Increase in Low-Frequency Oscillations in fNIRS as Cerebral Response to Auditory Stimulation with Familiar Music
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experiment Design
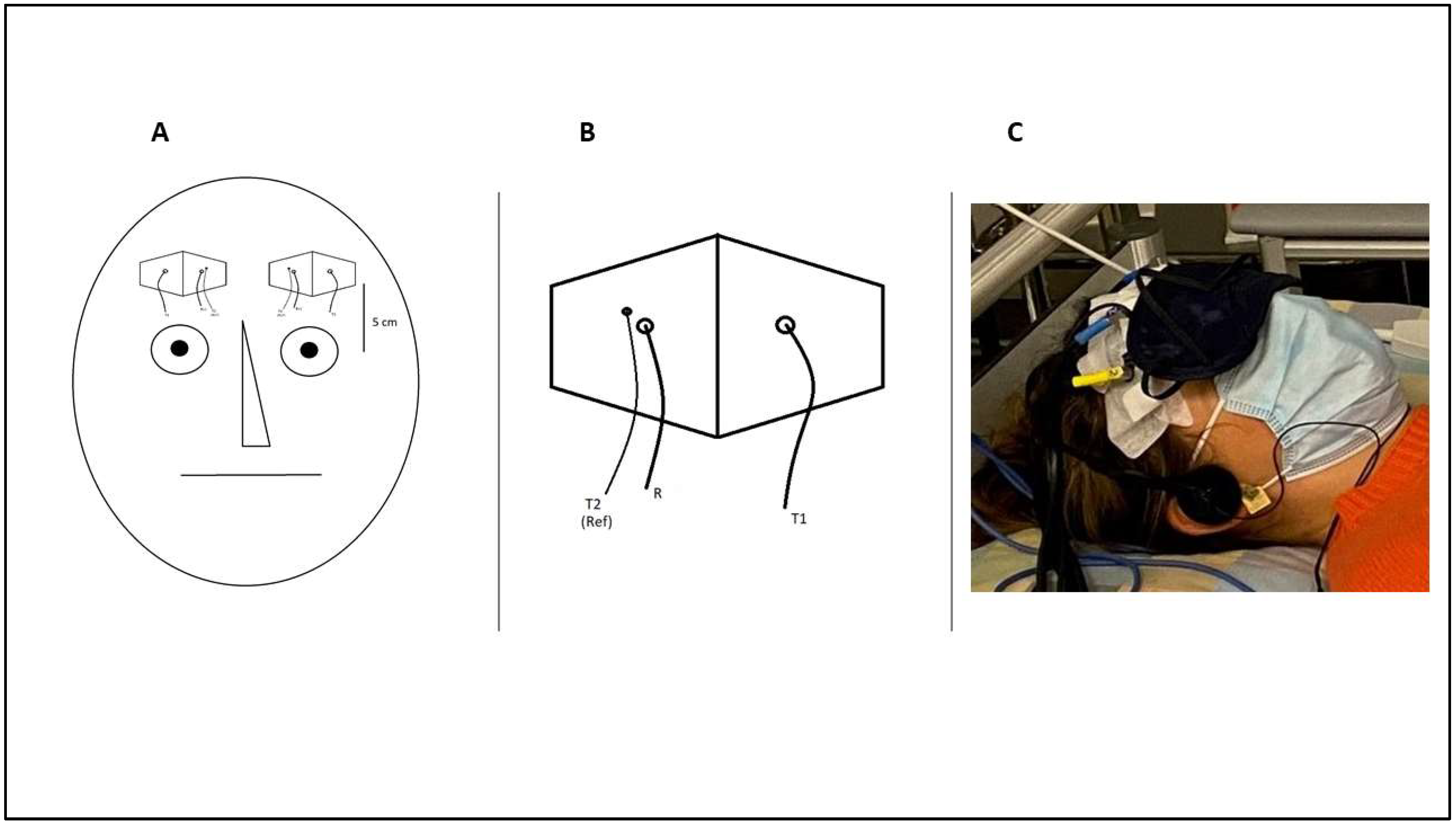
2.3. NIRS Measurement
2.4. Systemic Vital Parameters
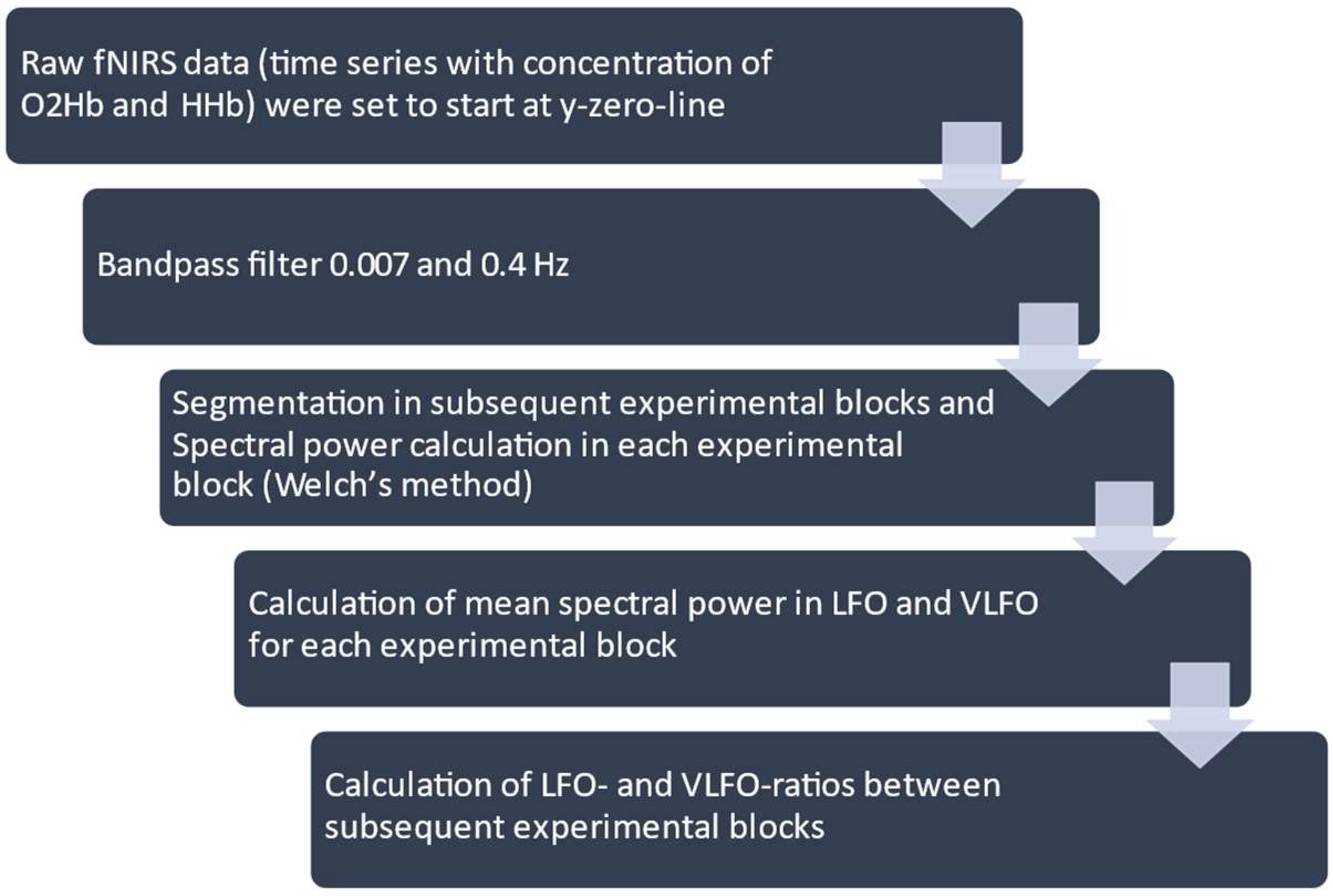
2.5. Processing of NIRS Data and Statistical Analysis
2.6. Statistical Tests
3. Results
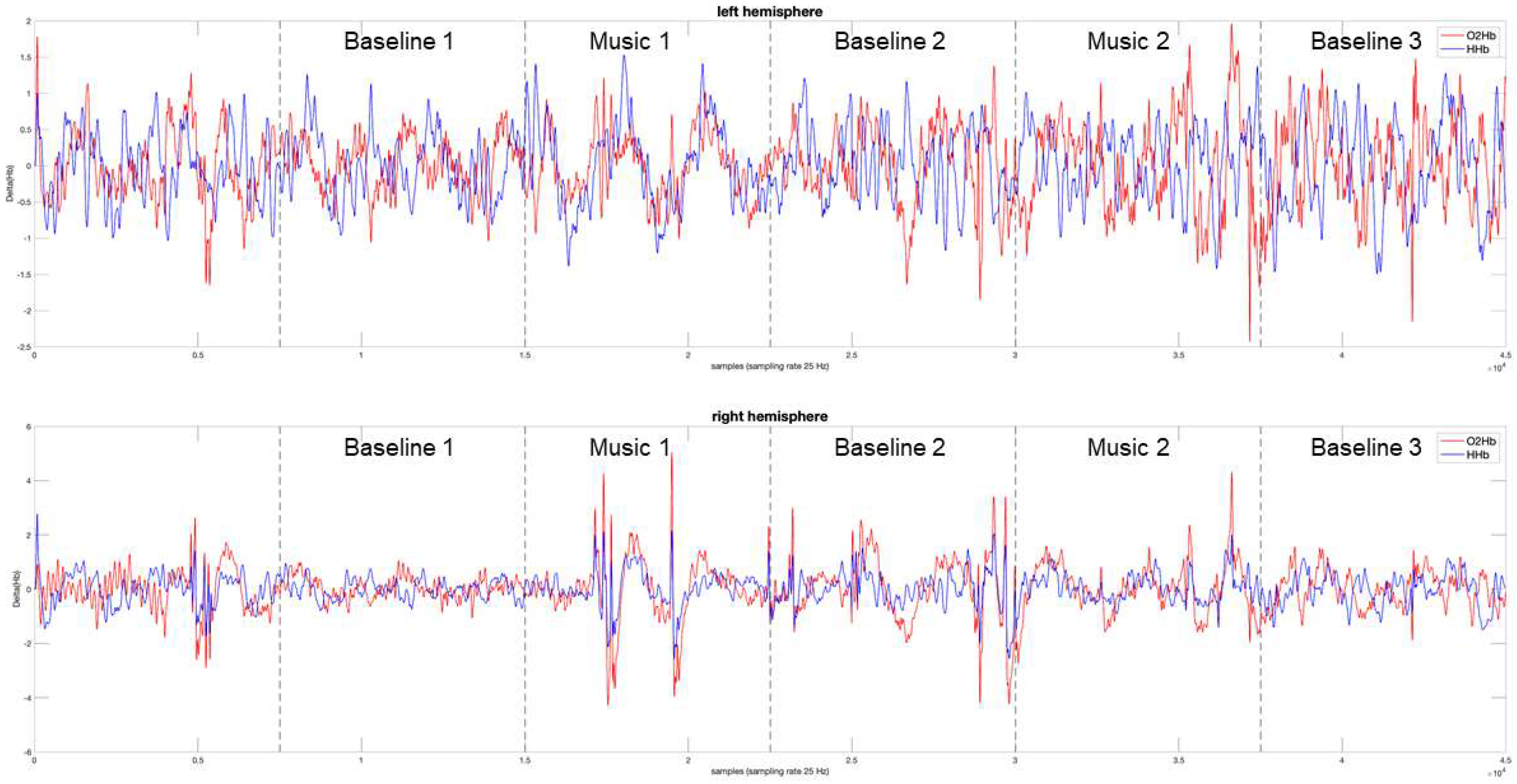
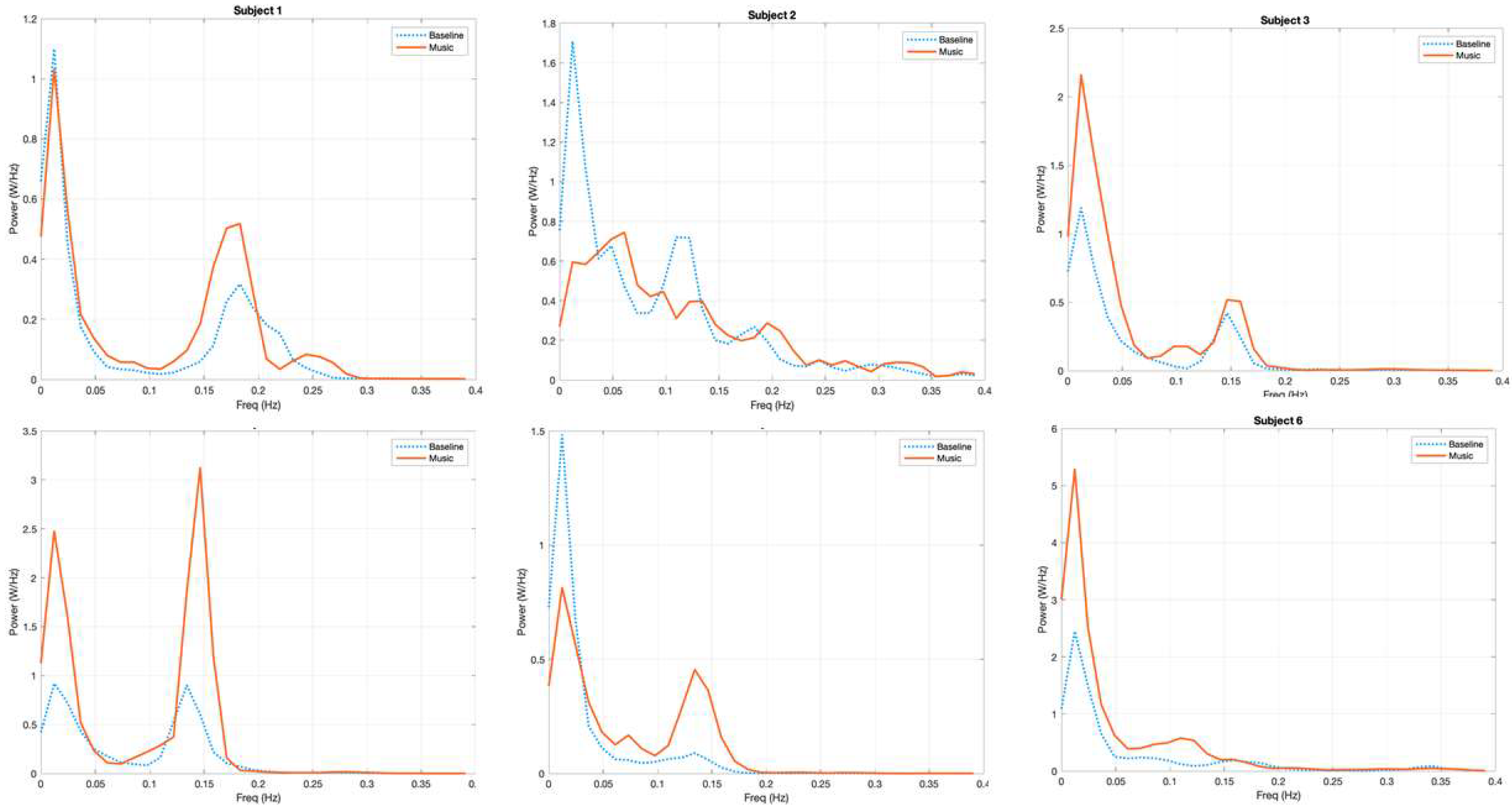
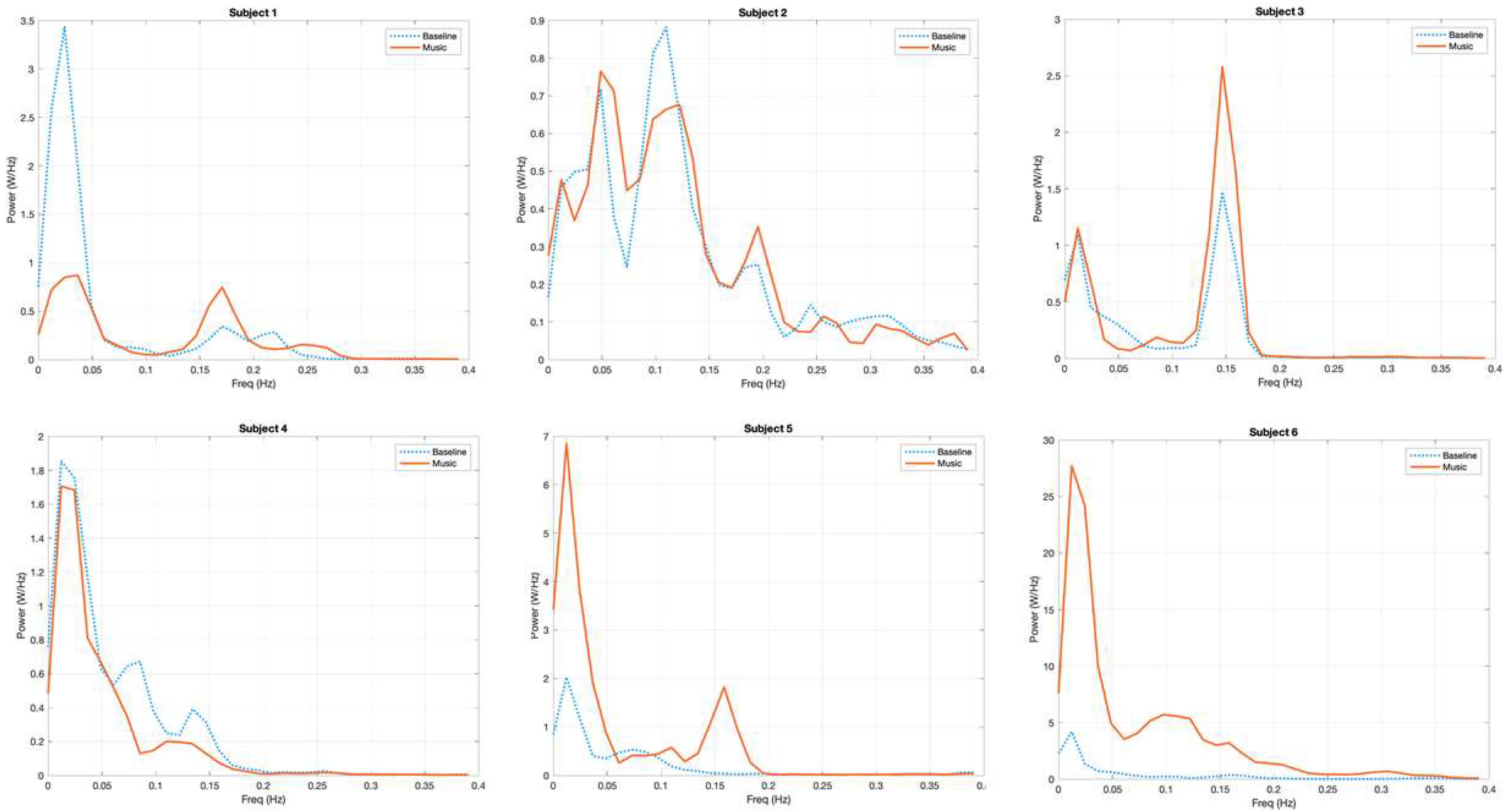
3.1. Power Spectrum Analysis, a Qualitative Assessment
3.2. Measuring the Response to Music, a Quantitative Approach
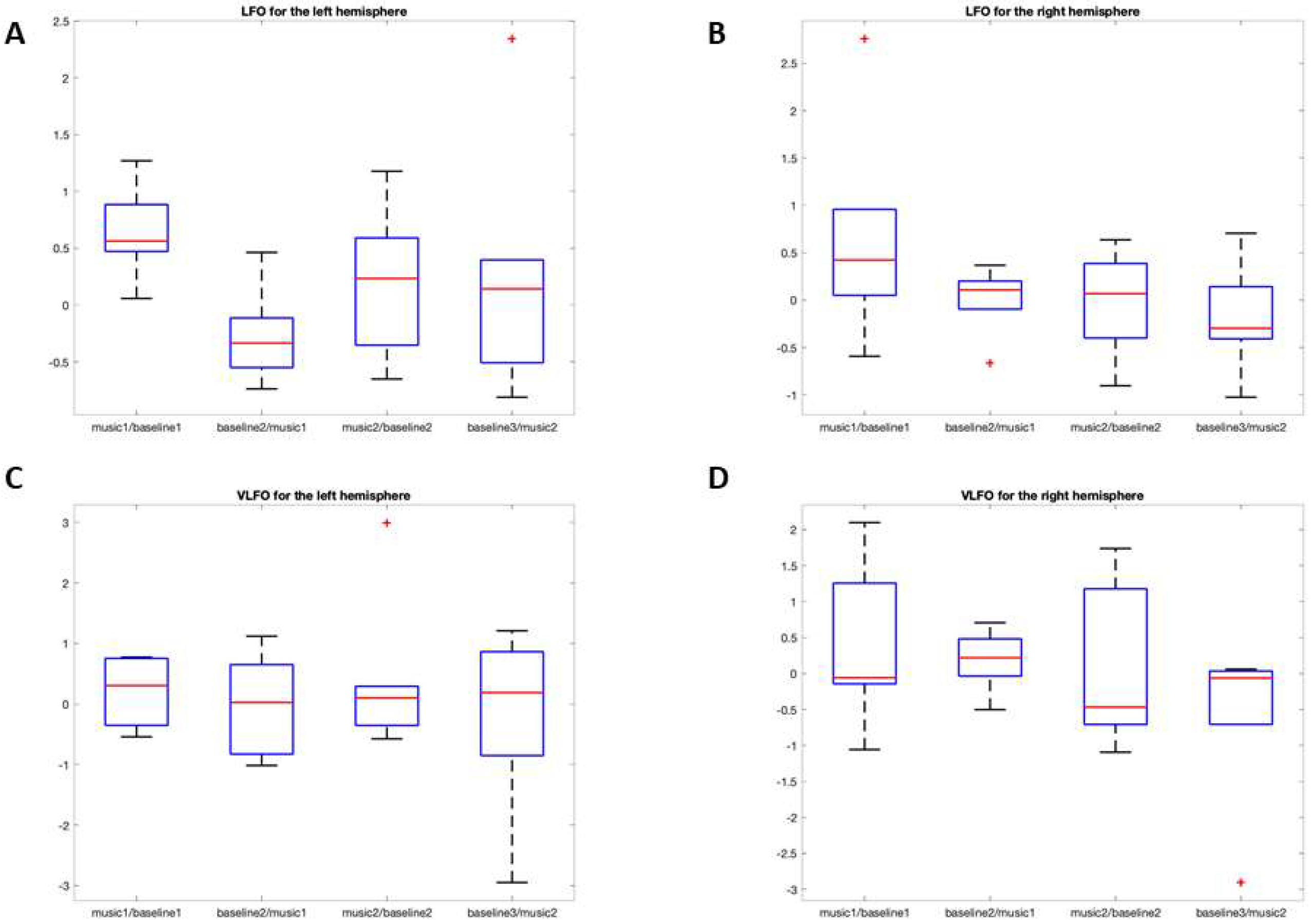
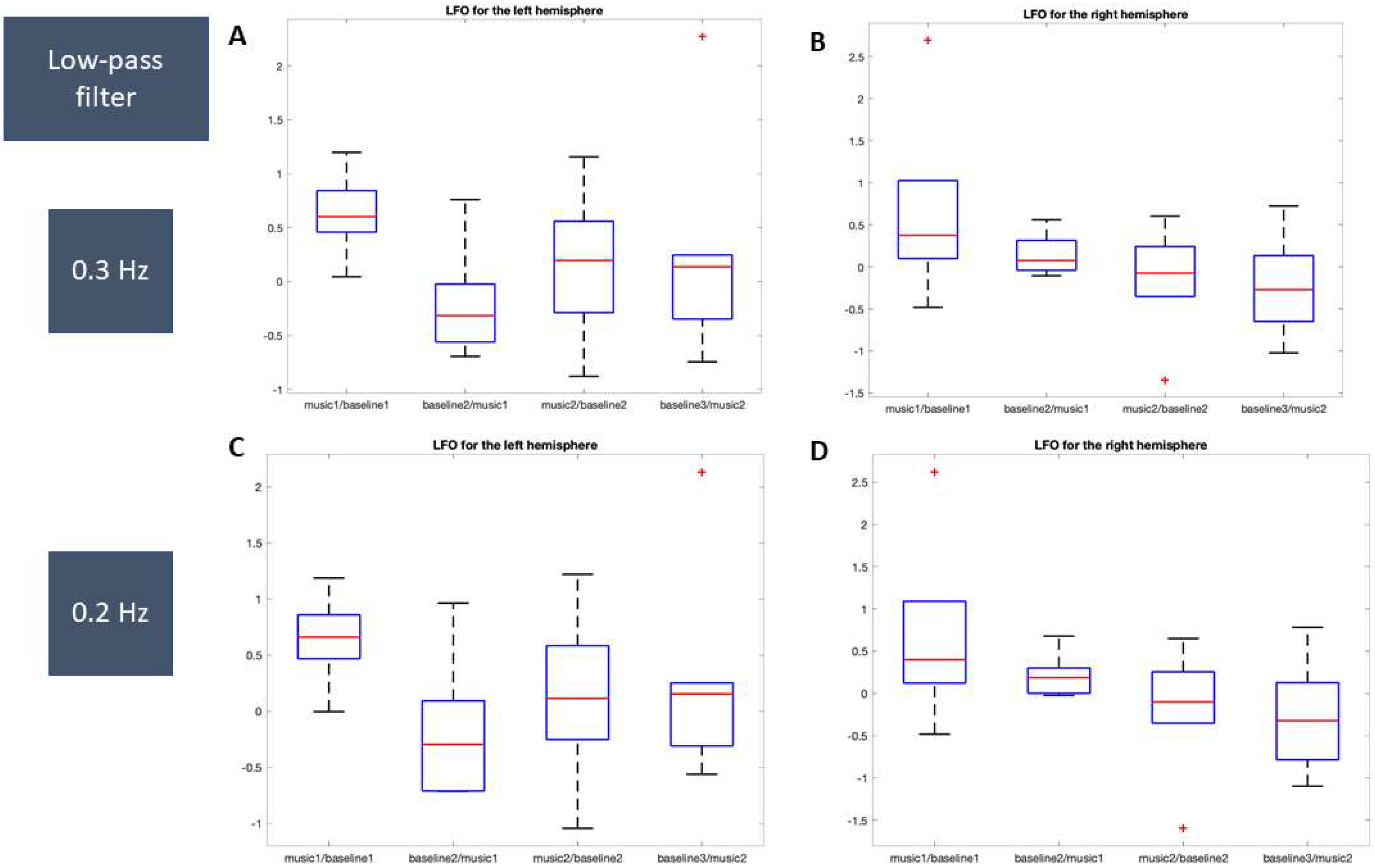
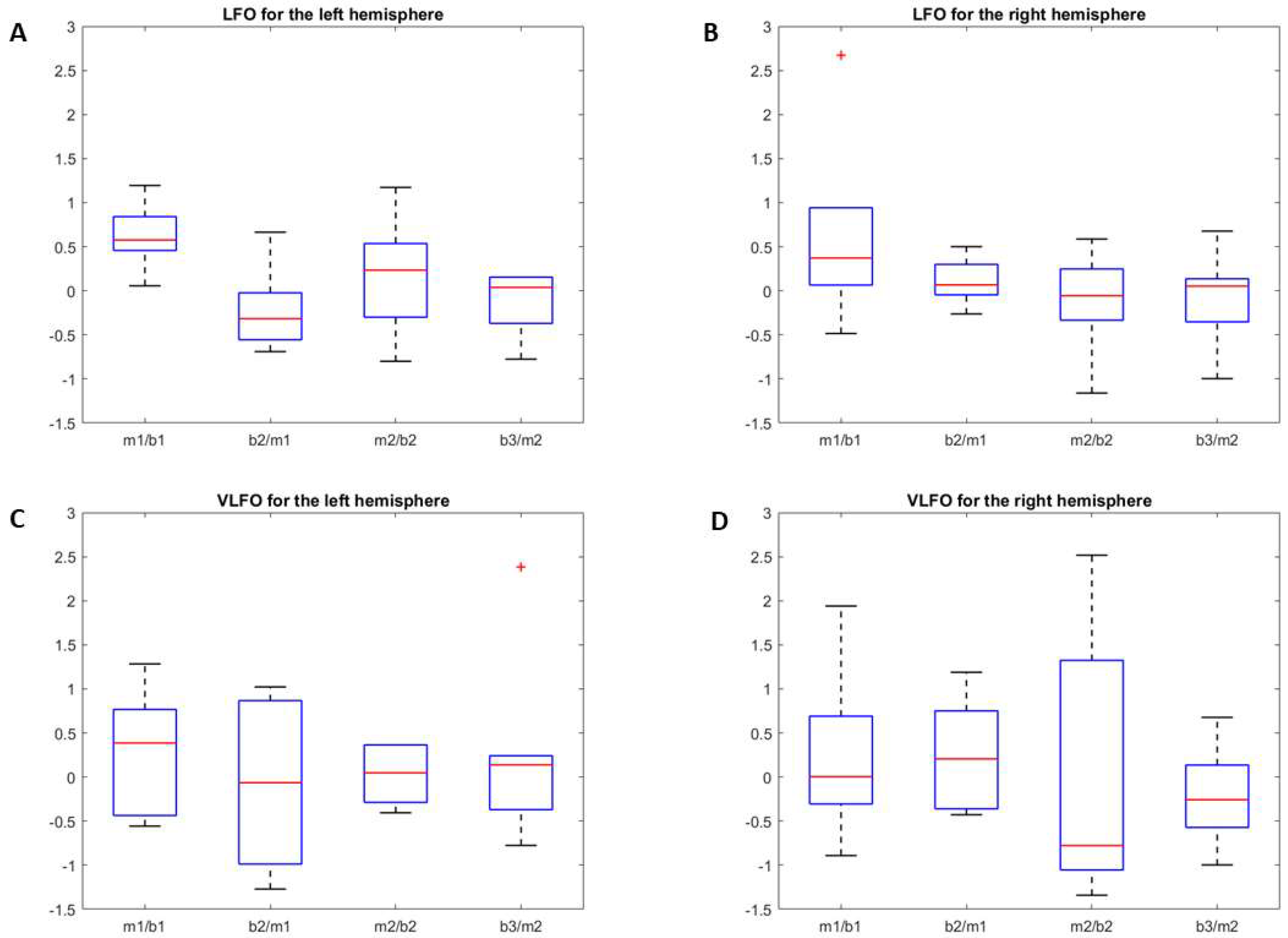
3.2.1. LFO
O2Hb
HHb
3.2.2. VLFO
O2Hb
HHb
3.2.3. Systemic Vital Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Steinhoff, N.; Heine, A.M.; Vogl, J.; Weiss, K.; Aschraf, A.; Hajek, P.; Schnider, P.; Tucek, G. A pilot study into the effects of music therapy on different areas of the brain of individuals with unresponsive wakefulness syndrome. Front. Neurosci. 2015, 9, 291. [Google Scholar] [CrossRef] [Green Version]
- Carrière, M.; Larroque, S.K.; Martial, C.; Bahri, M.A.; Aubinet, C.; Perrin, F.; Laureys, S.; Heine, L. An Echo of Consciousness: Brain Function During Preferred Music. Brain Connect. 2020, 10, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Verger, J.; Ruiz, S.; Tillmann, B.; Ben Romdhane, M.; De Quelen, M.; Castro, M.; Tell, L.; Luauté, J.; Perrin, F. Beneficial effect of preferred music on cognitive functions in minimally conscious state patients. Rev. Neurol. 2014, 170, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yan, Y.; Zhou, W.; Lin, Y.; Shen, Z.; Mou, X.; Ren, Y.; Hu, X.; Di, H. Clinical Research: Auditory Stimulation in the Disorders of Consciousness. Front. Hum. Neurosci. 2019, 13, 324. [Google Scholar] [CrossRef] [PubMed]
- De Salvo, S.; Caminiti, F.; Bonanno, L.; De Cola, M.C.; Corallo, F.; Caizzone, A.; Rifici, C.; Bramanti, P.; Marino, S. Neurophysiological assessment for evaluating residual cognition in vegetative and minimally conscious state patients: A pilot study. Funct. Neurol. 2015, 30, 237–244. [Google Scholar] [CrossRef]
