Pimavanserin and Parkinson’s Disease Psychosis: A Narrative Review
Abstract
1. Introduction
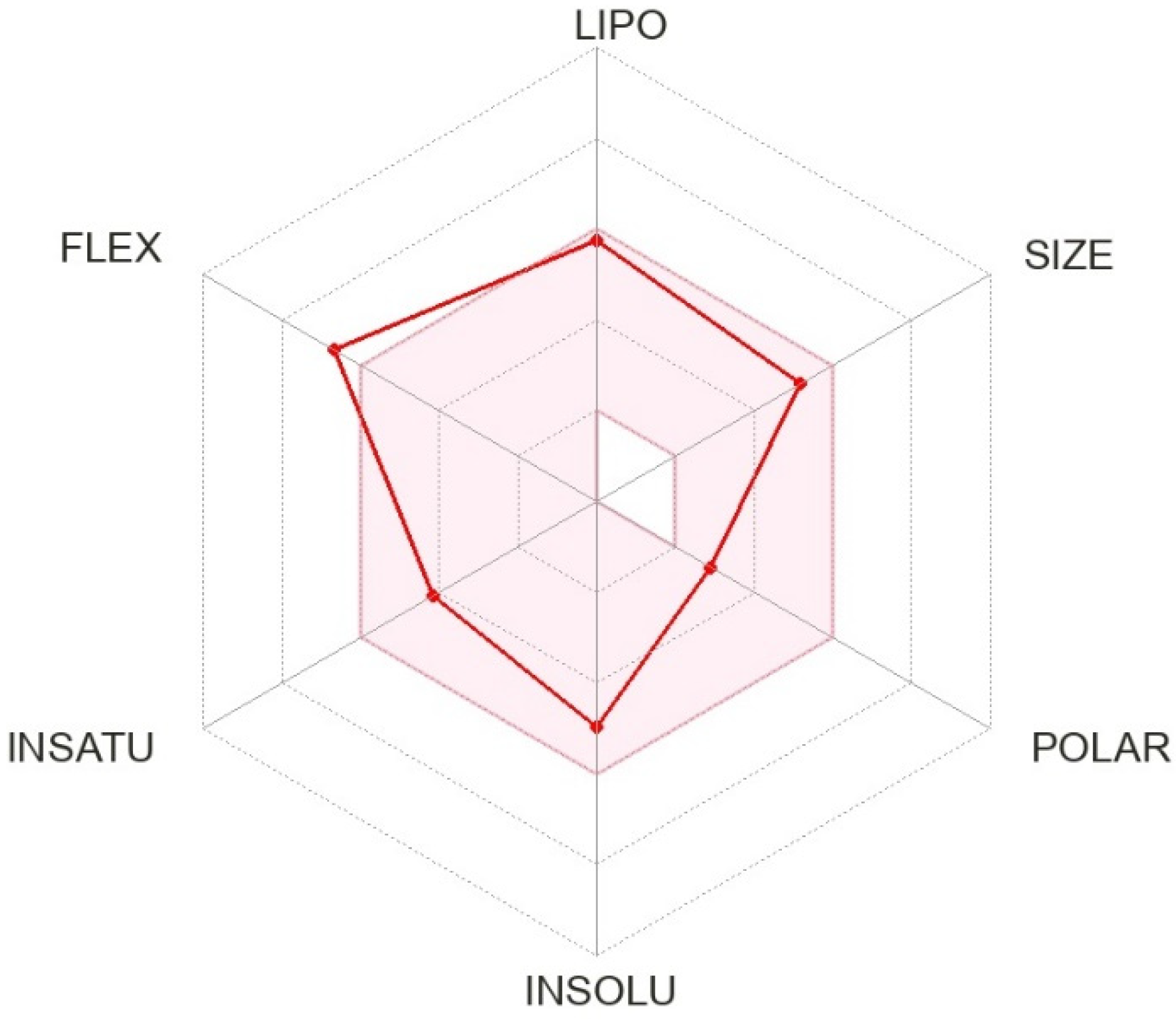
2. Pharmacological History of Pimavanserin
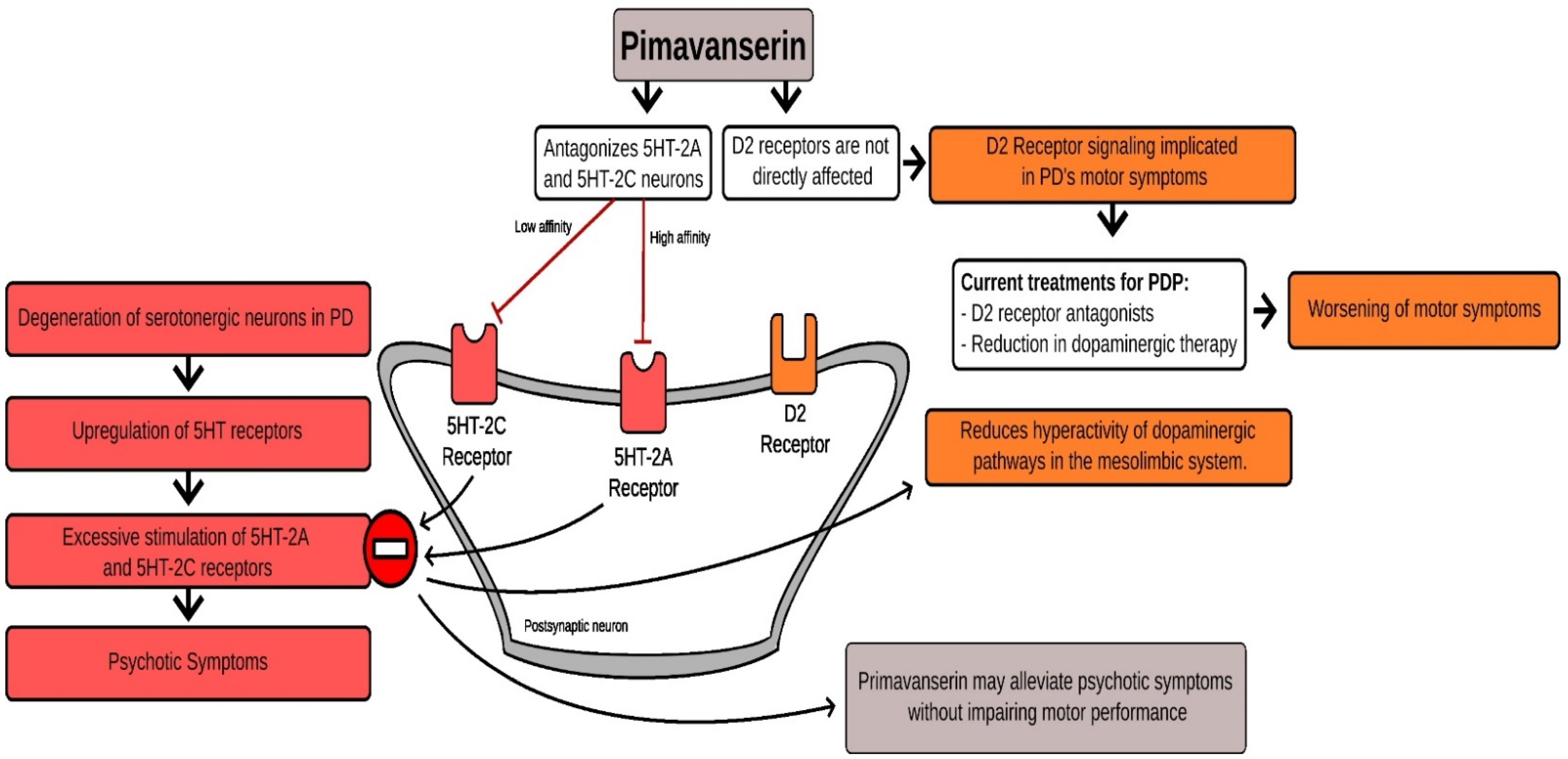
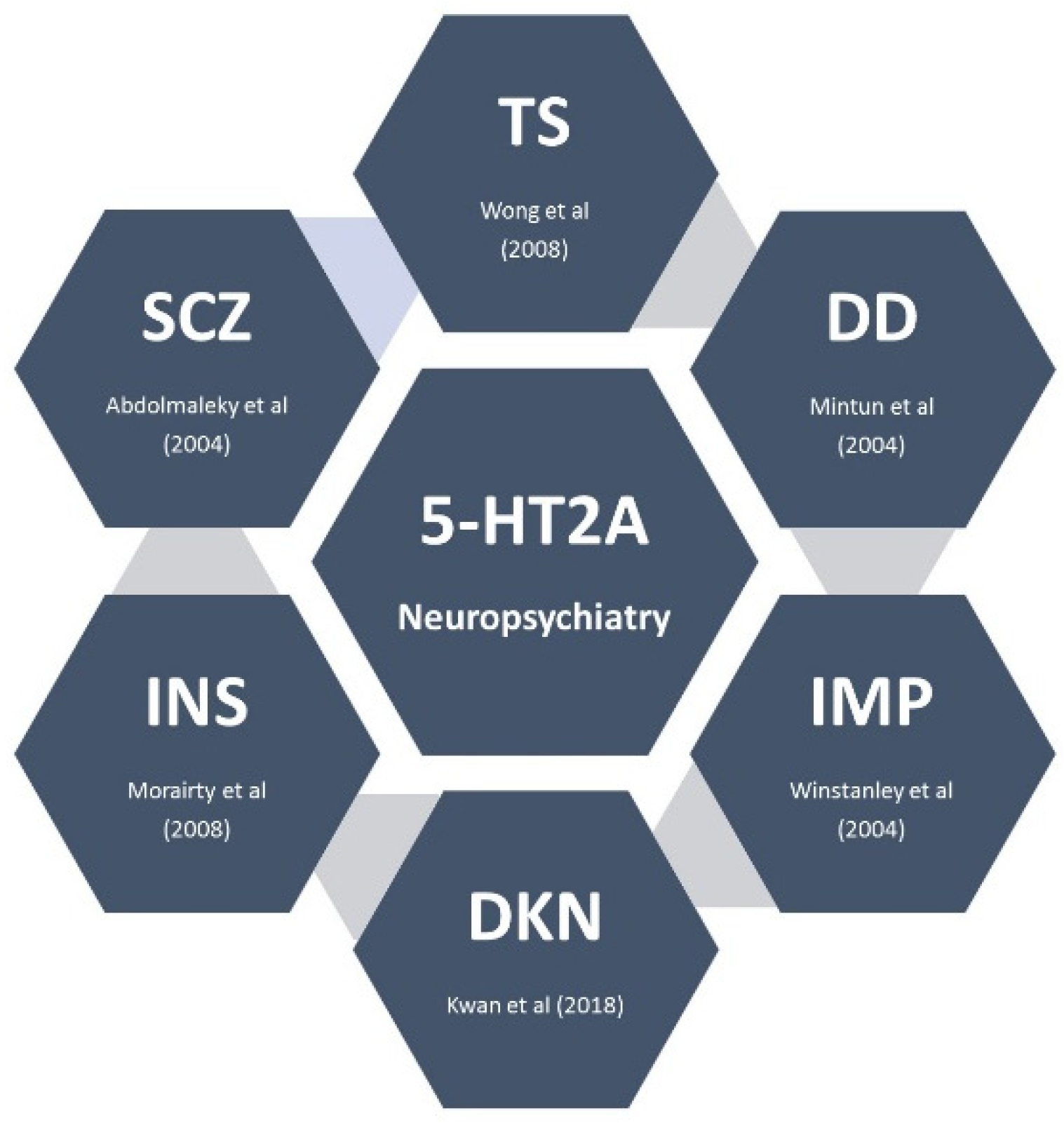
3. Mechanism of Action of Pimavanserin
4. Pimavanserin Clinical Trials
5. Pimavanserin Clinical Experience
5.1. Pimavanserin Efficacy
5.2. Switching from Off-Label Antipsychotics to Pimavanserin
5.3. Post-Marketing Surveillance and Experience
5.4. Pimavanserin Clinical Experience
6. Future Directions
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kang, G.A.; Bronstein, J.M. Psychosis in nursing home patients with Parkinson’s disease. J. Am. Med. Dir. Assoc. 2004, 5, 167–173. [Google Scholar] [CrossRef]
- Aarsland, D.; Larsen, J.P.; Tandberg, E.; Laake, K. Predictors of nursing home placement in Parkinson’s disease: A population-based, prospective study. J. Am. Geriatr. Soc. 2000, 48, 938–942. [Google Scholar] [CrossRef]
- Mantri, S.; Klawson, E.; Albert, S.; Rapoport, R.; Precht, C.; Glancey, S.; Daeschler, M.; Mamikonyan, E.; Kopil, C.M.; Marras, C.; et al. The experience of care partners of patients with Parkinson’s disease psychosis. PLoS ONE 2021, 16, e0248968. [Google Scholar] [CrossRef]
- Cummings, J.; Ballard, C.; Tariot, P.; Owen, R.; Foff, E.; Youakim, J.; Norton, J.; Stankovic, S. Pimavanserin: Potential Treatment for Dementia-Related Psychosis. J. Prev. Alzheimer’s Dis. 2018, 5, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Fox, S.H. Psychosis in Parkinson’s Disease: Epidemiology, Pathophysiology, and Management. Drugs 2016, 76, 1093–1118. [Google Scholar] [CrossRef] [PubMed]
- Lenka, A.; Gomathinayagam, V.; Bahroo, L. Approach to the management of psychosis in Parkinson’s disease. Ann. Mov. Disord. 2019, 2, 83. [Google Scholar] [CrossRef]
- Ravina, B.; Marder, K.; Fernandez, H.H.; Friedman, J.H.; McDonald, W.; Murphy, D.; Aarsland, D.; Babcock, D.; Cummings, J.; Endicott, J.; et al. Diagnostic criteria for psychosis in Parkinson’s disease: Report of an NINDS, NIMH work group. Mov. Disord. Off. J. Mov. Disord. Soc. 2007, 22, 1061–1068. [Google Scholar] [CrossRef]
- Segal, G.S.; Xie, S.J.; Paracha, S.U.; Grossberg, G.T. Psychosis in Parkinson’s Disease: Current Treatment Options and Impact on Patients and Caregivers. J. Geriatr. Psychiatry Neurol. 2021, 34, 274–279. [Google Scholar] [CrossRef]
- Fénelon, G. Psychosis in Parkinson’s disease: Phenomenology, frequency, risk factors, and current understanding of pathophysiologic mechanisms. CNS Spectr. 2008, 13, 18–25. [Google Scholar] [CrossRef]
