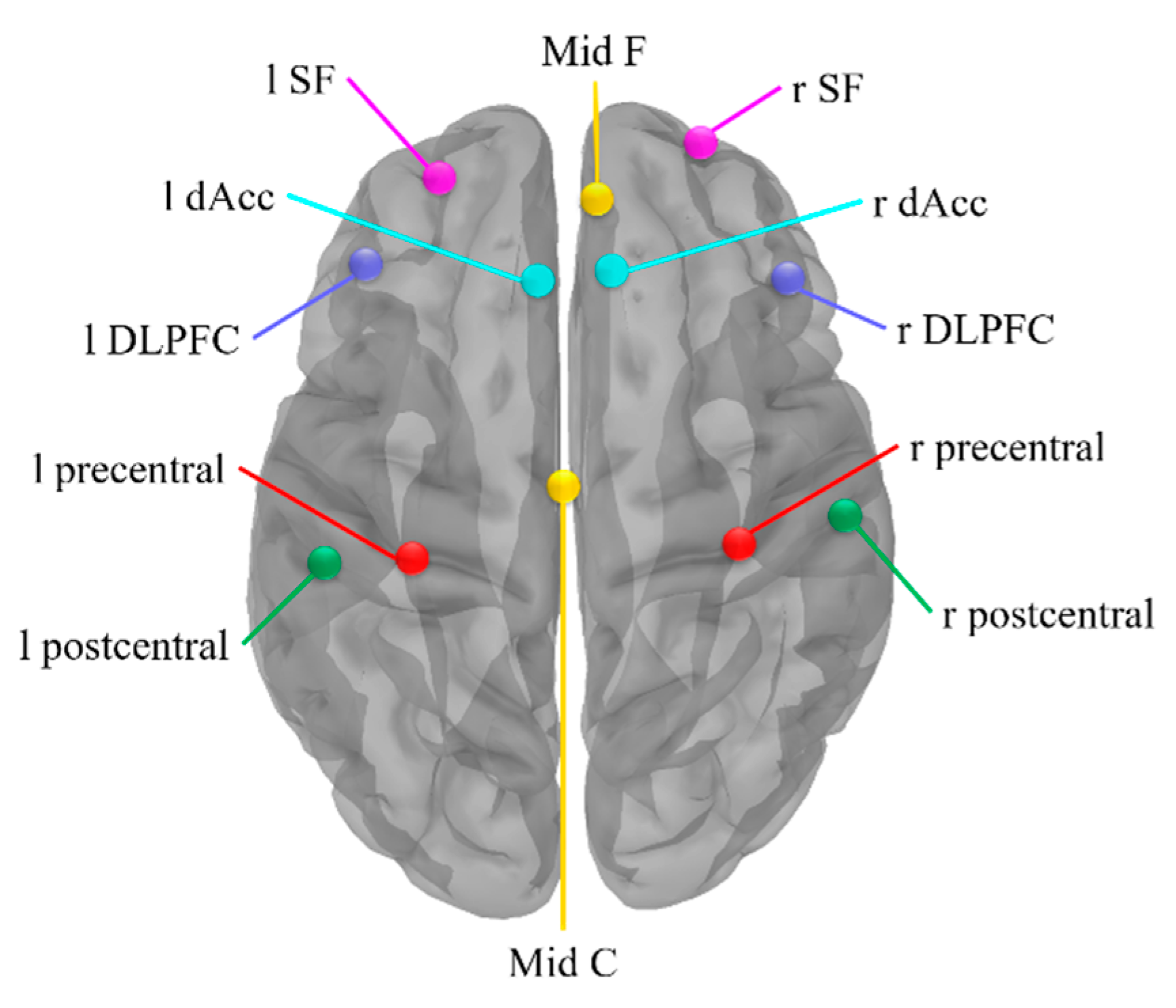
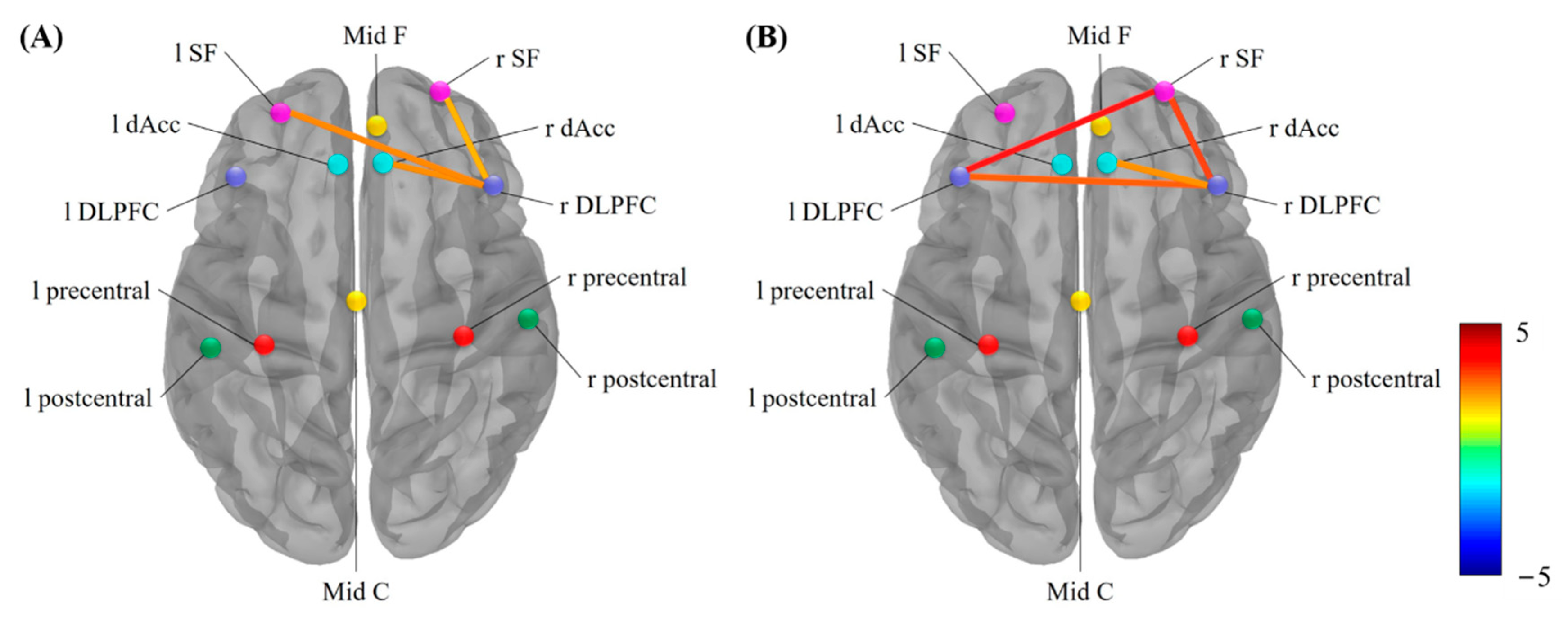
3.3. Functional Connectivity
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kraus, N.; Chandrasekaran, B. Music training for the development of auditory skills. Nat. Rev. Neurosci. 2010, 11, 599–605. [Google Scholar] [CrossRef]
- Rauscher, F.; Shaw, G.; Ky, C. Music and spatial task performance. Nature 1993, 365, 611. [Google Scholar] [CrossRef]
- Wan, C.; Schlaug, G. Music making as a tool for promoting brain plasticity across the life span. Neuroscientist 2010, 16, 566–577. [Google Scholar] [CrossRef]
- Aydin, S.; Guducu, C.; Kutluk, F.; Oniz, A.; Ozgoren, M. The impact of musical experience on neural sound encoding performance. Neurosci. Lett. 2019, 694, 124–128. [Google Scholar] [CrossRef]
- Bonetti, L.; Brattico, E.; Carlomagno, F.; Donati, G.; Cabral, J.; Haumann, N.T.; Deco, G.; Vuust, P.; Kringelbach, M.L. Rapid encoding of musical tones discovered in whole-brain connectivity. Neuroimage 2021, 245, 118735. [Google Scholar] [CrossRef]
- Gaser, C.; Schlaug, G. Brain structures differ between musicians and non-musicians. J. Neurosci. 2003, 23, 9240–9245. [Google Scholar] [CrossRef] [PubMed]
- Criscuolo, A.; Bonetti, L.; Sarkamo, T.; Kliuchko, M.; Brattico, E. On the Association between Musical Training, Intelligence and Executive Functions in Adulthood. Front. Psychol. 2019, 10, 1704. [Google Scholar] [CrossRef] [PubMed]
- Janus, M.; Lee, Y.; Moreno, S.; Bialystok, E. Effects of short-term music and second-language training on executive control. J. Exp. Child Psychol. 2016, 144, 84–97. [Google Scholar] [CrossRef]
- Collins, A.; Koechlin, E. Reasoning, learning, and creativity: Frontal lobe function and human decision-making. PLoS Biol. 2012, 10, e1001293. [Google Scholar] [CrossRef] [PubMed]
- Xi Xi, L.; Jiu Ju, W.; Dan, W.; Wen Xiang, Q.; Yan Ping, S.; Yu Xin, T.; Wen Tian, D. Comparative Analysis of Brainwave Music Translated from Spontaneous EEG between Major Depression Disorders and Healthy People. Brain Appar. Commun. J. Bacomics 2022, 1, 1–29. [Google Scholar] [CrossRef]
- Moreno, S.; Lee, Y.; Janus, M.; Bialystok, E. Short-term second language and music training induces lasting functional brain changes in early childhood. Child Dev. 2015, 86, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Hedden, T.; Gabrieli, J.D. Insights into the ageing mind: A view from cognitive neuroscience. Nat. Rev. Neurosci. 2004, 5, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Pallesen, K.J.; Brattico, E.; Bailey, C.J.; Korvenoja, A.; Koivisto, J.; Gjedde, A.; Carlson, S. Cognitive control in auditory working memory is enhanced in musicians. PLoS ONE 2010, 5, e11120. [Google Scholar] [CrossRef] [PubMed]
