Risk Factors of Transient Neurological Deficits and Perioperative Stroke after Revascularization in Patients with Moyamoya Disease
Abstract
1. Introduction
2. Methods
2.1. Patient Selection
2.2. Clinical Data
2.3. Definition of Complications
2.4. Definition of Influencing Factors
2.5. Operation
2.6. Follow-Up Method
2.7. Statistical Method
3. Results
3.1. Incidence of Complications
3.2. Statistical Outcomes
3.3. Follow-Up Results
4. Discussion
4.1. The Mechanism of TND
4.2. The Mechanism of Perioperative Cerebral Infarction
4.3. The Mechanism of Perioperative Cerebral Hemorrhage
4.4. Baseline Data of Patients
4.5. Moyamoya Disease Information
4.6. Surgery and Anesthesia Management
4.7. Perioperative Management Data
4.8. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suzuki, J.; Takaku, A. Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch. Neurol. 1969, 20, 288–299. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, G.; Feng, J.; Chen, L.; Liu, G.; Wang, J.; Wang, Q.; Yu, J.; Yang, X.; Yang, Z.; et al. Incidence and prevalence of moyamoya disease in urban China: A nationwide retrospective cohort study. Stroke Vasc. Neurol. 2021, 6. [Google Scholar] [CrossRef]
- Bao, X.; Wang, Q.; Zhang, Y.; Zhang, Q.; Li, D.; Yang, W.; Zhang, Z.; Zong, R.; Han, C.; Duan, L. Epidemiology of Moyamoya Disease in China: Single-Center, Population-Based Study. World Neurosurg. 2019, 122, e917–e923. [Google Scholar] [CrossRef]
- Pandey, P.; Steinberg, G. Neurosurgical advances in the treatment of moyamoya disease. Stroke 2011, 42, 3304–3310. [Google Scholar] [CrossRef]
- Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of Willis). Neurol. Med. Chir. 2012, 52, 245–266. [CrossRef]
- Li, Q.; Gao, Y.; Xin, W.; Zhou, Z.; Rong, H.; Qin, Y.; Li, K.; Zhou, Y.; Wang, J.; Xiong, J.; et al. Meta-Analysis of Prognosis of Different Treatments for Symptomatic Moyamoya Disease. World Neurosurg. 2019, 127, 354–361. [Google Scholar] [CrossRef]
- Fujimura, M.; Shimizu, H.; Inoue, T.; Mugikura, S.; Saito, A.; Tominaga, T. Significance of focal cerebral hyperperfusion as a cause of transient neurologic deterioration after extracranial-intracranial bypass for moyamoya disease: Comparative study with non-moyamoya patients using N-isopropyl-p-[(123)I]iodoamphetamine single-photon emission computed tomography. Neurosurgery 2011, 68, 957–964; discussion 955–964. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, J.; Li, J.; Zhang, J.; Chen, J. Cerebral Hyperperfusion Syndrome After Revascularization Surgery in Patients with Moyamoya Disease: Systematic Review and Meta-Analysis. World Neurosurg. 2020, 135, 357–366.e4. [Google Scholar] [CrossRef]
- Kuriyama, S.; Kusaka, Y.; Fujimura, M.; Wakai, K.; Tamakoshi, A.; Hashimoto, S.; Tsuji, I.; Inaba, Y.; Yoshimoto, T. Prevalence and clinicoepidemiological features of moyamoya disease in Japan: Findings from a nationwide epidemiological survey. Stroke 2008, 39, 42–47. [Google Scholar] [CrossRef]
