Age-Related Trajectories of Brain Structure–Function Coupling in Female Roller Derby Athletes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
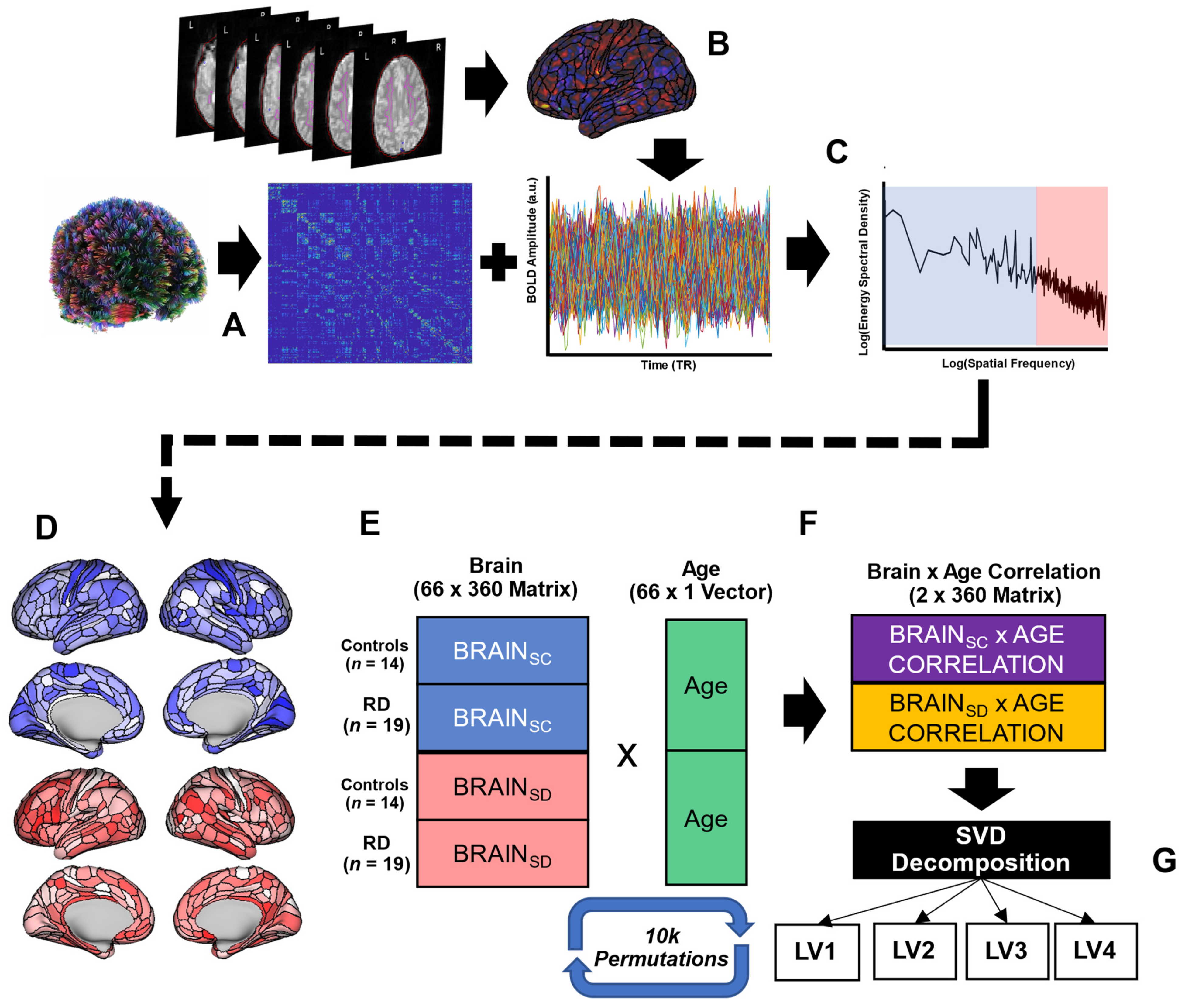
2.2. MRI Data Acquisition and Preprocessing
2.3. Structure–Function Coupling
2.4. Statistical Analysis
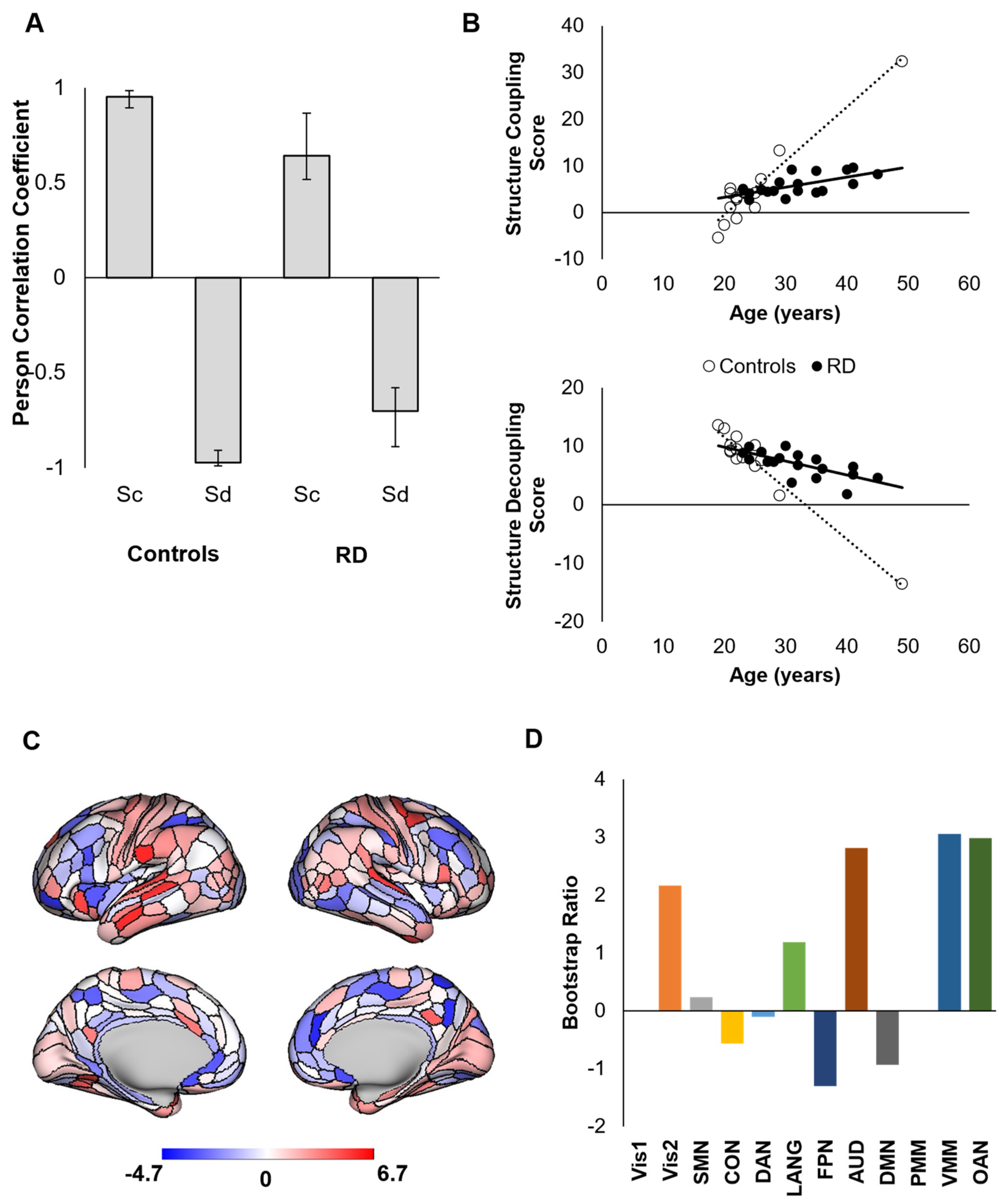
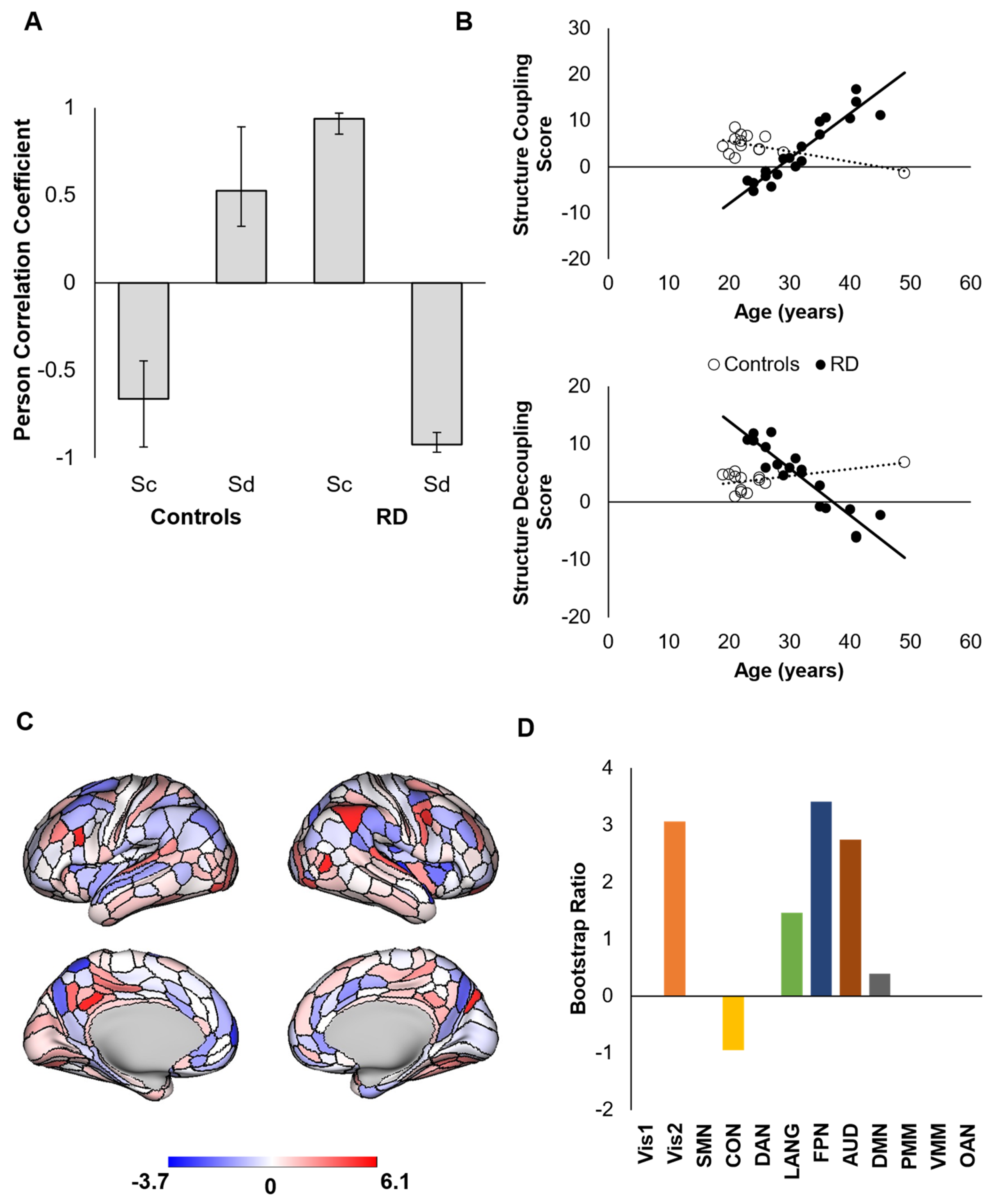
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bittner, N.; Jockwitz, C.; Mühleisen, T.W.; Hoffstaedter, F.; Eickhoff, S.B.; Moebus, S.; Bayen, U.J.; Cichon, S.; Zilles, K.; Amunts, K.; et al. Combining Lifestyle Risks to Disentangle Brain Structure and Functional Connectivity Differences in Older Adults. Nat. Commun. 2019, 10, 621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opel, N.; Martin, S.; Meinert, S.; Redlich, R.; Enneking, V.; Richter, M.; Goltermann, J.; Johnen, A.; Dannlowski, U.; Repple, J. White Matter Microstructure Mediates the Association between Physical Fitness and Cognition in Healthy, Young Adults. Sci. Rep. 2019, 9, 12885. [Google Scholar] [CrossRef] [Green Version]
- Ruotsalainen, I.; Gorbach, T.; Perkola, J.; Renvall, V.; Syväoja, H.J.; Tammelin, T.H.; Karvanen, J.; Parviainen, T. Physical Activity, Aerobic Fitness, and Brain White Matter: Their Role for Executive Functions in Adolescence. Dev. Cogn. Neurosci. 2020, 42, 100765. [Google Scholar] [CrossRef]
- Stillman, C.M.; Donofry, S.D.; Erickson, K.I. Exercise, Fitness and the Aging Brain: A Review of Functional Connectivity in Aging. Arch. Psychol. 2019, 3. [Google Scholar] [CrossRef]