- Isaacson, S.H.; Citrome, L. Hallucinations and delusions associated with Parkinson’s disease psychosis: Safety of current treatments and future directions. Expert Opin. Drug Saf. 2022, 21, 873–879. [Google Scholar] [CrossRef]
- Friedman, J.H. Parkinson’s disease psychosis 2010: A review article. Parkinsonism Relat. Disord. 2010, 16, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Schneider, R.B.; Iourinets, J.; Richard, I.H. Parkinson’s disease psychosis: Presentation, diagnosis and management. Neurodegener. Dis. Manag. 2017, 7, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.M. Parkinson’s disease psychosis as a serotonin-dopamine imbalance syndrome. CNS Spectr. 2016, 21, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Bashkatova, V. Metabotropic glutamate receptors and nitric oxide in dopaminergic neurotoxicity. World J. Psychiatry 2021, 11, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, T.; Berman, B.D. Pimavanserin: A novel therapeutic option for Parkinson disease psychosis. Neurology. Clin. Pract. 2017, 7, 157–162. [Google Scholar] [CrossRef]
- Cummings, J.; Isaacson, S.; Mills, R.; Williams, H.; Chi-Burris, K.; Corbett, A.; Dhall, R.; Ballard, C. Pimavanserin for patients with Parkinson’s disease psychosis: A randomised, placebo-controlled phase 3 trial. Lancet 2014, 383, 533–540. [Google Scholar] [CrossRef]
- Seppi, K.; Weintraub, D.; Coelho, M.; Perez-Lloret, S.; Fox, S.H.; Katzenschlager, R.; Hametner, E.M.; Poewe, W.; Rascol, O.; Goetz, C.G.; et al. The Movement Disorder Society Evidence-Based Medicine Review Update: Treatments for the non-motor symptoms of Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2011, 26 (Suppl. S3), S42–S80. [Google Scholar] [CrossRef]
- Kurlan, R.; Cummings, J.; Raman, R.; Thal, L. Quetiapine for agitation or psychosis in patients with dementia and parkinsonism. Neurology 2007, 68, 1356–1363. [Google Scholar] [CrossRef]
- Wagner, E.; Siafis, S.; Fernando, P.; Falkai, P.; Honer, W.G.; Röh, A.; Siskind, D.; Leucht, S.; Hasan, A. Efficacy and safety of clozapine in psychotic disorders-a systematic quantitative meta-review. Transl. Psychiatry 2021, 11, 487. [Google Scholar] [CrossRef]
- Hacksell, U.; Burstein, E.S.; McFarland, K.; Mills, R.G.; Williams, H. On the discovery and development of pimavanserin: A novel drug candidate for Parkinson’s psychosis. Neurochem. Res. 2014, 39, 2008–2017. [Google Scholar] [CrossRef]
- Martin, P.; Waters, N.; Waters, S.; Carlsson, A.; Carlsson, M.L. MK-801-induced hyperlocomotion: Differential effects of M100907, SDZ PSD 958 and raclopride. Eur. J. Pharmacol. 1997, 335, 107–116. [Google Scholar] [CrossRef]