- Im, S.; Yim, S.; Cho, C.; Joo, W.; Chough, C.; Park, H.; Lee, K.; Rha, H. Prevalence and epidemiological features of moyamoya disease in Korea. J. Cerebrovasc. Endovasc. Neurosurg. 2012, 14, 75–78. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, D.; Wang, S.; Zhao, Y.; Teo, M.; Wang, R.; Cao, Y.; Ye, X.; Kang, S.; Zhao, J. Clinical features and long-term outcomes of moyamoya disease: A single-center experience with 528 cases in China. J. Neurosurg. 2015, 122, 392–399. [Google Scholar] [CrossRef]
- Bao, X.; Duan, L.; Li, D.; Yang, W.; Sun, W.; Zhang, Z.; Zong, R.; Han, C. Clinical features, surgical treatment and long-term outcome in adult patients with Moyamoya disease in China. Cerebrovasc. Dis. 2012, 34, 305–313. [Google Scholar] [CrossRef]
- Zhai, X.; Mao, L.; Wang, H.; Zhang, X.; Hang, C.; Wu, W.; Jia, Y.; Liu, L. Risk Factors Associated with Neurologic Deterioration After Combined Direct and Indirect Revascularization in Patients with Moyamoya Disease on the East Coast of China. World Neurosurg. 2018, 118, e92–e98. [Google Scholar] [CrossRef]
- Kim, J.; Oh, C.; Kwon, O.; Park, S.; Kim, S.; Kim, Y. Transient hyperperfusion after superficial temporal artery/middle cerebral artery bypass surgery as a possible cause of postoperative transient neurological deterioration. Cerebrovasc. Dis. 2008, 25, 580–586. [Google Scholar] [CrossRef]
- Ohue, S.; Kumon, Y.; Kohno, K.; Watanabe, H.; Iwata, S.; Ohnishi, T. Postoperative temporary neurological deficits in adults with moyamoya disease. Surg. Neurol. 2008, 69, 281–286; discussion 286–287. [Google Scholar] [CrossRef]
- Fujimura, M.; Kaneta, T.; Mugikura, S.; Shimizu, H.; Tominaga, T. Temporary neurologic deterioration due to cerebral hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in patients with adult-onset moyamoya disease. Surg. Neurol. 2007, 67, 273–282. [Google Scholar] [CrossRef]
- Fujimura, M.; Tominaga, T. Lessons learned from moyamoya disease: Outcome of direct/indirect revascularization surgery for 150 affected hemispheres. Neurol. Med. Chir. 2012, 52, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Iwama, T.; Hashimoto, N.; Tsukahara, T.; Murai, B. Peri-operative complications in adult moyamoya disease. Acta Neurochir. 1995, 132, 26–31. [Google Scholar] [CrossRef]
- Uno, M.; Nakajima, N.; Nishi, K.; Shinno, K.; Nagahiro, S. Hyperperfusion syndrome after extracranial-intracranial bypass in a patient with moyamoya disease—Case report. Neurol. Med. Chir. 1998, 38, 420–424. [Google Scholar] [CrossRef]
- Hayashi, T.; Shirane, R.; Fujimura, M.; Tominaga, T. Postoperative neurological deterioration in pediatric moyamoya disease: Watershed shift and hyperperfusion. J. Neurosurg. Pediatr. 2010, 6, 73–81. [Google Scholar] [CrossRef]
- Mukerji, N.; Cook, D.; Steinberg, G. Is local hypoperfusion the reason for transient neurological deficits after STA-MCA bypass for moyamoya disease? J. Neurosurg. 2015, 122, 90–94. [Google Scholar] [CrossRef]