- Harmon, K.G.; Drezner, J.; Gammons, M.; Guskiewicz, K.; Halstead, M.; Herring, S.; Kutcher, J.; Pana, A.; Putukian, M.; Roberts, W. American Medical Society for Sports Medicine Position Statement: Concussion in Sport. Clin. J. Sport Med. 2013, 23, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Huber, C.M.; Patton, D.A.; McDonald, C.C.; Jain, D.; Simms, K.; Lallo, V.A.; Margulies, S.S.; Master, C.L.; Arbogast, K.B. Sport- and Gender-Based Differences in Head Impact Exposure and Mechanism in High School Sports. Orthop. J. Sports Med. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.E.; Urban, J.E.; Davenport, E.M.; Powers, A.K.; Whitlow, C.T.; Maldjian, J.A.; Stitzel, J.D. Brain Strain: Computational Model-Based Metrics for Head Impact Exposure and Injury Correlation. Ann. Biomed. Eng. 2021, 49, 1083–1096. [Google Scholar] [CrossRef]
- Guskiewicz, K.M.; Marshall, S.W.; Bailes, J.; McCrea, M.; Cantu, R.C.; Randolph, C.; Jordan, B.D. Association between Recurrent Concussion and Late-Life Cognitive Impairment in Retired Professional Football Players. Neurosurgery 2005, 57, 719–726. [Google Scholar] [CrossRef]
- Montenigro, P.H.; Alosco, M.L.; Martin, B.M.; Daneshvar, D.H.; Mez, J.; Chaisson, C.E.; Nowinski, C.J.; Au, R.; McKee, A.C.; Cantu, R.C.; et al. Cumulative Head Impact Exposure Predicts Later-Life Depression, Apathy, Executive Dysfunction, and Cognitive Impairment in Former High School and College Football Players. J. Neurotrauma 2017, 34, 328–340. [Google Scholar] [CrossRef]
- Alosco, M.L.; Kasimis, A.B.; Stamm, J.M.; Chua, A.S.; Baugh, C.M.; Daneshvar, D.H.; Robbins, C.A.; Mariani, M.; Hayden, J.; Conneely, S.; et al. Age of First Exposure to American Football and Long-Term Neuropsychiatric and Cognitive Outcomes. Transl. Psychiatry 2017, 7, e1236. [Google Scholar] [CrossRef] [Green Version]