- Bugos, J.; DeMarie, D. The effects of a short-term music program on preschool children’s executive functions. Psychol. Music 2017, 45, 855–867. [Google Scholar] [CrossRef]
- Winsler, A.; Ducenne, L.; Koury, A. Singing One’s Way to Self-Regulation: The Role of Early Music and Movement Curricula and Private Speech. Early Educ. Dev. 2011, 22, 274–304. [Google Scholar] [CrossRef]
- Moreno, S.; Bidelman, G.M. Examining neural plasticity and cognitive benefit through the unique lens of musical training. Hear. Res. 2014, 308, 84–97. [Google Scholar] [CrossRef]
- Zatorre, R. Music, the food of neuroscience? Nature 2005, 434, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C. Music Performance. Annu. Rev. Psychol. 1997, 48, 115–138. [Google Scholar] [CrossRef]
- Chong, H.J.; Kim, S.J.; Yoo, G.E. Differential effects of type of keyboard playing task and tempo on surface EMG amplitudes of forearm muscles. Front. Psychol. 2015, 6, 1277. [Google Scholar] [CrossRef]
- Kilincer, O.; Ustun, E.; Akpinar, S.; Kaya, E.E. Motor Lateralization May Be Influenced by Long-Term Piano Playing Practice. Percept. Mot. Ski. 2019, 126, 25–39. [Google Scholar] [CrossRef]
- Gorniak, S.; Collins, E.; Staines, G.; Brooks, F.; Young, R. The Impact of Musical Training on Hand Biomechanics in String Musicians. HAND 2019, 14, 823–829. [Google Scholar] [CrossRef]
- Mizrahi, J. Neuro-mechanical aspects of playing-related mobility disorders in orchestra violinists and upper strings players: A review. Eur. J. Transl. Myol. 2020, 30, 9095. [Google Scholar] [CrossRef] [PubMed]
- Rensing, N.; Schemmann, H.; Zalpour, C. Musculoskeletal Demands in Violin and Viola Playing: A Literature Review. Med. Probl. Perform. Artist. 2018, 33, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Bangert, M.; Schlaug, G. Specialization of the specialized in features of external human brain morphology. Eur. J. Neurosci. 2006, 24, 1832–1834. [Google Scholar] [CrossRef] [PubMed]
- Elbert, T.; Pantev, C.; Wienbruch, C.; Rockstroh, B.; Taub, E. Increased cortical representation of the fingers of the left hand in string players. Science 1995, 270, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Cantou, P.; Platel, H.; Desgranges, B.; Groussard, M. How motor, cognitive and musical expertise shapes the brain: Focus on fMRI and EEG resting-state functional connectivity. J. Chem. Neuroanat. 2018, 89, 60–68. [Google Scholar] [CrossRef]