- Vanover, K.E.; Weiner, D.M.; Makhay, M.; Veinbergs, I.; Gardell, L.R.; Lameh, J.; Del Tredici, A.L.; Piu, F.; Schiffer, H.H.; Ott, T.R.; et al. Pharmacological and behavioral profile of N-(4-fluorophenylmethyl)-N-(1-methylpiperidin-4-yl)-N’-(4-(2-methylpropyloxy)phenylmethyl) carbamide (2R,3R)-dihydroxybutanedioate (2:1) (ACP-103), a novel 5-hydroxytryptamine(2A) receptor inverse agonist. J. Pharmacol. Exp. Ther. 2006, 317, 910–918. [Google Scholar] [CrossRef] [PubMed]
- McFarland, K.; Price, D.L.; Bonhaus, D.W. Pimavanserin, a 5-HT2A inverse agonist, reverses psychosis-like behaviors in a rodent model of Parkinson’s disease. Behav. Pharmacol. 2011, 22, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Vanover, K.E.; Robbins-Weilert, D.; Wilbraham, D.G.; Mant, T.G.; van Kammen, D.P.; Davis, R.E.; Weiner, D.M. Pharmacokinetics, tolerability, and safety of ACP-103 following single or multiple oral dose administration in healthy volunteers. J. Clin. Pharmacol. 2007, 47, 704–714. [Google Scholar] [CrossRef]
- Meltzer, H.Y.; Mills, R.; Revell, S.; Williams, H.; Johnson, A.; Bahr, D.; Friedman, J.H. Pimavanserin, a serotonin(2A) receptor inverse agonist, for the treatment of parkinson’s disease psychosis. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2010, 35, 881–892. [Google Scholar] [CrossRef]
- Wirth, R.; Dziewas, R.; Beck, A.M.; Clavé, P.; Hamdy, S.; Heppner, H.J.; Langmore, S.; Leischker, A.H.; Martino, R.; Pluschinski, P.; et al. Oropharyngeal dysphagia in older persons—From pathophysiology to adequate intervention: A review and summary of an international expert meeting. Clin. Interv. Aging 2016, 11, 189–208. [Google Scholar] [CrossRef]
- Forman, M.; Kouassi, A.; Brandt, T.; Barsky, L.; Zamora, C.; Dekarske, D. Palatability and Swallowability of Pimavanserin When Mixed with Selected Food Vehicles: An Exploratory Open-Label Crossover Study. Geriatrics 2021, 6, 61. [Google Scholar] [CrossRef]
- Hunter, N.S.; Anderson, K.C.; Cox, A. Pimavanserin. Drugs Today 2015, 51, 645–652. [Google Scholar] [CrossRef]
- Webster, P. Pimavanserin evaluated by the FDA. Lancet 2018, 391, 1762. [Google Scholar] [CrossRef]
- Davis, J.; Zamora, D.; Horowitz, M.; Leucht, S. Evaluating pimavanserin as a treatment for psychiatric disorders: A pharmacological property in search of an indication. Expert Opin. Pharmacother. 2021, 22, 1651–1660. [Google Scholar] [CrossRef]
- Stahl, S.M. Mechanism of action of pimavanserin in Parkinson’s disease psychosis: Targeting serotonin 5HT2A and 5HT2C receptors. CNS Spectr. 2016, 21, 271–275. [Google Scholar] [CrossRef]