- Claassen, J.; Doyle, K.; Matory, A.; Couch, C.; Burger, K.M.; Velazquez, A.; Okonkwo, J.U.; King, J.-R.; Park, S.; Agarwal, S.; et al. Detection of Brain Activation in Unresponsive Patients with Acute Brain Injury. N. Engl. J. Med. 2019, 380, 2497–2505. [Google Scholar] [CrossRef] [PubMed]
- Stender, J.; Gosseries, O.; Bruno, M.A.; Charland-Verville, V.; Vanhaudenhuyse, A.; Demertzi, A.; Chatelle, C.; Thonnard, M.; Thibaut, A.; Heine, L.; et al. Diagnostic precision of PET imaging and functional MRI in disorders of consciousness: A clinical validation study. Lancet 2014, 384, 514–522. [Google Scholar] [CrossRef]
- Molteni, E.; Arrigoni, F.; Bardoni, A.; Galbiati, S.; Villa, F.; Colombo, K.; Strazzer, S. Bedside assessment of residual functional activation in minimally conscious state using NIRS and general linear models. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 3551–3554. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Si, J.; Xia, X.; He, J.; Jiang, T. Influence of inter-stimulus interval of spinal cord stimulation in patients with disorders of consciousness: A preliminary functional near-infrared spectroscopy study. Neuroimage Clin. 2018, 17, 1–9. [Google Scholar] [CrossRef]
- Kempny, A.M.; James, L.; Yelden, K.; Duport, S.; Farmer, S.; Playford, E.D.; Leff, A.P. Functional near infrared spectroscopy as a probe of brain function in people with prolonged disorders of consciousness. Neuroimage Clin. 2016, 12, 312–319. [Google Scholar] [CrossRef] [Green Version]
- Meder, U.; Tarjanyi, E.; Kovacs, K.; Szakmar, E.; Cseko, A.J.; Hazay, T.; Belteki, G.; Szabo, M.; Jermendy, A. Cerebral oxygenation in preterm infants during maternal singing combined with skin-to-skin care. Pediatr. Res. 2020, 90, 809–814. [Google Scholar] [CrossRef]
- Chen, S.; Sakatani, K.; Lichty, W.; Ning, P.; Zhao, S.; Zuo, H. Auditory-evoked cerebral oxygenation changes in hypoxic-ischemic encephalopathy of newborn infants monitored by near infrared spectroscopy. Early Hum. Dev. 2002, 67, 113–121. [Google Scholar] [CrossRef]
- Ferreri, L.; Bigand, E.; Perrey, S.; Muthalib, M.; Bard, P.; Bugaiska, A. Less Effort, Better Results: How Does Music Act on Prefrontal Cortex in Older Adults during Verbal Encoding? An fNIRS Study. Front. Hum. Neurosci. 2014, 8, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saitou, H.; Yanagi, H.; Hara, S.; Tsuchiya, S.; Tomura, S. Cerebral blood volume and oxygenation among poststroke hemiplegic patients: Effects of 13 rehabilitation tasks measured by near-infrared spectroscopy. Arch. Phys. Med. Rehabil. 