- Phi, J.; Lee, S.; Kang, H.; Kim, J.; Kim, S.; Cho, W.; Lee, S. Postoperative Transient Neurologic Dysfunction: A Proposal for Pathophysiology. J. Clin. Neurol. 2018, 14, 393–400. [Google Scholar] [CrossRef]
- Ishii, D.; Okazaki, T.; Matsushige, T.; Shinagawa, K.; Ichinose, N.; Sakamoto, S.; Kurisu, K. Postoperative Dilatation of Superficial Temporal Artery Associated with Transient Neurologic Symptoms After Direct Bypass Surgery for Moyamoya Angiopathy. World Neurosurg. 2017, 106, 435–441. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, J.; Liu, N.; Xue, Y.; Ren, X.; Fu, J. Postoperative Functional Outcomes and Prognostic Factors in Two Types of Adult Moyamoya Diseases. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2020, 29, 104846. [Google Scholar] [CrossRef]
- Kazumata, K.; Ito, M.; Tokairin, K.; Ito, Y.; Houkin, K.; Nakayama, N.; Kuroda, S.; Ishikawa, T.; Kamiyama, H. The frequency of postoperative stroke in moyamoya disease following combined revascularization: A single-university series and systematic review. J. Neurosurg. 2014, 121, 432–440. [Google Scholar] [CrossRef]
- Zhao, M.; Deng, X.; Zhang, D.; Wang, S.; Zhang, Y.; Wang, R.; Zhao, J. Risk factors for and outcomes of postoperative complications in adult patients with moyamoya disease. J. Neurosurg. 2018, 130, 531–542. [Google Scholar] [CrossRef]
- Muraoka, S.; Araki, Y.; Kondo, G.; Kurimoto, M.; Shiba, Y.; Uda, K.; Ota, S.; Okamoto, S.; Wakabayashi, T. Postoperative Cerebral Infarction Risk Factors and Postoperative Management of Pediatric Patients with Moyamoya Disease. World Neurosurg. 2018, 113, e190–e199. [Google Scholar] [CrossRef]
- Park, W.; Ahn, J.; Lee, H.; Park, J.; Kwun, B. Risk Factors for Newly Developed Cerebral Infarction After Surgical Revascularization for Adults with Moyamoya Disease. World Neurosurg. 2016, 92, 65–73. [Google Scholar] [CrossRef]
- Fujimura, M.; Kaneta, T.; Shimizu, H.; Tominaga, T. Cerebral ischemia owing to compression of the brain by swollen temporal muscle used for encephalo-myo-synangiosis in moyamoya disease. Neurosurg. Rev. 2009, 32, 245–249; discussion 249. [Google Scholar] [CrossRef]
- Sussman, E.; Madhugiri, V.; Teo, M.; Nielsen, T.; Furtado, S.; Pendharkar, A.; Ho, A.; Esparza, R.; Azad, T.; Zhang, M.; et al. Contralateral acute vascular occlusion following revascularization surgery for moyamoya disease. J. Neurosurg. 2018, 131, 1702–1708. [Google Scholar] [CrossRef]
- Tokairin, K.; Kazumata, K.; Uchino, H.; Ito, M.; Ono, K.; Tatezawa, R.; Shindo, T.; Kawabori, M.; Nakayama, N.; Houkin, K. Postoperative Intracerebral Hemorrhage After Combined Revascularization Surgery in Moyamoya Disease: Profiles and Clinical Associations. World Neurosurg. 2018, 120, e593–e600. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, L.; Lu, J.; Chen, X.; Ye, X.; Zhang, D.; Zhang, Y.; Wang, R.; Zhao, Y. Postoperative hemorrhage during the acute phase after direct or combined revascularization for moyamoya disease: Risk factors, prognosis, and literature review. J. Neurosurg. 2019, 133, 1450–1459. [Google Scholar] [CrossRef]