- Schmidt, C.J.; Sorensen, S.M.; Kehne, J.H.; Carr, A.A.; Palfreyman, M.G. The role of 5-HT2A receptors in antipsychotic activity. Life Sci. 1995, 56, 2209–2222. [Google Scholar] [CrossRef]
- Geyer, M.A. Lysergic Acid Diethylamide and Psilocybin Revisited. Biol. Psychiatry 2015, 78, 516–518. [Google Scholar] [CrossRef] [PubMed]
- Diez-Alarcia, R.; Muguruza, C.; Rivero, G.; García-Bea, A.; Gómez-Vallejo, V.; Callado, L.F.; Llop, J.; Martín, A.; Meana, J.J. Opposite alterations of 5-HT(2A) receptor brain density in subjects with schizophrenia: Relevance of radiotracers pharmacological profile. Transl. Psychiatry 2021, 11, 302. [Google Scholar] [CrossRef] [PubMed]
- Pourhamzeh, M.; Moravej, F.G.; Arabi, M.; Shahriari, E.; Mehrabi, S.; Ward, R.; Ahadi, R.; Joghataei, M.T. The Roles of Serotonin in Neuropsychiatric Disorders. Cell. Mol. Neurobiol. 2022, 42, 1671–1692. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Winstanley, C.A.; Theobald, D.E.; Dalley, J.W.; Glennon, J.C.; Robbins, T.W. 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: Interactions with global 5-HT depletion. Psychopharmacology 2004, 176, 376–385. [Google Scholar] [CrossRef]
- Morairty, S.R.; Hedley, L.; Flores, J.; Martin, R.; Kilduff, T.S. Selective 5HT2A and 5HT6 receptor antagonists promote sleep in rats. Sleep 2008, 31, 34–44. [Google Scholar] [CrossRef]
- Mintun, M.A.; Sheline, Y.I.; Moerlein, S.M.; Vlassenko, A.G.; Huang, Y.; Snyder, A.Z. Decreased hippocampal 5-HT2A receptor binding in major depressive disorder: In vivo measurement with [18F]altanserin positron emission tomography. Biol. Psychiatry 2004, 55, 217–224. [Google Scholar] [CrossRef]
- Kwan, C.; Frouni, I.; Bédard, D.; Nuara, S.G.; Gourdon, J.C.; Hamadjida, A.; Huot, P. 5-HT(2A) blockade for dyskinesia and psychosis in Parkinson’s disease: Is there a limit to the efficacy of this approach? A study in the MPTP-lesioned marmoset and a literature mini-review. Exp. Brain Res. 2019, 237, 435–442. [Google Scholar] [CrossRef]
- Abdolmaleky, H.M.; Faraone, S.V.; Glatt, S.J.; Tsuang, M.T. Meta-analysis of association between the T102C polymorphism of the 5HT2a receptor gene and schizophrenia. Schizophr. Res. 2004, 67, 53–62. [Google Scholar] [CrossRef]
- Wong, D.F.; Brasić, J.R.; Singer, H.S.; Schretlen, D.J.; Kuwabara, H.; Zhou, Y.; Nandi, A.; Maris, M.A.; Alexander, M.; Ye, W.; et al. Mechanisms of dopaminergic and serotonergic neurotransmission in Tourette syndrome: Clues from an in vivo neurochemistry study with PET. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2008, 33, 1239–1251. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martín, P.; Rojo-Abuin, J.M.; Rodríguez-Violante, M.; Serrano-Dueñas, M.; Garretto, N.; Martínez-Castrillo, J.C.; Arillo, V.C.; Fernández, W.; Chaná-Cuevas, P.; Arakaki, T.; et al. Analysis of four scales for global severity evaluation in Parkinson’s disease. NPJ Parkinson’s Dis. 2016, 2, 16007. [Google Scholar] [CrossRef] [PubMed]