2000, 81, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Fantini, S. Dynamic model for the tissue concentration and oxygen saturation of hemoglobin in relation to blood volume, flow velocity, and oxygen consumption: Implications for functional neuroimaging and coherent hemodynamics spectroscopy (CHS). Neuroimage 2014, 85 Pt 1, 202–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buzsáki, G.; Watson, B.O. Brain rhythms and neural syntax: Implications for efficient coding of cognitive content and neuropsychiatric disease. Dialogues Clin. Neurosci. 2012, 14, 345–367. [Google Scholar]
- Sasai, S.; Homae, F.; Watanabe, H.; Taga, G. Frequency-specific functional connectivity in the brain during resting state revealed by NIRS. Neuroimage 2011, 56, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Yuen, N.H.; Osachoff, N.; Chen, J.J. Intrinsic Frequencies of the Resting-State fMRI Signal: The Frequency Dependence of Functional Connectivity and the Effect of Mode Mixing. Front. Neurosci. 2019, 13, 900. [Google Scholar] [CrossRef]
- Zuo, X.N.; Di Martino, A.; Kelly, C.; Shehzad, Z.E.; Gee, D.; Klein, D.F.; Castellanos, F.; Biswal, B.B.; Milham, M.P. The oscillating brain: Complex and reliable. Neuroimage 2010, 49, 1432–1445. [Google Scholar] [CrossRef] [Green Version]
- Thompson, W.H.; Fransson, P. The frequency dimension of fMRI dynamic connectivity: Network connectivity, functional hubs and integration in the resting brain. Neuroimage 2015, 121, 227–242. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Lin, Y.; Hu, C.; Zhang, J.; Yi, Y.; Dai, Z. Integrated and segregated frequency architecture of the human brain network. Brain Struct. Funct. 2021, 226, 335–350. [Google Scholar] [CrossRef]
- Smitha, K.A.; Akhil Raja, K.; Arun, K.M.; Rajesh, P.G.; Thomas, B.; Kapilamoorthy, T.R.; Kesavadas, C. Resting state fMRI: A review on methods in resting state connectivity analysis and resting state networks. Neuroradiol. J. 2017, 30, 305–317. [Google Scholar] [CrossRef]
- Ferrari, M.; Quaresima, V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. Neuroimage 2012, 63, 921–935. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Zhang, Y.J.; Zhu, C.Z. Quantitative comparison of resting-state functional connectivity derived from fNIRS and fMRI: A simultaneous recording study. Neuroimage 2012, 60, 2008–2018. [Google Scholar] [CrossRef]