- Tremblay, S.; De Beaumont, L.; Henry, L.C.; Boulanger, Y.; Evans, A.C.; Bourgouin, P.; Poirier, J.; Théoret, H.; Lassonde, M. Sports Concussions and Aging: A Neuroimaging Investigation. Cereb. Cortex 2013, 23, 1159–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackay, D.F.; Russell, E.R.; Stewart, K.; MacLean, J.A.; Pell, J.P.; Stewart, W. Neurodegenerative Disease Mortality among Former Professional Soccer Players. N. Engl. J. Med. 2019, 381, 1801–1808. [Google Scholar] [CrossRef]
- McKee, A.C.; Cairns, N.J.; Dickson, D.W.; Folkerth, R.D.; Dirk Keene, C.; Litvan, I.; Perl, D.P.; Stein, T.D.; Vonsattel, J.-P.; Stewart, W.; et al. The First NINDS/NIBIB Consensus Meeting to Define Neuropathological Criteria for the Diagnosis of Chronic Traumatic Encephalopathy. Acta Neuropathol. 2016, 131, 75–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitsis, E.M.; Riggio, S.; Kostakoglu, L.; Dickstein, D.L.; Machac, J.; Delman, B.; Goldstein, M.; Jennings, D.; D’Antonio, E.; Martin, J.; et al. Tauopathy PET and Amyloid PET in the Diagnosis of Chronic Traumatic Encephalopathies: Studies of a Retired NFL Player and of a Man with FTD and a Severe Head Injury. Transl. Psychiatry 2014, 4, e441. [Google Scholar] [CrossRef] [Green Version]
- Alosco, M.L.; Tripodis, Y.; Jarnagin, J.; Baugh, C.M.; Martin, B.; Chaisson, C.E.; Estochen, N.; Song, L.; Cantu, R.C.; Jeromin, A.; et al. Repetitive Head Impact Exposure and Later-Life Plasma Total Tau in Former National Football League Players. Alzheimers Dement. Diagn. Assess. Dis. Monit. 2017, 7, 33–40. [Google Scholar] [CrossRef]
- Monroe, D.C.; Blumenfeld, R.S.; Keator, D.B.; Solodkin, A.; Small, S.L. One Season of Head-to-Ball Impact Exposure Alters Functional Connectivity in a Central Autonomic Network. NeuroImage 2020, 223, 117306. [Google Scholar] [CrossRef] [PubMed]