- Bergman, J.; Lerner, P.P.; Sokolik, S.; Lerner, V.; Kreinin, A.; Miodownik, C. Successful Use of Escitalopram for the Treatment of Visual Hallucinations in Patients with Parkinson Disease. Clin. Neuropharmacol. 2017, 40, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bhatia, M.; Behari, M. Excessive daytime sleepiness in Parkinson’s disease as assessed by Epworth Sleepiness Scale (ESS). Sleep Med. 2003, 4, 339–342. [Google Scholar] [CrossRef]
- Aarsland, D.; Muniz, G.; Matthews, F. Nonlinear decline of mini-mental state examination in Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2011, 26, 334–337. [Google Scholar] [CrossRef]
- Rissardo, J.P.; Caprara, A.L.F. Parkinson’s disease rating scales: A literature review. Ann. Mov. Disord. 2020, 3, 3–22. [Google Scholar] [CrossRef]
- Friedberg, G.; Zoldan, J.; Weizman, A.; Melamed, E. Parkinson Psychosis Rating Scale: A practical instrument for grading psychosis in Parkinson’s disease. Clin. Neuropharmacol. 1998, 21, 280–284. [Google Scholar] [PubMed]
- Voss, T.; Bahr, D.; Cummings, J.; Mills, R.; Ravina, B.; Williams, H. Performance of a shortened Scale for Assessment of Positive Symptoms for Parkinson’s disease psychosis. Parkinsonism Relat. Disord. 2013, 19, 295–299. [Google Scholar] [CrossRef]
- Schubmehl, S.; Sussman, J. Perspective on Pimavanserin and the SAPS-PD: Novel Scale Development as a Means to FDA Approval. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 2018, 26, 1007–1011. [Google Scholar] [CrossRef]
- Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease. The Unified Parkinson’s Disease Rating Scale (UPDRS): Status and recommendations. Mov. Disord. Off. J. Mov. Disord. Soc. 2003, 18, 738–750. [Google Scholar] [CrossRef] [PubMed]
- Giguère-Rancourt, A.; Plourde, M.; Racine, E.; Couture, M.; Langlois, M.; Dupré, N.; Simard, M. Altered Theory of Mind in Parkinson’s Disease and Impact on Caregivers: A Pilot Study. Can. J. Neurol. Sci. J. Can. Des Sci. Neurol. 2022, 49, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Mansuri, Z.; Reddy, A.; Vadukapuram, R.; Trivedi, C.; Amara, A. Pimavanserin in the Treatment of Parkinson’s Disease Psychosis: Meta-analysis and Meta-regression of Randomized Clinical Trials. Innov. Clin. Neurosci. 2022, 19, 46–51. [Google Scholar] [PubMed]