- Obrig, H.; Neufang, M.; Wenzel, R.; Kohl, M.; Steinbrink, J.; Einhäupl, K.; Villringer, A. Spontaneous low frequency oscillations of cerebral hemodynamics and metabolism in human adults. Neuroimage 2000, 12, 623–639. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.V.; Simonsen, S.A.; Schytz, H.W.; Iversen, H.K. Assessing low-frequency oscillations in cerebrovascular diseases and related conditions with near-infrared spectroscopy: A plausible method for evaluating cerebral autoregulation? Neurophotonics 2018, 5, 030901. [Google Scholar] [CrossRef]
- Pinti, P.; Cardone, D.; Merla, A. Simultaneous fNIRS and thermal infrared imaging during cognitive task reveal autonomic correlates of prefrontal cortex activity. Sci. Rep. 2015, 5, 17471. [Google Scholar] [CrossRef]
- Salvador, R.; Suckling, J.; Schwarzbauer, C.; Bullmore, E. Undirected graphs of frequency-dependent functional connectivity in whole brain networks. Philos. Trans. R. Soc. B 2005, 360, 937–946. [Google Scholar] [CrossRef] [Green Version]
- Sassaroli, A.; Pierro, M.; Bergethon, P.; Fantini, S. Low-Frequency Spontaneous Oscillations of Cerebral Hemodynamics Investigated with Near-Infrared Spectroscopy: A Review. IEEE J. Sel. Top. Quantum Electron. 2012, 18, 1478–1492. [Google Scholar] [CrossRef]
- Chen, W.L.; Wagner, J.; Heugel, N.; Sugar, J.; Lee, Y.-W.; Conant, L.; Malloy, M.; Heffernan, J.; Quirk, B.; Zinos, A.; et al. Functional Near-Infrared Spectroscopy and Its Clinical Application in the Field of Neuroscience: Advances and Future Directions. Front. Neurosci. 2020, 14, 724. [Google Scholar] [CrossRef] [PubMed]
- Pinti, P.; Scholkmann, F.; Hamilton, A.; Burgess, P.; Tachtsidis, I. Current Status and Issues Regarding Pre-processing of fNIRS Neuroimaging Data: An Investigation of Diverse Signal Filtering Methods within a General Linear Model Framework. Front. Hum. Neurosci. 2018, 12, 505. [Google Scholar] [CrossRef] [Green Version]
- Pierro, M.L.; Sassaroli, A.; Bergethon, P.R.; Ehrenberg, B.L.; Fantini, S. Phase-amplitude investigation of spontaneous low-frequency oscillations of cerebral hemodynamics with near-infrared spectroscopy: A sleep study in human subjects. Neuroimage 2012, 63, 1571–1584. [Google Scholar] [CrossRef] [Green Version]