- Frischen, U.; Schwarzer, G.; Dege, F. Comparing the Effects of Rhythm-Based Music Training and Pitch-Based Music Training on Executive Functions in Preschoolers. Front. Integr. Neurosci. 2019, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- George, E.M.; Coch, D. Music training and working memory: An ERP study. Neuropsychologia 2011, 49, 1083–1094. [Google Scholar] [CrossRef]
- Veale, J. Edinburgh Handedness Inventory-Short Form: A revised version based on confirmatory factor analysis. Laterality 2014, 19, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Jegede, R.O. Psychometric attributes of the self-rating anxiety scale. Psychol. Rep. 1977, 40, 303–306. [Google Scholar] [CrossRef]
- Zung, W.W. A self-rating depression scale. Arch. Gen. Psychiatry 1965, 12, 63–70. [Google Scholar] [CrossRef] [PubMed]
- John, O.; Donahue, E.; Kentle, R. Big five inventory. J. Personal. Soc. Psychol. 1991. [Google Scholar] [CrossRef]
- Mas-Herrero, E.; Marco-Pallares, J.; Lorenzo-Seva, U.; Zatorre, R.J.; Rodriguez-Fornells, A. Individual differences in music reward experiences. Music Percept. 2012, 31, 118–138. [Google Scholar] [CrossRef]
- Peretz, I.; Gosselin, N.; Nan, Y.; Caron-Caplette, E.; Trehub, S.E.; Béland, R. A novel tool for evaluating children’s musical abilities across age and culture. Front. Syst. Neurosci. 2013, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Tadel, F.; Baillet, S.; Mosher, J.C.; Pantazis, D.; Leahy, R.M. Brainstorm: A user-friendly application for MEG/EEG analysis. Comput. Intell. Neurosci. 2011, 2011, 879716. [Google Scholar] [CrossRef]
- Dong, L.; Li, F.; Liu, Q.; Wen, X.; Lai, Y.; Xu, P.; Yao, D. MATLAB toolboxes for reference electrode standardization technique (REST) of scalp EEG. Front. Neurosci. 2017, 11, 601. [Google Scholar] [CrossRef]
- Beaty, R.E.; Benedek, M.; Silvia, P.J.; Schacter, D.L. Creative Cognition and Brain Network Dynamics. Trends Cogn. Sci. 2016, 20, 87–95. [Google Scholar] [CrossRef]
- Menon, V.; D’Esposito, M. The role of PFC networks in cognitive control and executive function. Neuropsychopharmacol. Rep. 2022, 47, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hu, G.; Yu, Y.; Jiang, Z.; Yang, K.; Hu, X.; Li, Z.; Liu, D.; Zou, Y.; Liu, H. Structural and functional reorganization within cognitive control network associated with protection of executive function in patients with unilateral frontal gliomas. Front. Oncol. 2020, 10, 794. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zhang, L.; Song, A.; Wu, C.; Li, W.; Zhang, D.; Xu, G.; Li, H.; Zeng, H. Wavelet transform time-frequency image and convolutional network-based motor imagery EEG classification. IEEE Access 2018, 7, 6084–6093. [Google Scholar] [CrossRef]