- Iketani, R.; Furushima, D.; Imai, S.; Yamada, H. Efficacy and safety of atypical antipsychotics for psychosis in Parkinson’s disease: A systematic review and Bayesian network meta-analysis. Parkinsonism Relat. Disord. 2020, 78, 82–90. [Google Scholar] [CrossRef]
- Isaacson, S.H.; Coate, B.; Norton, J.; Stankovic, S. Blinded SAPS-PD Assessment after 10 Weeks of Pimavanserin Treatment for Parkinson’s Disease Psychosis. J. Parkinson’s Dis. 2020, 10, 1389–1396. [Google Scholar] [CrossRef]
- Sellers, J.; Darby, R.R.; Farooque, A.; Claassen, D.O. Pimavanserin for Psychosis in Parkinson’s Disease-Related Disorders: A Retrospective Chart Review. Drugs Aging 2019, 36, 647–653. [Google Scholar] [CrossRef]
- Wei, L.; Wang, Z.; Huang, Y.; Farias, S.; Duffy, A.; Shahlaie, K.; Wheelock, V.; Zhang, L. A Retrospective Study of Pimavanserin in Patients with Parkinson’s Disease: A Single-center Experience. (2083). Neurology 2021, 96, 2083. [Google Scholar]
- Black, K.J.; Nasrallah, H.; Isaacson, S.; Stacy, M.; Pahwa, R.; Adler, C.H.; Alva, G.; Cooney, J.W.; Kremens, D.; Menza, M.A.; et al. Guidance for switching from off-label antipsychotics to pimavanserin for Parkinson’s disease psychosis: An expert consensus. CNS Spectr. 2018, 23, 402–413. [Google Scholar] [CrossRef]
- Schneider, L.S. The Safety of Pimavanserin for Parkinson’s Disease and Efforts to Reduce Antipsychotics for People with Dementia. Am. J. Psychiatry 2022, 179, 519–521. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Alexander, G.C.; An, H.; Moore, T.J.; Mehta, H.B. Risk of Hospitalization and Death Associated with Pimavanserin Use in Older Adults with Parkinson Disease. Neurology 2021, 97, e1266–e1275. [Google Scholar] [CrossRef]
- Mosholder, A.D.; Ma, Y.; Akhtar, S.; Podskalny, G.D.; Feng, Y.; Lyu, H.; Liao, J.; Wei, Y.; Wernecke, M.; Leishear, K.; et al. Mortality among Parkinson’s Disease Patients Treated with Pimavanserin or Atypical Antipsychotics: An Observational Study in Medicare Beneficiaries. Am. J. Psychiatry 2022, 179, 553–561. [Google Scholar] [CrossRef]
- Brown, J.D.; Cicali, B.; Henriksen, C.; Malaty, I.; Okun, M.S.; Armstrong, M.J. Comparative pharmacovigilance assessment of mortality with pimavanserin in Parkinson disease-related psychosis. J. Manag. Care Spec. Pharm. 2021, 27, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Ballard, C.G.; Kreitzman, D.L.; Isaacson, S.; Liu, I.Y.; Norton, J.C.; Demos, G.; Fernandez, H.H.; Ilic, T.V.; Azulay, J.P.; Ferreira, J.J.; et al. Long-term evaluation of open-label pimavanserin safety and tolerability in Parkinson’s disease psychosis. Parkinsonism Relat. Disord. 