- Park, W.; Park, E.; Lee, S.; Park, J.; Chung, J.; Lee, J.; Ahn, J. Intracranial Hemorrhage After Superficial Temporal Artery-Middle Cerebral Artery Direct Anastomosis for Adults with Moyamoya Disease. World Neurosurg. 2018, 119, e774–e782. [Google Scholar] [CrossRef]
- Kazumata, K.; Tha, K.; Uchino, H.; Shiga, T.; Shichinohe, H.; Ito, M.; Nakayama, N.; Abumiya, T. Topographic changes in cerebral blood flow and reduced white matter integrity in the first 2 weeks following revascularization surgery in adult moyamoya disease. J. Neurosurg. 2017, 127, 260–269. [Google Scholar] [CrossRef]
- Fujimura, M.; Shimizu, H.; Mugikura, S.; Tominaga, T. Delayed intracerebral hemorrhage after superficial temporal artery-middle cerebral artery anastomosis in a patient with moyamoya disease: Possible involvement of cerebral hyperperfusion and increased vascular permeability. Surg. Neurol. 2009, 71, 223–227; discussion 227. [Google Scholar] [CrossRef]
- Fujimura, M.; Inoue, T.; Shimizu, H.; Saito, A.; Mugikura, S.; Tominaga, T. Efficacy of prophylactic blood pressure lowering according to a standardized postoperative management protocol to prevent symptomatic cerebral hyperperfusion after direct revascularization surgery for moyamoya disease. Cerebrovasc. Dis. 2012, 33, 436–445. [Google Scholar] [CrossRef]
- Zhao, M.; Deng, X.; Gao, F.; Zhang, D.; Wang, S.; Zhang, Y.; Wang, R.; Zhao, J. Ischemic Stroke in Young Adults with Moyamoya Disease: Prognostic Factors for Stroke Recurrence and Functional Outcome after Revascularization. World Neurosurg. 2017, 103, 161–167. [Google Scholar] [CrossRef]
- Shou, J.; Zhou, L.; Zhu, S.; Zhang, X. Diabetes is an Independent Risk Factor for Stroke Recurrence in Stroke Patients: A Meta-analysis. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2015, 24, 1961–1968. [Google Scholar] [CrossRef]
- Hyun, S.; Kim, J.; Hong, S. Prognostic factors associated with perioperative ischemic complications in adult-onset moyamoya disease. Acta Neurochir. 2010, 152, 1181–1188. [Google Scholar] [CrossRef]
- Miyamoto, S.; Kikuchi, H.; Karasawa, J.; Nagata, I.; Ikota, T.; Takeuchi, S. Study of the posterior circulation in moyamoya disease. Clinical and neuroradiological evaluation. J. Neurosurg. 1984, 61, 1032–1037. [Google Scholar] [CrossRef]
- Yu, L.; Ma, L.; Huang, Z.; Shi, Z.; Wang, R.; Zhao, Y.; Zhang, D. Revascularization Surgery in Patients with Ischemic-Type Moyamoya Disease: Predictors for Postoperative Stroke and Long-Term Outcomes. World Neurosurg. 2019, 128, e582–e596. [Google Scholar] [CrossRef]
- Jeon, J.P.; Kim, J.E.; Cho, W.S.; Bang, J.S.; Son, Y.J.; Oh, C.W. Meta-analysis of the surgical outcomes of symptomatic moyamoya disease in adults. J. Neurosurg. 2018, 128, 793–799. [Google Scholar] [CrossRef]