- Lacourse, M.G.; Orr, E.L.; Cramer, S.C.; Cohen, M.J. Brain activation during execution and motor imagery of novel and skilled sequential hand movements. Neuroimage 2005, 27, 505–519. [Google Scholar] [CrossRef]
- Popovych, S.; Rosjat, N.; Toth, T.I.; Wang, B.A.; Liu, L.; Abdollahi, R.O.; Viswanathan, S.; Grefkes, C.; Fink, G.R.; Daun, S. Movement-related phase locking in the delta-theta frequency band. Neuroimage 2016, 139, 439–449. [Google Scholar] [CrossRef]
- Ketenci, S.; Kayikcioglu, T. Investigation of Theta Rhythm Effect in Detection of Finger Movement. J. Exp. Neurosci. 2019, 13, 1179069519828737. [Google Scholar] [CrossRef] [PubMed]
- Kormendi, J.; Ferentzi, E.; Weiss, B.; Nagy, Z. Topography of Movement-Related Delta and Theta Brain Oscillations. Brain Topogr. 2021, 34, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Marqui, R.D. Standardized low-resolution brain electromagnetic tomography (sLORETA): Technical details. Methods Find Exp. Clin. Pharmacol. 2002, 24 (Suppl. D), 5–12. [Google Scholar] [PubMed]
- Luo, C.; Tu, S.; Peng, Y.; Gao, S.; Li, J.; Dong, L.; Li, G.; Lai, Y.; Li, H.; Yao, D. Long-term effects of musical training and functional plasticity in salience system. Neural Plast. 2014, 2014, 180138. [Google Scholar] [CrossRef] [PubMed]
- Leonard, M.K.; Desai, M.; Hungate, D.; Cai, R.; Singhal, N.S.; Knowlton, R.C.; Chang, E.F. Direct cortical stimulation of inferior frontal cortex disrupts both speech and music production in highly trained musicians. Cogn. Neuropsychol. 2019, 36, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Harpaintner, M.; Sim, E.-J.; Trumpp, N.M.; Ulrich, M.; Kiefer, M. The grounding of abstract concepts in the motor and visual system: An fMRI study. Cortex 2020, 124, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Ramnani, N.; Owen, A.M. Anterior prefrontal cortex: Insights into function from anatomy and neuroimaging. Nat. Rev. Neurosci. 2004, 5, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Sims, J.R.; Chen, A.M.; Sun, Z.; Deng, W.; Colwell, N.A.; Colbert, M.K.; Zhu, J.; Sainulabdeen, A.; Faiq, M.A.; Bang, J.W. Role of structural, metabolic, and functional MRI in monitoring visual system impairment and recovery. J. Magn. Reson. Imaging 2021, 54, 1706–1729. [Google Scholar] [CrossRef] [PubMed]