- Monroe, D.C.; Cecchi, N.J.; Gerges, P.; Phreaner, J.; Hicks, J.W.; Small, S.L. A Dose Relationship Between Brain Functional Connectivity and Cumulative Head Impact Exposure in Collegiate Water Polo Players. Front. Neurol. 2020, 11, 218. [Google Scholar] [CrossRef] [Green Version]
- Brett, B.L.; Koch, K.M.; Muftuler, L.T.; Budde, M.; McCrea, M.A.; Meier, T.B. Association of Head Impact Exposure with White Matter Macrostructure and Microstructure Metrics. J. Neurotrauma 2021, 38, 474–484. [Google Scholar] [CrossRef]
- Holcomb, J.; Fisicaro, R.A.; Miller, L.E.; Yu, F.F.; Davenport, E.M.; Xi, Y.; Urban, J.E.; Wagner, B.C.; Powers, A.K.; Whitlow, C.T.; et al. Regional White Matter Diffusion Changes Associated with the Cumulative Tensile Strain and Strain Rate in Non-Concussed Youth Football Players. J. Neurotrauma 2021, 38, 2763–2771. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.D.; Varangis, E.M.L.; Champagne, A.A.; Giovanello, K.S.; Shi, F.; Kerr, Z.Y.; Smith, J.K.; Guskiewicz, K.M. Effects of Career Duration, Concussion History, and Playing Position on White Matter Microstructure and Functional Neural Recruitment in Former College and Professional Football Athletes. Radiology 2018, 286, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R. Frontotemporal Correlates of Impulsivity and Machine Learning in Retired Professional Athletes with a History of Multiple Concussions. Brain Struct. Funct. 2016, 221, 1911–1925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hart, J., Jr.; Kraut, M.A.; Womack, K.B.; Strain, J.; Didehbani, N.; Bartz, E.; Conover, H.; Mansinghani, S.; Lu, H.; Cullum, C.M. Neuroimaging of Cognitive Dysfunction and Depression in Aging Retired National Football League Players: A Cross-Sectional Study. JAMA Neurol. 