- Kameyama, M.; Fujimura, M.; Tashiro, R.; Sato, K.; Endo, H.; Niizuma, K.; Mugikura, S.; Tominaga, T. Significance of Quantitative Cerebral Blood Flow Measurement in the Acute Stage after Revascularization Surgery for Adult Moyamoya Disease: Implication for the Pathological Threshold of Local Cerebral Hyperperfusion. Cerebrovasc. Dis. 2019, 48, 217–225. [Google Scholar] [CrossRef]
- Hwang, J.W.; Yang, H.M.; Lee, H.; Lee, H.K.; Jeon, Y.T.; Kim, J.E.; Lim, Y.J.; Park, H.P. Predictive factors of symptomatic cerebral hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in adult patients with moyamoya disease. Br. J. Anaesth. 2013, 110, 773–779. [Google Scholar] [CrossRef]
- Li, C.; Zhang, N.; Yu, S.; Xu, Y.; Yao, Y.; Zeng, M.; Li, D.; Xia, C. Individualized Perioperative Blood Pressure Management for Adult Moyamoya Disease: Experience from 186 Consecutive Procedures. J. Stroke Cerebrovasc. Dis. 2021, 30, 105413. [Google Scholar] [CrossRef]
- Saito, M.; Saga, T.; Hayashi, H.; Noro, S.; Wada, H.; Kamada, K. Quantitative Blood Flow Assessment by Multiparameter Analysis of Indocyanine Green Video Angiography. World Neurosurg. 2018, 116, e187–e193. [Google Scholar] [CrossRef]
- Zhang, X.; Ni, W.; Feng, R.; Li, Y.; Lei, Y.; Xia, D.; Gao, P.; Yang, S.; Gu, Y. Evaluation of Hemodynamic Change by Indocyanine Green-FLOW 800 Videoangiography Mapping: Prediction of Hyperperfusion Syndrome in Patients with Moyamoya Disease. Oxid. Med. Cell. Longev. 2020, 2020, 8561609. [Google Scholar] [CrossRef]
- Rennert, R.C.; Strickland, B.A.; Ravina, K.; Bakhsheshian, J.; Russin, J.J. Assessment of Hemodynamic Changes and Hyperperfusion Risk After Extracranial-to-Intracranial Bypass Surgery Using Intraoperative Indocyanine Green-Based Flow Analysis. World Neurosurg. 2018, 114, 352–360. [Google Scholar] [CrossRef]
- Tu, X.K.; Fujimura, M.; Rashad, S.; Mugikura, S.; Sakata, H.; Niizuma, K.; Tominaga, T. Uneven cerebral hemodynamic change as a cause of neurological deterioration in the acute stage after direct revascularization for moyamoya disease: Cerebral hyperperfusion and remote ischemia caused by the ‘watershed shift’. Neurosurg. Rev. 2017, 40, 507–512. [Google Scholar] [CrossRef]
- Sakamoto, T.; Kawaguchi, M.; Kurehara, K.; Kitaguchi, K.; Furuya, H.; Karasawa, J. Postoperative neurological deterioration following the revascularization surgery in children with moyamoya disease. J. Neurosurg. Anesthesiol. 1998, 10, 37–41. [Google Scholar] [CrossRef]
- Uchino, H.; Nakayama, N.; Kazumata, K.; Kuroda, S.; Houkin, K. Edaravone Reduces Hyperperfusion-Related Neurological Deficits in Adult Moyamoya Disease: Historical Control Study. Stroke 2016, 47, 1930–1932. [Google Scholar] [CrossRef]
- Fujimura, M.; Niizuma, K.; Inoue, T.; Sato, K.; Endo, H.; Shimizu, H.; Tominaga, T. Minocycline prevents focal neurological deterioration due to cerebral hyperperfusion after extracranial-intracranial bypass for moyamoya disease. Neurosurgery 2014, 74, 163–170; discussion 170. [Google Scholar] [CrossRef]