- Van Donkelaar, P.; Stein, J.; Passingham, R.; Miall, R. Neuronal activity in the primate motor thalamus during visually triggered and internally generated limb movements. J. Neurophysiol. 1999, 82, 934–945. [Google Scholar] [CrossRef]
- Li, J.; Luo, C.; Peng, Y.; Xie, Q.; Gong, J.; Dong, L.; Lai, Y.; Li, H.; Yao, D. Probabilistic diffusion tractography reveals improvement of structural network in musicians. PLoS ONE 2014, 9, e105508. [Google Scholar] [CrossRef]
- Shaffer, L. Rhythm and timing in skill. Psychol. Rev. 1982, 89, 109. [Google Scholar] [CrossRef] [PubMed]
- Norton, A.; Winner, E.; Cronin, K.; Overy, K.; Lee, D.J.; Schlaug, G. Are there pre-existing neural, cognitive, or motoric markers for musical ability? Brain Cogn. 2005, 59, 124–134. [Google Scholar] [CrossRef]
- Oikawa, N.; Tsubota, S.; Chikenji, T.; Chin, G.; Aoki, M. Wrist Positioning and Muscle Activities in the Wrist Extensor and Flexor during Piano Playing. Hong Kong J. Occup. Ther. 2011, 21, 41–46. [Google Scholar] [CrossRef]
- Degrave, V.; Verdugo, F.; Pelletier, J.; Traube, C.; Begon, M. Time history of upper-limb muscle activity during isolated piano keystrokes. J. Electromyogr. Kinesiol. 2020, 54, 102459. [Google Scholar] [CrossRef] [PubMed]
- Bugos, J.A. The Effects of Bimanual Coordination in Music Interventions on Executive Functions in Aging Adults. Front. Integr. Neurosci. 2019, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Zuk, J.; Benjamin, C.; Kenyon, A.; Gaab, N. Behavioral and neural correlates of executive functioning in musicians and non-musicians. PLoS ONE 2014, 9, e99868. [Google Scholar] [CrossRef] [PubMed]
- Mizuhara, H.; Yamaguchi, Y. Human cortical circuits for central executive function emerge by theta phase synchronization. Neuroimage 2007, 36, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hou, Y.; Ren, Y.; Tian, X.; Song, Y. Alterations of theta oscillation in executive control in temporal lobe epilepsy patients. Epilepsy Res. 2018, 140, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Pennekamp, P.; Bösel, R.; Mecklinger, A.; Ott, H. Differences in EEG-theta for responded and omitted targets in a sustained attention task. J. Psychophysiol. 1994, 8, 131–134. [Google Scholar]
- Makeig, S.; Delorme, A.; Westerfield, M.; Jung, T.-P.; Townsend, J.; Courchesne, E.; Sejnowski, T.J.; Goebel, R. Electroencephalographic brain dynamics following manually responded visual targets. PLoS Biol. 2004, 2, e176. [Google Scholar] [CrossRef] [PubMed]