2013, 70, 326–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koerte, I.K.; Mayinger, M.; Muehlmann, M.; Kaufmann, D.; Lin, A.P.; Steffinger, D.; Fisch, B.; Rauchmann, B.-S.; Immler, S.; Karch, S.; et al. Cortical Thinning in Former Professional Soccer Players. Brain Imaging Behav. 2016, 10, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-H.; Chen, T.-F.; Chiu, M.-J.; Yen, R.-F.; Shih, M.-C.; Lin, C.-H. Integrated 18F-T807 Tau PET, Structural MRI, and Plasma Tau in Tauopathy Neurodegenerative Disorders. Front. Aging Neurosci. 2021, 13, 133. [Google Scholar] [CrossRef]
- Anckaerts, C.; Blockx, I.; Summer, P.; Michael, J.; Hamaide, J.; Kreutzer, C.; Boutin, H.; Couillard-Després, S.; Verhoye, M.; Van der Linden, A. Early Functional Connectivity Deficits and Progressive Microstructural Alterations in the TgF344-AD Rat Model of Alzheimer’s Disease: A Longitudinal MRI Study. Neurobiol. Dis. 2019, 124, 93–107. [Google Scholar] [CrossRef] [Green Version]
- Baum, G.L.; Cui, Z.; Roalf, D.R.; Ciric, R.; Betzel, R.F.; Larsen, B.; Cieslak, M.; Cook, P.A.; Xia, C.H.; Moore, T.M.; et al. Development of Structure–Function Coupling in Human Brain Networks during Youth. Proc. Natl. Acad. Sci. USA 2020, 117, 771–778. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, J.; Ritter, P.; Shen, K.; Rothmeier, S.; Schirner, M.; McIntosh, A.R. Structural Architecture Supports Functional Organization in the Human Aging Brain at a Regionwise and Network Level. Hum. Brain Mapp. 2016, 37, 2645–2661. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, Z.; Gong, G.; Zhou, C.; He, Y. Understanding Structural-Functional Relationships in the Human Brain: A Large-Scale Network Perspective. Neuroscientist 2015, 21, 290–305. [Google Scholar] [CrossRef]