2020, 77, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Layton, J.B.; Forns, J.; Turner, M.E.; Dempsey, C.; Bartsch, J.L.; Anthony, M.S.; Danysh, H.E.; Ritchey, M.E.; Demos, G. Falls and Fractures in Patients with Parkinson’s Disease-Related Psychosis Treated with Pimavanserin vs Atypical Antipsychotics: A Cohort Study. Drug-Real World Outcomes 2022, 9, 9–22. [Google Scholar] [CrossRef]
- Dashtipour, K.; Gupta, F.; Hauser, R.A.; Karunapuzha, C.A.; Morgan, J.C. Pimavanserin Treatment for Parkinson’s Disease Psychosis in Clinical Practice. Parkinson’s Dis. 2021, 2021, 2603641. [Google Scholar] [CrossRef]
- Livezey, S.; Shah, N.B.; McCormick, R.; DeClercq, J.; Choi, L.; Zuckerman, A.D. Specialty pharmacist integration into an outpatient neurology clinic improves pimavanserin access. Ment. Health Clin. 2021, 11, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Lake, A.; Hill, M.; Lunsford, J. “Managing the menagerie in my home”, successful clozapine titration for “pimavanserin-resistant” parkinson’s disease psychosis. Am. J. Geriatr. Psychiatry 2020, 28, S117–S118. [Google Scholar] [CrossRef]
- Bokser, A.D.; Adegbenle, Y.H.; Stoisavljevic, V.; Norton, J.C. In Vitro Stability and Recovery Studies of Pimavanserin in Water and in Different Vehicles Orally Administered. Drugs RD 2022, 22, 95–104. [Google Scholar] [CrossRef]
- Bugarski-Kirola, D.; Nunez, R.; Odetalla, R.; Liu, I.Y.; Turner, M.E. Effects of adjunctive pimavanserin and current antipsychotic treatment on QT interval prolongation in patients with schizophrenia. Front. Psychiatry 2022, 13, 892199. [Google Scholar] [CrossRef]
The presence of at least one of the following symptoms of psychosis:
| 1. A primary diagnosis of PD |
| 2. Symptoms of psychosis occur after the onset of PD | |
| 3. The duration of symptoms of psychosis are recurrent or continuous for at least one month | |
| 4. Exclusion of alternative diagnosis |
| Medication | Current Evidence |
|---|---|
| Pimavanserin | Level B (should be considered) |
| Clozapine | Level B (should be considered) |
| Quetiapine | Level C (may be considered) |
| Study | NCT00658567 | NCT00477672 | Meltzer et al. | Cumming et al. (NCT01174004) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Year | 2009 | 2009 | 2010 | 2014 | ||||||
| Type of study | Randomized parallel assignment, quadruple masking | Randomized parallel assignment, quadruple masking | Multicenter, randomized, placebo-controlled, double-blind | Randomized parallel assignment, quadruple masking | ||||||
| Total number of participants | 123 | 298 | 60 | 199 | ||||||