- Gomarus, H.K.; Althaus, M.; Wijers, A.A.; Minderaa, R.B. The effects of memory load and stimulus relevance on the EEG during a visual selective memory search task: An ERP and ERD/ERS study. Clin. Neurophysiol. 2006, 117, 871–884. [Google Scholar] [CrossRef] [PubMed]
- Clayton, M.S.; Yeung, N.; Kadosh, R.C. The roles of cortical oscillations in sustained attention. Trends Cogn. Sci. 2015, 19, 188–195. [Google Scholar] [CrossRef]
- Sauseng, P.; Hoppe, J.; Klimesch, W.; Gerloff, C.; Hummel, F.C. Dissociation of sustained attention from central executive functions: Local activity and interregional connectivity in the theta range. Eur. J. Neurosci. 2007, 25, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Moreno, S.; Bialystok, E.; Barac, R.; Schellenberg, E.G.; Cepeda, N.J.; Chau, T. Short-term music training enhances verbal intelligence and executive function. Psychol. Sci. 2011, 22, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Jaschke, A.C.; Honing, H.; Scherder, E.J. Longitudinal analysis of music education on executive functions in primary school children. Front. Neurosci. 2018, 12, 103. [Google Scholar] [CrossRef]
- Slevc, L.R.; Davey, N.S.; Buschkuehl, M.; Jaeggi, S.M. Tuning the mind: Exploring the connections between musical ability and executive functions. Cognition 2016, 152, 199–211. [Google Scholar] [CrossRef]
- Colombo, P.J.; Habibi, A.; Alain, C. Music Training, Neural Plasticity, and Executive Function. Front. Integr. Neurosci. 2020, 14, 41. [Google Scholar] [CrossRef] [PubMed]
- Bugos, J.A.; Wang, Y. Piano Training Enhances Executive Functions and Psychosocial Outcomes in Aging: Results of a Randomized Controlled Trial. J. Gerontol. B Psychol. Sci. Soc. Sci. 2022, 77, 1625–1636. [Google Scholar] [CrossRef] [PubMed]
- Lesiuk, T.; Bugos, J.A.; Murakami, B. In A rationale for music training to enhance executive functions in Parkinson’s disease: An overview of the problem. Healthcare 2018, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Moussard, A.; Guo, S.; Lee, Y.; Bidelman, G.M.; Moreno, S.; Skrotzki, C.; Bugos, J.; Shen, D.; Yao, D.; et al. Music training modulates theta brain oscillations associated with response suppression. Ann. N. Y. Acad. Sci. 2022, 1513, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Koshimori, Y.; Thaut, M.H. New perspectives on music in rehabilitation of executive and attention functions. Front. Neurosci. 2019, 13, 1245. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.K.; Cohen, J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef] [PubMed]