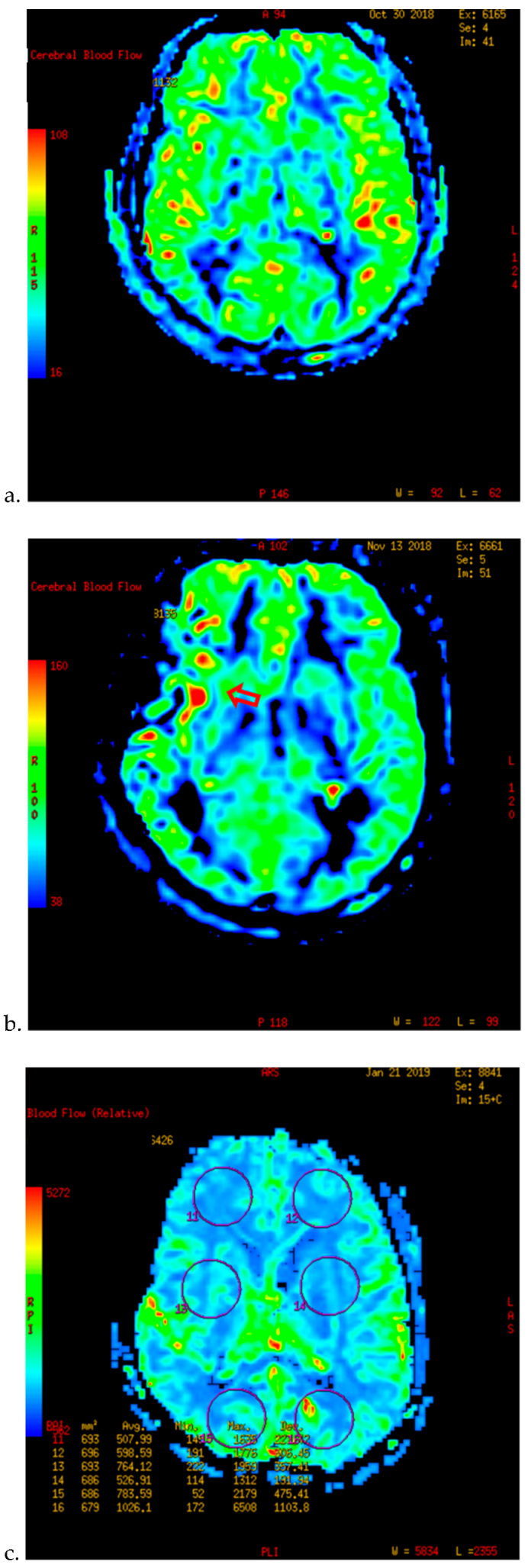
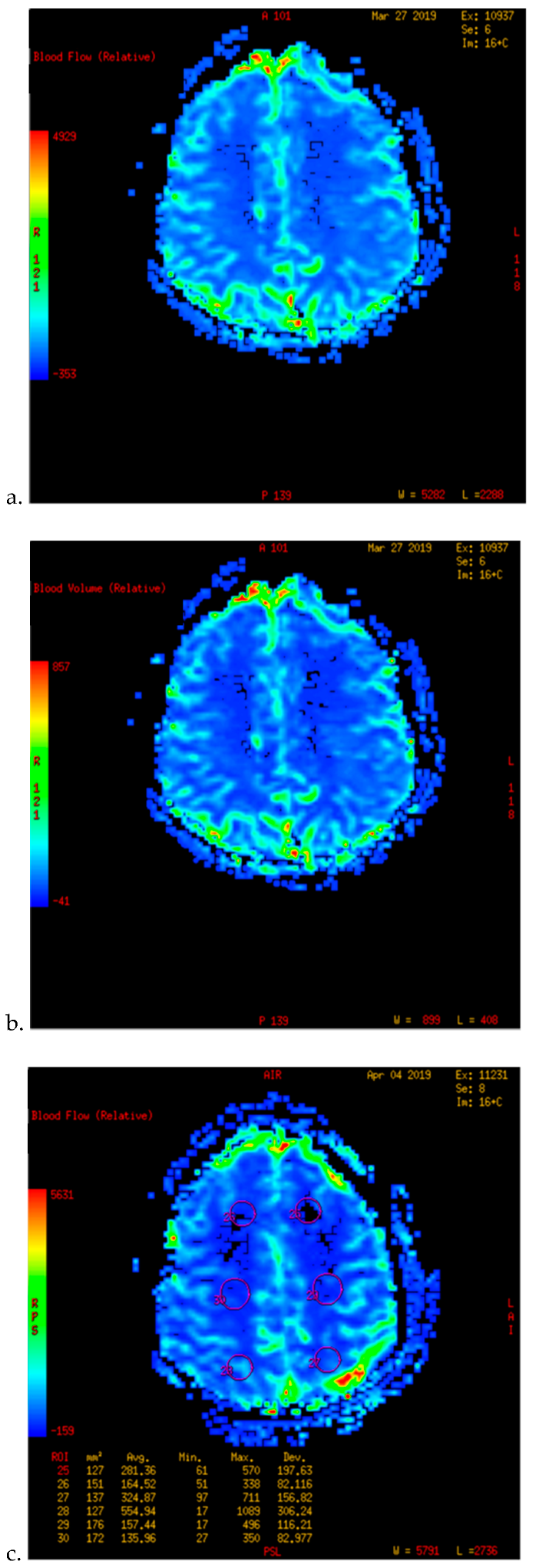
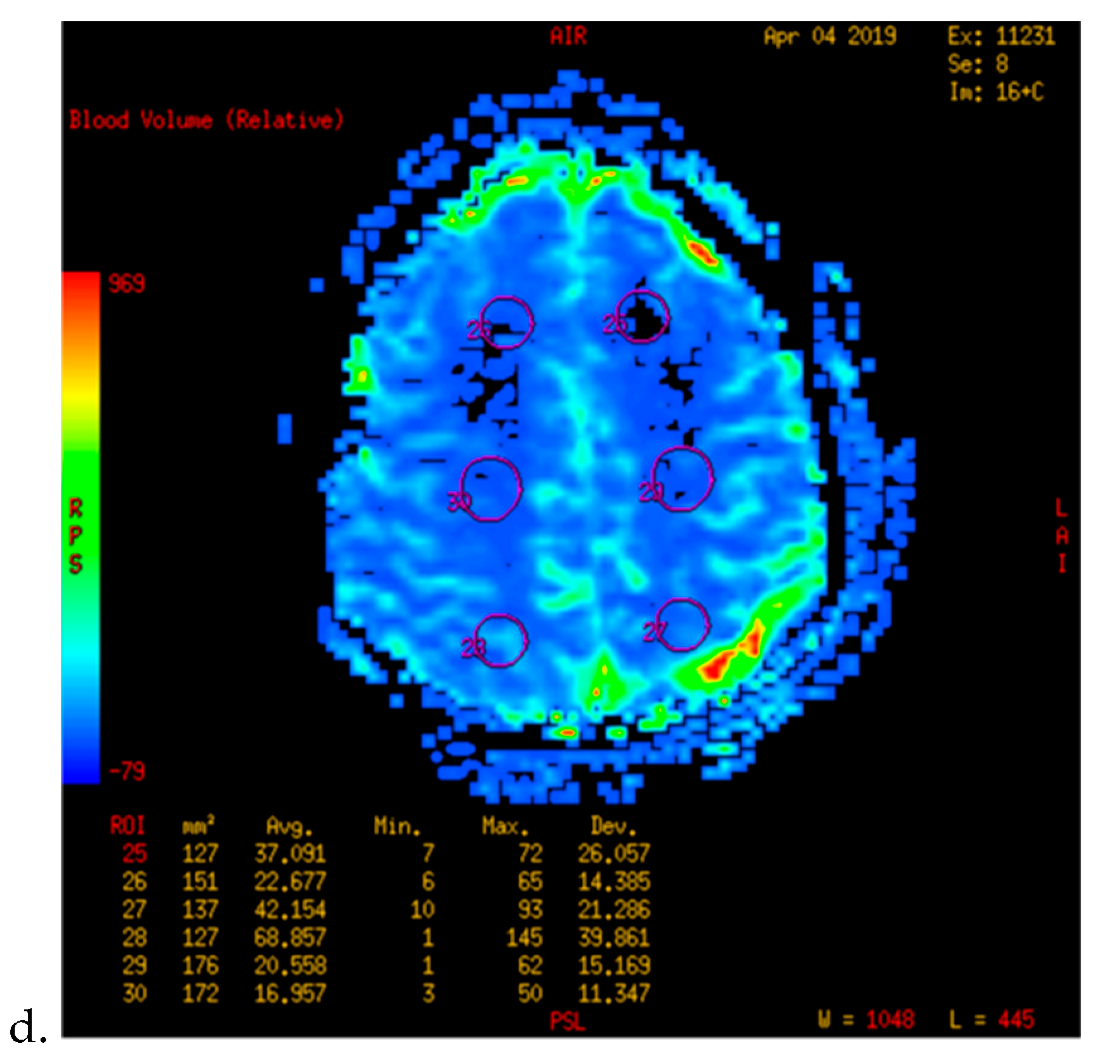
| Characteristics | n (%) |
|---|---|
| Gender | |
| Male | 110 (54.2) |
| Female | 93 (45.8) |
| Age (, years) | 46.76 ± 11.15 |
| History | |
| Hypertension | 72 (35.5) |
| Diabetes | 25 (12.3) |
| Aneurysm | 22 (10.8) |
| Moyamoya disease information | |
| Main clinical symptom | |
| Cerebral hemorrhage | 92 (45.3) |
| Cerebral infarction | 54 (26.6) |
| TIA | 31 (15.3) |
| Chronic cerebral ischemia | 26 (12.8) |
| Preoperative attack times | |
| One time | 160 (78.8) |
| Two times and more | 43 (21.2) |
| Interval between the last attack and operation | |
| Acute operation | 41 (20.2) |
| Stable operation | 94 (46.3) |
| Long-term operation | 68 (33.5) |
| Stage classification | |
| Early stage | 16 (7.9) |
| Middle stage | 142 (70) |
| Late stage | 45 (22.2) |
| Lesions involving the posterior circulation | 54 (26.6) |
| Admission mRS score (, marks) | 1.82 ± 1.02 |
| Follow-up mRS score (, marks) | 1.34 ± 1.18 |
| Information | n (%) |
|---|---|
| Operation method | |
| EDMS + STA–MCA bypass | 196 (96.6) |
| EDMS | 7 (3.4) |
| Operation side | |
| Left | 95 (46.8) |
| Right | 108 (53.2) |
| Time of bypass | |
| First time | 172 (84.7) |
| Second time | 31 (15.3) |
| Duration of surgery [(Md, IQR), minute] | 218 (71) |
| Intraoperative blood loss (, mL) | 138.77 ± 129.76 |
| Intraoperative PCO2 | |
| Low | 40 (19.7) |
| Normal | 161 (79.3) |
| High | 2 (1) |
| Perioperative blood pressure | |
| Preoperative | |
| Normal | 179 (88.2) |
| High | 24 (11.8) |
| Intraoperative | |
| Normal | 186 (91.6) |
| High | 17 (8.4) |
| Postoperative | |
| Normal | 161 (79.3) |
| High | 42 (20.7) |
| 24 h cranial drainage volume after operation (, mL) | 161.63 ± 81.09 |
| Complications | Total (case) | n (%) | Dominant Hemispheric Surgery (%) |
|---|---|---|---|
| Perioperative period | |||
| TND | 26 (12.8%) | ||
| Aphasia | 7 (26.9) | 7 (100) | |
| Epilepsy | 4 (15.4) | 1 (25) | |
| Muscle weakness + Aphasia | 3 (11.5) | 3 (100) | |
| Severe vomiting + Vertigo | 3 (11.5) | 0 (0) | |
| Muscle weakness | 2 (7.7) | 0 (0) | |
| Central facial paralysis | 2 (7.7) | 0 (0) | |
| Consciousness disorders | 2 (7.7) | 1 (50) | |
| Acroparesthesia | 1 (3.8) | 0 (0) | |
| Blurred vision | 1 (3.8) | 1 (100) | |
| Drinking water cough + Epilepsy | 1 (3.8) | 0 (0) | |
| Stroke | 12 (5.9%) | Surgical side hemisphere (%) | |
| Infarction | 10 (83.3) | 7 (70) | |
| Hemorrhage | 2 (16.7) | 2 (100) | |
| Long-term | |||
| Cerebral rebleeding | 8 (3.9%) | 2 (25) |
| Factors | TND | Perioperative Stroke | ||||
|---|---|---|---|---|---|---|
| TND (+) n (%) (n = 26) | TND (-) n (%) (n = 177) | p Value | Stroke (+) n (%) (n = 12) | Stroke (−) n (%) (n = 191) | p Value | |
| Gender (male/female) † | 13/13 | 97/80 | 0.646 | 8/4 | 102/89 | 0.371 |