- Huntenburg, J.M.; Bazin, P.-L.; Margulies, D.S. Large-Scale Gradients in Human Cortical Organization. Trends Cogn. Sci. 2018, 22, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Hui, E.S.; Cao, P.; Mak, H.K.F. Relationship between Amyloid-Beta Deposition and the Coupling between Structural and Functional Brain Networks in Patients with Mild Cognitive Impairment and Alzheimer’s Disease. Brain Sci. 2021, 11, 1535. [Google Scholar] [CrossRef]
- Mesulam, M. From Sensation to Cognition. Brain 1998, 121, 1013–1052. [Google Scholar] [CrossRef]
- Gu, Z.; Jamison, K.W.; Sabuncu, M.R.; Kuceyeski, A. Heritability and Interindividual Variability of Regional Structure-Function Coupling. Nat. Commun. 2021, 12, 4894. [Google Scholar] [CrossRef]
- Phipps, H.; Mondello, S.; Wilson, A.; Dittmer, T.; Rohde, N.N.; Schroeder, P.J.; Nichols, J.; McGirt, C.; Hoffman, J.; Tanksley, K.; et al. Characteristics and Impact of U.S. Military Blast-Related Mild Traumatic Brain Injury: A Systematic Review. Front. Neurol. 2020, 11, 559318. [Google Scholar] [CrossRef]
- Danielsen, T.; Hauch, C.; Kelly, L.; White, C.L. Chronic Traumatic Encephalopathy (CTE)-Type Neuropathology in a Young Victim of Domestic Abuse. J. Neuropathol. Exp. Neurol. 2021, 80, 624–627. [Google Scholar] [CrossRef]
- NPR/Robert Wood Johnson Foundation/Harvard TH Chan School of Public Health. Sports and Health in America. 2015. Available online: https://media.npr.org/documents/2015/june/sportsandhealthpoll.pdf (accessed on 1 November 2021).