- Bajaj, S.; Drake, D.; Butler, A.J.; Dhamala, M. Oscillatory motor network activity during rest and movement: An fNIRS study. Front. Syst. Neurosci. 2014, 8, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satoh, M.; Takeda, K.; Nagata, K.; Shimosegawa, E.; Kuzuhara, S. Positron-emission tomography of brain regions activated by recognition of familiar music. Am. J. Neuroradiol. 2006, 27, 1101–1106. [Google Scholar] [PubMed]
- Freitas, C.; Manzato, E.; Burini, A.; Taylor, M.J.; Lerch, J.P.; Anagnostou, E. Neural Correlates of Familiarity in Music Listening: A Systematic Review and a Neuroimaging Meta-Analysis. Front. Neurosci. 2018, 12, 686. [Google Scholar] [CrossRef] [Green Version]
- Wolf, U.; Toronov, V.; Choi, J.H.; Gupta, R.; Michalos, A.; Gratton, E.; Wolf, M. Correlation of functional and resting state connectivity of cerebral oxy-, deoxy-, and total hemoglobin concentration changes measured by near-infrared spectrophotometry. J. Biomed. Opt. 2011, 16, 087013. [Google Scholar] [CrossRef] [PubMed]
- Grill-Spector, K.; Henson, R.; Martin, A. Repetition and the brain: Neural models of stimulus-specific effects. Trends Cogn. Sci. 2006, 10, 14–23. [Google Scholar] [CrossRef]
- Lee, S.M.; Henson, R.N.; Lin, C.Y. Neural Correlates of Repetition Priming: A Coordinate-Based Meta-Analysis of fMRI Studies. Front. Hum. Neurosci. 2020, 14, 565114. [Google Scholar] [CrossRef]
- Kawakubo, Y.; Yanagi, M.; Tsujii, N.; Shirakawa, O. Repetition of verbal fluency task attenuates the hemodynamic activation in the left prefrontal cortex: Enhancing the clinical usefulness of near-infrared spectroscopy. PLoS ONE 2018, 13, e0193994. [Google Scholar] [CrossRef]
- Bernardi, L.; Porta, C.; Sleight, P. Cardiovascular, cerebrovascular, and respiratory changes induced by different types of music in musicians and non-musicians: The importance of silence. Heart 2006, 92, 445–452. [Google Scholar] [CrossRef] [Green Version]
- Malliani, A.; Pagani, M.; Lombardi, F.; Cerutti, S. Cardiovascular neural regulation explored in the frequency domain. Circulation 1991, 84, 482–492. [Google Scholar] [CrossRef] [Green Version]