| Age (, years) ‡ | 43.2 ± 12.9 | 47.3 ± 10.8 | 0.081 | 56 ± 8.7 | 46.2 ± 11.1 | 0.003 * |
| History † | ||||||
| Hypertension | 7 (26.9) | 65 (36.7) | 0.329 | 8 (66.7) | 64 (33.5) | 0.044 * |
| Diabetes | 7 (26.9) | 18 (10.2) | 0.035 * | 4 (33.3) | 21 (11) | 0.067 |
| Aneurysm | 2 (7.7) | 20 (11.3) | 0.83 | 3 (25) | 19 (9.9) | 0.251 |
| Main clinical symptom † | 0.328 | <0.001 * | ||||
| Cerebral hemorrhage | 9 (34.6) | 83 (46.9) | 1 (8.3) | 91 (47.6) | ||
| Cerebral infarction | 7 (26.9) | 47 (26.6) | 11 (91.7) | 43 (22.5) | ||
| TIA | 7 (26.9) | 24 (13.6) | 0 (0) | 31 (16.2) | ||
| Chronic cerebral ischemia | 3 (11.5) | 23 (13) | 0 (0) | 26 (13.6) | ||
| Preoperative attack times † | 0.005 * | 0.976 | ||||
| One time | 15 (57.7) | 145 (81.9) | 10 (83.3) | 150 (78.5) | ||
| Two times and more | 11 (42.3) | 32 (18.1) | 2 (16.7) | 41 (21.5) | ||
| Interval between the last attack and operation § | 0.876 | 0.237 | ||||
| Acute operation | 5 (19.2) | 36 (20.3) | 2 (16.7) | 39 (20.4) | ||
| Stable operation | 12 (46.2) | 82 (46.3) | 9 (75) | 85 (44.5) | ||
| Long-term operation | 9 (34.6) | 59 (33.3) | 1 (8.3) | 67 (35.1) | ||
| Stage classification § | 0.086 | 0.064 | ||||
| Early stage | 1 (3.8) | 15 (8.5) | 0 (0) | 16 (8.4) | ||
| Middle stage | 16 (61.5) | 126 (71.2) | 7 (58.3) | 135 (70.7) | ||
| Late stage | 9 (34.6) | 36 (20.3) | 5 (41.7) | 40 (20.9) | ||
| Lesions involving the posterior circulation † | 13 (50) | 41 (23.2) | 0.004 * | 7 (58.3) | 47 (24.6) | 0.026 * |
| Admission mRS score § | 0.136 | 0.073 | ||||
| 0~1 | 13 (50) | 62 (35) | 2 (16.7) | 73 (38.2) | ||
| 2~3 | 12 (46.2) | 103 (58.2) | 8 (66.7) | 107 (56) | ||
| ≥4 | 1 (3.8) | 12 (6.8) | 2 (16.7) | 11 (5.8) | ||
| Operation method II | 0.221 | 1 | ||||
| EDMS + STA–MCA bypass | 24 (92.3) | 172 (97.2) | 12 (100) | 184 (96.3) | ||
| EDMS | 2 (7.7) | 5 (2.8) | 0 (0) | 7 (3.7) | ||
| Operation side † | 0.726 | 0.819 | ||||
| Left | 13 (50) | 82 (46.3) | 6 (50) | 89 (46.6) | ||
| Right | 13 (50) | 95 (53.7) | 6 (50) | 102 (53.4) | ||
| Time of bypass † | 0.784 | 0.27 | ||||
| First time | 23 (88.5) | 149 (84.2) | 12 (100) | 160 (83.3) | ||
| Second time | 3 (11.5) | 28 (15.8) | 0 (0) | 31 (16.2) | ||
| Duration of surgery [(Md, IQR), minute] § | 232 (65) | 243 (77) | 0.179 | 232 (101) | 217 (64) | 0.201 |
| Cerebrovascular occlusion times † | 0.927 | 0.058 | ||||
| <20 min | 9 (34.6) | 57 (32.2) | 3 (25) | 72 (37.7) | ||
| 20~40 min | 14 (53.8) | 95 (53.7) | 5 (41.7) | 99 (51.8) | ||
| >40 min | 3 (11.5) | 25 (14.1) | 4 (33.3) | 20 (10.5) | ||
| Intraoperative blood loss (, mL) § | 126.9 ± 145.8 | 140.5 ± 127.6 | 0.399 | 162.5 ± 143.2 | 137.3 ± 129.1 | 0.406 |
| Intraoperative PCO2 § | 0.115 | 0.596 | ||||
| <35 mmHg | 8 (30.8) | 32 (18.1) | 3 (25) | 37 (19.4) | ||
| 35~45 mmHg | 18 (69.2) | 143 (80.8) | 9 (75) | 152 (79.6) | ||
| >45 mmHg | 0 (0) | 2 (1.1) | 0 (0) | 2 (1) | ||
| Perioperative blood pressure † | ||||||