| Intervention | Pimavanserin 10–20 mg and placebo | Pimavanserin 10–40 mg and placebo | Pimavanserin 20–60 mg | Pimavanserin 40 mg and placebo | ||||||
| Primary outcome | Change in SAPS score from baseline to day 42 | Change in SAPS score from baseline to day 42 | Change in SAPS score from baseline to day 28 | Change in SAPS score from baseline to day 43 | ||||||
| Secondary outcome | Change in UPDRS II/III score from baseline to day 42 | Change in UPDRS II/III score from baseline to day 42 | Change in UPDRS II/III score from baseline to day 28 | Change in UPDRS II/III score from baseline to day 43 | ||||||
| Groups | PMV10 | PMV20 | Pla | PMV10 | PMV40 | Pla | PMV | Pla | PMV | Pla |
| N | 41 | 41 | 39 | 99 | 98 | 98 | 29 | 31 | 95 | 90 |
| Age mean (SD) | 71 (7.4) | 72.1 (8.2) | 73 (7.9) | 69.0 (8.6) | 69.4 (7.8) | 69.6 (9.7) | 72.3 (1.4) | 69.6 (1.6) | 72.4 (6.6) | 72.4 (7.9) |
| Sex (male) | 26 | 24 | 27 | 63 | 74 | 51 | 26 | 20 | 64 | 52 |
| Race (white) | - | - | - | - | - | - | 28 | 31 | 90 | 85 |
| Primary endpoint | - | −6.5 | −4.4 | −5.8 | −6.7 | −5.9 | −1.9 | −0.2 | −5.7 | −2.7 |
| Secondary endpoint | - | −3.9 | −1.8 | −1.4 | −3.1 | −2.9 | −3.0 | −3.8 | −1.6 | −1.4 |
| 1. Low PMV dose (17 mg/day; 10 mg/day) may be effective in some patients |
| 2. PMV may be added without disrupting or adversely affecting other multidrug PD regimens |
| 3. PMV may be effective for managing PD psychosis in individuals with deep brain stimulation |
| 4. PMV and another antipsychotic may be necessary to manage PD psychosis |
| 5. After controlling PD psychosis symptoms with PMV, clinicians can increase the dose of dopaminergic drugs for better motor control |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rissardo, J.P.; Durante, Í.; Sharon, I.; Fornari Caprara, A.L. Pimavanserin and Parkinson’s Disease Psychosis: A Narrative Review. Brain Sci. 2022, 12, 1286. https://doi.org/10.3390/brainsci12101286
Rissardo JP, Durante Í, Sharon I, Fornari Caprara AL. Pimavanserin and Parkinson’s Disease Psychosis: A Narrative Review. Brain Sciences. 2022; 12(10):1286. https://doi.org/10.3390/brainsci12101286
Chicago/Turabian StyleRissardo, Jamir Pitton, Ícaro Durante, Idan Sharon, and Ana Letícia Fornari Caprara. 2022. "Pimavanserin and Parkinson’s Disease Psychosis: A Narrative Review" Brain Sciences 12, no. 10: 1286. https://doi.org/10.3390/brainsci12101286
APA StyleRissardo, J. P., Durante, Í., Sharon, I., & Fornari Caprara, A. L. (2022). Pimavanserin and Parkinson’s Disease Psychosis: A Narrative Review. Brain Sciences, 12(10), 1286. https://doi.org/10.3390/brainsci12101286