- Brancucci, A.; Lugli, V.; Perrucci, M.G.; Del Gratta, C.; Tommasi, L. A frontal but not parietal neural correlate of auditory consciousness. Brain Struct. Funct. 2016, 221, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Carrière, M.; Cassol, H.; Aubinet, C.; Panda, R.; Thibaut, A.; Larroque, S.K.; Simon, J.; Martial, C.; Bahri, M.A.; Chatelle, C.; et al. Auditory localization should be considered as a sign of minimally conscious state based on multimodal findings. Brain Commun. 2020, 2, fcaa195. [Google Scholar] [CrossRef] [PubMed]
- Lancioni, G.E.; Singh, N.N.; O’Reilly, M.F.; Sigafoos, J.; Desideri, L. Music Stimulation for People with Disorders of Consciousness: A Scoping Review. Brain Sci. 2021, 11, 858. [Google Scholar] [CrossRef] [PubMed]

| O2Hb | ||||||||
|---|---|---|---|---|---|---|---|---|
| Left Hemisphere | Right Hemisphere | |||||||
| m1/b1 | b2/m1 | m2/b2 | b3/m2 | m1/b1 | b2/m1 | m2/b2 | b3/m2 | |
| Subj 1 | 0.458 | −0.556 | 1.172 | −0.776 | 0.297 | 0.299 | 0.248 | −0.352 |
| Subj 2 | 0.056 | −0.136 | 0.536 | 0.123 | 0.065 | −0.007 | −0.333 | 0.677 |
| Subj 3 | 0.498 | −0.495 | −0.300 | 0.155 | 0.445 | −0.046 | −0.119 | 0.136 |
| Subj 4 | 0.841 | −0.690 | −0.040 | −0.370 | −0.484 | 0.141 | 0.587 | −0.997 |
| Subj 5 | 1.194 | 0.665 | −0.800 | 4.012 | 0.942 | 0.501 | 0.006 | 0.107 |
| Subj 6 | 0.653 | −0.024 | 0.508 | −0.046 | 2.670 | −0.262 | −1.161 | −0.002 |
| Mean (SD) | 0.617 (0.384) | −0.206 (0.498) | 0.179 (0.701) | 0.516 (1.748) | 0.655 (1.09) | 0.104 (0.270) | −0.128 (0.597) | −0.072 (0.561) |
| two-sided Wilcoxon signed rank test | m1/b1 − 2/m1, p = 0.031 rs = 21 | b2/m1 − m2/b2, p = 0.313 rs = 5 | b3/m2 − m2/b2, p = 0.844 rs = 12 | m1/b1 − b2/m1, p = 0.438 rs = 15 | b2/m1 − m2/b2, p = 0.219 rs = 17 | b3/m2 − m2/b2, p = 0.844 rs = 9 | ||
| HHb | ||||||||
| Left Hemisphere | Right Hemisphere | |||||||
| m1/b1 | b2/m1 | m2/b2 | b3/m2 | m1/b1 | b2/m1 | m2/b2 | b3/m2 | |
| Subj 1 | 0.421 | −0.037 | 0.098 | −0.32588 | 0.305 | 0.172 | −0.085 | −0.241 |
| Subj 2 | 0.284 | 0.355 | 0.214 | 0.006273 | 0.110 | 0.050 | -0.556 | 0.666 |
| Subj 3 | 0.702 | 0.132 | −0.594 | 0.20135 | 0.876 | −0.442 | −0.334 | 0.180 |
| Subj 4 | −0.108 | −0.054 | 0.799 | −0.63941 | −0.015 | 0.079 | 0.267 | −0.290 |
| Subj 5 | 0.344 | 2.850 | −2.46 | 3.654578 | 0.645 | 1.581 | −1.431 | 0.695 |
| Subj 6 | −0.039 | 0.777 | −0.242 | −0.30515 | 1.581 | −0.054 | −0.864 | −0.351 |
| Mean (SD) | 0.267 (0.301) | 0.670 (1.110) | −0.363 (1.126) | 0.432 (1.605) | 0.583 (0.591) | 0.230 (0.695) | −0.500 (0.599) | −0.110 (0.480) |