- Puig, M.V.; Miller, E.K. Neural substrates of dopamine D2 receptor modulated executive functions in the monkey prefrontal cortex. Cereb. Cortex 2015, 25, 2980–2987. [Google Scholar] [CrossRef]
- Salehinejad, M.A.; Ghanavati, E.; Rashid, M.H.A.; Nitsche, M.A. Hot and cold executive functions in the brain: A prefrontal-cingular network. Brain Neurosci. Adv. 2021, 5, 23982128211007769. [Google Scholar] [CrossRef]
- Friedman, N.P.; Robbins, T.W. The role of prefrontal cortex in cognitive control and executive function. Neuropsychopharmacol. Rep. 2022, 47, 72–89. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, C.-Y.C.; Wager, T.D.; Lacey, S.C.; Hernandez, L.; Nichols, T.E.; Smith, E.E.; Jonides, J. Switching attention and resolving interference: fMRI measures of executive functions. Neuropsychologia 2003, 41, 357–370. [Google Scholar] [CrossRef]
- Derrfuss, J.; Brass, M.; Von Cramon, D.Y. Cognitive control in the posterior frontolateral cortex: Evidence from common activations in task coordination, interference control, and working memory. Neuroimage 2004, 23, 604–612. [Google Scholar] [CrossRef]
- Collette, F.; Van der Linden, M.; Laureys, S.; Delfiore, G.; Degueldre, C.; Luxen, A.; Salmon, E. Exploring the unity and diversity of the neural substrates of executive functioning. Hum. Brain Mapp. 2005, 25, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Niendam, T.A.; Laird, A.R.; Ray, K.L.; Dean, Y.M.; Glahn, D.C.; Carter, C.S. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn. Affect. Behav. Neurosci. 2012, 12, 241–268. [Google Scholar] [CrossRef]
- Panikratova, Y.R.; Vlasova, R.M.; Akhutina, T.V.; Korneev, A.A.; Sinitsyn, V.E.; Pechenkova, E.V. Functional connectivity of the dorsolateral prefrontal cortex contributes to different components of executive functions. Int. J. Psychophysiol. 2020, 151, 70–79. [Google Scholar] [CrossRef]
- Petersen, S.E.; Posner, M.I. The attention system of the human brain: 20 years after. Annu. Rev. Neurosci. 2012, 35, 73. [Google Scholar] [CrossRef]
- Shenhav, A.; Botvinick, M.M.; Cohen, J.D. The expected value of control: An integrative theory of anterior cingulate cortex function. Neuron 2013, 79, 217–240. [Google Scholar] [CrossRef] [PubMed]
- Gevins, A.; Smith, M.E.; McEvoy, L.; Yu, D. High-resolution EEG mapping of cortical activation related to working memory: Effects of task difficulty, type of processing, and practice. Cereb. Cortex 1997, 7, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Onton, J.; Delorme, A.; Makeig, S. Frontal midline EEG dynamics during working memory. Neuroimage 2005, 27, 341–356. [Google Scholar] [CrossRef] [PubMed]