| High preoperative blood pressure | 6 (23.1) | 18 (10.2) | 0.115 | 5 (41.7) | 19 (9.9) | 0.005 * |
| High intraoperative blood pressure | 3 (11.5) | 14 (7.9) | 0.807 | 4 (33.3) | 13 (6.8) | 0.007 * |
| High postoperative blood pressure | 11 (42.3) | 31 (17.5) | 0.004 * | 8 (66.7) | 34 (17.8) | <0.001 * |
| 24 h cranial drainage volume after operation (, mL) ‡ | 161.9 ± 77.4 | 161.6 ± 81.8 | 0.984 | 165 ± 65.9 | 161.4 ± 82.1 | 0.882 |
| Risk Factors | B Value | OR | 95% CI | p Value |
|---|---|---|---|---|
| TND | ||||
| History of diabetes | −0.806 | 0.446 | 0.142~1.401 | 0.167 |
| Multiple episodes of preoperative symptoms | 1.142 | 3.134 | 1.236~7.943 | 0.016 * |
| Lesions involving the posterior circulation | −1.119 | 0.327 | 0.133~0.8 | 0.014 * |
| High postoperative blood pressure | 0.831 | 2.296 | 0.868~6.077 | 0.094 |
| Perioperative stroke | ||||
| Older age | 0.06 | 1.061 | 0.988~1.141 | 0.104 |
| History of hypertension | −0.048 | 0.953 | 0.146~6.229 | 0.96 |
| Main clinical symptom | −1.565 | 0.209 | 0.059~0.738 | 0.015 * |
| Lesions involving the posterior circulation | −1.353 | 0.258 | 0.062~1.079 | 0.064 |
| Perioperative blood pressure | ||||
| High preoperative blood pressure | −0.229 | 0.795 | 0.106~5.942 | 0.823 |
| High intraoperative blood pressure | 0.804 | 2.235 | 0.351~14.237 | 0.395 |
| High postoperative blood pressure | 1.074 | 2.928 | 0.585~14.645 | 0.191 |
| Changes in Long-Term Prognosis | ||
|---|---|---|
| Improvement of Prognosis ¶ | Deterioration of Prognosis # | |
| Perioperative complications | ||
| Occurred | 30 (78.9) | 8 (21.1) |
| Did not occur | 149 (90.3) | 16 (9.7) |
| p value | 0.094 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Yang, Y.; Gan, C.; He, X.; Liu, Y.; Huang, Y.; Ma, X.; Wang, S.; Shu, K.; Lei, T.; et al. Risk Factors of Transient Neurological Deficits and Perioperative Stroke after Revascularization in Patients with Moyamoya Disease. Brain Sci. 2022, 12, 1285. https://doi.org/10.3390/brainsci12101285
Zhang X, Yang Y, Gan C, He X, Liu Y, Huang Y, Ma X, Wang S, Shu K, Lei T, et al. Risk Factors of Transient Neurological Deficits and Perioperative Stroke after Revascularization in Patients with Moyamoya Disease. Brain Sciences. 2022; 12(10):1285. https://doi.org/10.3390/brainsci12101285
Chicago/Turabian StyleZhang, Xincheng, Yiping Yang, Chao Gan, Xuejun He, Yanchao Liu, Yimin Huang, Xiaopeng Ma, Sheng Wang, Kai Shu, Ting Lei, and et al. 2022. "Risk Factors of Transient Neurological Deficits and Perioperative Stroke after Revascularization in Patients with Moyamoya Disease" Brain Sciences 12, no. 10: 1285. https://doi.org/10.3390/brainsci12101285
APA StyleZhang, X., Yang, Y., Gan, C., He, X., Liu, Y., Huang, Y., Ma, X., Wang, S., Shu, K., Lei, T., & Zhang, H. (2022). Risk Factors of Transient Neurological Deficits and Perioperative Stroke after Revascularization in Patients with Moyamoya Disease. Brain Sciences, 12(10), 1285. https://doi.org/10.3390/brainsci12101285