| two-sided Wilcoxon signed rank test | m1/b1 − b2/m1, p = 0.562 rs = 7 | b2/m1 − m2/b2, p = 0.313 rs = 16 | b3/m2 − m2/b2, p = 1 rs = 11 | m1/b1 − b2/m1, p = 0.437 rs = 15 | b2/m1 − m2/b2, p = 0.156 rs = 18 | b3/m2 − m2/b2, p = 0.313 rs = 5 | ||
| LFO | ||||||||
|---|---|---|---|---|---|---|---|---|
| O2Hb | HHb | |||||||
| left | right | left | right | |||||
| Cohen’s d | Cliff’s delta | Cohen’s d | Cliff’s delta | Cohen’s d | Cliff’s delta | Cohen’s d | Cliff’s delta | |
| m1 vs. b1 | 1.0 | 0.6 | 0.4 | 0.4 | 0.6 | 0.2 | 0.6 | 0.4 |
| b2 vs. m1 | −0.3 | −0.3 | 0.3 | 0.0 | 0.4 | 0.4 | 0.2 | 0.2 |
| m2 vs. b2 | 0.2 | 0.2 | −0.1 | −0.1 | −0.2 | −0.2 | −0.5 | −0.4 |
| b3 vs. m2 | 0.2 | 0.3 | −0.2 | −0.2 | 0.2 | 0.2 | 0.1 | 0.1 |
| VLFO | ||||||||
| O2Hb | HHb | |||||||
| left | right | left | right | |||||
| Cohen’s d | Cliff’s delta | Cohen’s d | Cliff’s delta | Cohen’s d | Cliff’s delta | Cohen’s d | Cliff’s delta | |
| m1 vs. b1 | 0.3 | 0.2 | 0.2 | 0.0 | −0.2 | 0.0 | −0.2 | −0.1 |
| b2 vs. m1 | −0.1 | −0.1 | 0.3 | 0.2 | 0.3 | 0.2 | 0.5 | 0.3 |
| m2 vs. b2 | 0.2 | 0.2 | 0.0 | 0.0 | −0.1 | −0.3 | −0.4 | −0.2 |
| b3 vs. m2 | −0.1 | 0.0 | −0.3 | −0.3 | 0.3 | 0.4 | 0.0 | 0.0 |
| O2Hb | ||||||||
|---|---|---|---|---|---|---|---|---|
| Left Hemisphere | Right Hemisphere | |||||||
| m1/b1 | b2/m1 | m2/b2 | b3/m2 | m1/b1 | b2/m1 | m2/b2 | b3/m2 | |
| Subj 1 | 0.255 | −1.27 | 0.365 | −0.776 | −0.890 | −0.427 | 1.323 | −0.352 |
| Subj 2 | −0.557 | 0.627 | −0.287 | 0.123 | 0.171 | 1.189 | −1.054 | 0.677 |
| Subj 3 | 0.766 | −0.987 | −0.405 | 0.155 | −0.162 | 0.467 | -0.853 | 0.136 |
| Subj 4 | 1.283 | −0.755 | 3.411 | −0.370 | −0.305 | −0.360 | 2.516 | −0.997 |
| Subj 5 | −0.436 | 1.023 | −0.012 | 2.386 | 0.691 | −0.054 | −0.700 | −0.162 |
| Subj 6 | 0.518 | 0.867 | 0.106 | 0.241 | 1.94 | 0.751 | −1.34 | −0.571 |
| Mean (SD) | 0.304 (0.708) | −0.081 (1.029) | 0.529 (1.438) | 0.293 (1.100) | 0.240 (0.981) | 0.261 (0.648) | −0.017 (1.561) | −0.212 (0.579) |
| two-sided Wilcoxon signed rank test | m1/b1 − 2/m1, p = 0.437 rs = 15 | b2/m1 − m2/b2, p = 0.844 rs = 9 | b3/m2 − m2/b2, p = 1 rs = 10 | m1/b1 − b2/m1, p = 1 rs = 11 | b2/m1 − m2/b2, p = 0.844 rs = 12 | b3/m2 − m2/b2, p = 1 rs = 10 | ||
| HHb | ||||||||
| Left Hemisphere | Right Hemisphere | |||||||
| m1/b1 | b2/m1 | m2/b2 | b3/m2 | m1/b1 | b2/m1 | m2/b2 | b3/m2 | |
| Subj 1 | 0.283 | −0.377 | 0.251 | −0.326 | 0.616 | −0.521 | 0.300 | −0.241 |