| String | Piano | Control | |
|---|---|---|---|
| n | 18 | 20 | 19 |
| Male/Female | 11/7 | 11/9 | 11/8 |
| Age (years) | |||
| 21.76 ± 4.92 | 20.75 ± 2.45 | 20.68 ± 1.34 | |
| Education Level (years) | |||
| 14.12 ± 1.20 | 14.35 ± 2.30 | 14.5 ± 1.31 | |
| Age of Musical Training Onset (years) | |||
| 7.38 ± 3.36 | 5.7 ± 1.87 | - | |
| Formal Training (years) | |||
| 12.05 ± 4.92 | 11.23 ± 4.55 | - | |
| Self-rating Anxiety Scale (SAS) | |||
| 29.53 ± 7.83 | 27.11 ± 3.78 | 31.15 ± 6.65 | |
| Self-rating Depression (SDS) | |||
| 30.69 ± 7.08 | 30.5 ± 5.68 | 32.53 ± 6.83 | |
| Edinburgh Handedness Inventory | |||
| 10 | 10 | 10 | |
| The Big Five | |||
| The Big Five-Neuroticism | |||
| 30.27 ± 4.93 | 30.70 ± 5.85 | 31.22 ± 4.93 | |
| The Big Five-Extraversion | |||
| 27.27 ± 6.52 | 25.75 ± 6.51 | 25.89 ± 4.23 | |
| The Big Five-Openness | |||
| 43.80 ± 4.98 | 42 ± 3.50 | 38.56 ± 3.50 | |
| The Big Five-Agreeableness | |||
| 33.27 ± 5.50 | 35.55 ± 4.24 | 33.83 ± 3.73 | |
| The Big Five-Conscientiousness | |||
| 35.13 ± 5.08 | 32.90 ± 4.75 | 33.78 ± 4.51 | |
| Barcelona Music Reward Questionnaire (BMRQ) | |||
| BMRQ-Emotional Evocation | |||
| 18.47 ± 1.77 | 17.15 ± 2.21 | 15.89 ± 2.14 | |
| BMRQ-Sensory Motor | |||
| 16.73 ± 2.02 | 15.95 ± 3.19 | 13.39 ± 3.11 | |
| BMRQ-Mood Regulation | |||
| 17.87 ± 1.55 | 17.10 ± 2.10 | 15.56 ± 1.89 | |
| BMRQ-Musical Seeking | |||
| 17.67 ± 1.72 | 16.80 ± 1.90 | 14.56 ± 2.38 | |
| BMRQ-Social Reward | |||
| 17.27 ± 1.22 | 16.55 ± 2.61 | 14.16 ± 2.62 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Xu, R.; Guo, X.; Guo, S.; Zhou, J.; Lu, J.; Yao, D. Different Music Training Modulates Theta Brain Oscillations Associated with Executive Function. Brain Sci. 2022, 12, 1304. https://doi.org/10.3390/brainsci12101304
Wang J, Xu R, Guo X, Guo S, Zhou J, Lu J, Yao D. Different Music Training Modulates Theta Brain Oscillations Associated with Executive Function. Brain Sciences. 2022; 12(10):1304. https://doi.org/10.3390/brainsci12101304
Chicago/Turabian StyleWang, Junce, Ruijie Xu, Xiaolong Guo, Sijia Guo, Junchen Zhou, Jing Lu, and Dezhong Yao. 2022. "Different Music Training Modulates Theta Brain Oscillations Associated with Executive Function" Brain Sciences 12, no. 10: 1304. https://doi.org/10.3390/brainsci12101304
APA StyleWang, J., Xu, R., Guo, X., Guo, S., Zhou, J., Lu, J., & Yao, D. (2022). Different Music Training Modulates Theta Brain Oscillations Associated with Executive Function. Brain Sciences, 12(10), 1304. https://doi.org/10.3390/brainsci12101304