| Subj 2 | −0.789 | 1.56 | −0.661 | 0.006 | −1.071 | 1.962 | −1.627 | 0.666 |
| Subj 3 | −0.247 | 0.648 | −0.685 | 0.201 | −1.223 | 0.359 | −1.296 | 0.180 |
| Subj 4 | −0.913 | 0.148 | 1.94 | -0.639 | −0.332 | 0.802 | 0.732 | −0.290 |
| Subj 5 | −0.866 | 2.225 | −2.445 | 4.106 | −0.579 | 0.917 | −0.861 | 0.695 |
| Subj 6 | 0.903 | −1.004 | −0.118 | −0.305 | 1.059 | 0.162 | −0.629 | −0.351 |
| Mean (SD) | −0.271 (0.738) | −0.533 (1.21) | −0.287 (1.429) | 0.507 (1.787) | −0.255 (0.916) | 0.613 (0.837) | −0.563 (0.915) | 0.110 (0.480) |
| two-sided Wilcoxon signed rank test | m1/b1 − b2/m1, p = 0.312 rs = 5 | b2/m1 − m2/b2, p = 0.562 rs = 14 | b3/m2 − m2/b2, p = 0.687 rs = 8 | m1/b1 − b2/m1, p = 0.218 rs = 4 | b2/m1 − m2/b2, p = 0.156 rs = 18 | b3/m2 − m2/b2, p = 0.312 rs = 5 | ||
| b1 vs. m1 | m1 vs. b2 | b1 vs. b2 | |
|---|---|---|---|
| Heart rate | rs = 8.5, p = 0.84375 | rs = 5, p = 0.3125 | rs = 1, p = 0.61 |
| SpO2 | rs = 9.5, p = 1 | rs = 1, p = 0.65 | rs = 5, p = 0.3125 |
| Respiratory rate | rs = 0.0, p = 0.04 | rs = 1.5, p = 0.1974 | rs =2, p = 0.5637 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bicciato, G.; Keller, E.; Wolf, M.; Brandi, G.; Schulthess, S.; Friedl, S.G.; Willms, J.F.; Narula, G. Increase in Low-Frequency Oscillations in fNIRS as Cerebral Response to Auditory Stimulation with Familiar Music. Brain Sci. 2022, 12, 42. https://doi.org/10.3390/brainsci12010042
Bicciato G, Keller E, Wolf M, Brandi G, Schulthess S, Friedl SG, Willms JF, Narula G. Increase in Low-Frequency Oscillations in fNIRS as Cerebral Response to Auditory Stimulation with Familiar Music. Brain Sciences. 2022; 12(1):42. https://doi.org/10.3390/brainsci12010042
Chicago/Turabian StyleBicciato, Giulio, Emanuela Keller, Martin Wolf, Giovanna Brandi, Sven Schulthess, Susanne Gabriele Friedl, Jan Folkard Willms, and Gagan Narula. 2022. "Increase in Low-Frequency Oscillations in fNIRS as Cerebral Response to Auditory Stimulation with Familiar Music" Brain Sciences 12, no. 1: 42. https://doi.org/10.3390/brainsci12010042
APA StyleBicciato, G., Keller, E., Wolf, M., Brandi, G., Schulthess, S., Friedl, S. G., Willms, J. F., & Narula, G. (2022). Increase in Low-Frequency Oscillations in fNIRS as Cerebral Response to Auditory Stimulation with Familiar Music. Brain Sciences, 12(1), 42. https://doi.org/10.3390/brainsci12010042