- Ritchie, S.J.; Cox, S.R.; Shen, X.; Lombardo, M.V.; Reus, L.M.; Alloza, C.; Harris, M.A.; Alderson, H.L.; Hunter, S.; Neilson, E.; et al. Sex Differences in the Adult Human Brain: Evidence from 5216 UK Biobank Participants. Cereb. Cortex 2018, 28, 2959–2975. [Google Scholar] [CrossRef]
- Scheinost, D.; Finn, E.S.; Tokoglu, F.; Shen, X.; Papademetris, X.; Hampson, M.; Constable, R.T. Sex Differences in Normal Age Trajectories of Functional Brain Networks. Hum. Brain Mapp. 2015, 36, 1524–1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarnutzer, A.A.; Straumann, D.; Brugger, P.; Feddermann-Demont, N. Persistent Effects of Playing Football and Associated (Subconcussive) Head Trauma on Brain Structure and Function: A Systematic Review of the Literature. Br. J. Sports Med. 2017, 51, 1592–1604. [Google Scholar] [CrossRef] [PubMed]
- Kearney, M.C. Tough Girls in a Rough Game. Fem. Media Stud. 2011, 11, 283–301. [Google Scholar] [CrossRef]
- Cathorall, M.L.; Peachey, A.A. Incidence and Predictors of Roller Derby Injuries among Female Roller Derby Athletes. Int. J. Inj. Contr. Saf. Promot. 2018, 25, 387–392. [Google Scholar] [CrossRef]
- Hoskins, A.; Hooker, R. Annals of Sports Medicine and Research Sudden Impact: Concussion in Female Roller Derby Athletes. Ann. Sports Med. Res. 2015, 2, 1041. [Google Scholar]
- Esteban, O.; Markiewicz, C.J.; Blair, R.W.; Moodie, C.A.; Isik, A.I.; Erramuzpe, A.; Kent, J.D.; Goncalves, M.; DuPre, E.; Snyder, M.; et al. FMRIPrep: A Robust Preprocessing Pipeline for Functional MRI. Nat. Methods 2019, 16, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Dale, A.M.; Fischl, B.; Sereno, M.I. Cortical Surface-Based Analysis—I. Segmentation and Surface Reconstruction. Neuroimage 1999, 9, 179–194. [Google Scholar] [CrossRef]
- Fonov, V.S.; Evans, A.C.; McKinstry, R.C.; Almli, C.R.; Collins, D.L. Unbiased Nonlinear Average Age-Appropriate Brain Templates from Birth to Adulthood. NeuroImage 2009, 47 (Suppl. S1), S102. [Google Scholar] [CrossRef]
- Avants, B.B.; Tustison, N.; Johnson, H. Advanced Normalization Tools (ANTS). Insight J. 2009, 2, 1–35. [Google Scholar]
- Greve, D.N.; Fischl, B. Accurate and Robust Brain Image Alignment Using Boundary-Based Registration. NeuroImage 2009, 48, 63–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Power, J.D.; Barnes, K.A.; Snyder, A.Z.; Schlaggar, B.L.; Petersen, S.E. Spurious but Systematic Correlations in Functional Connectivity MRI Networks Arise from Subject Motion. NeuroImage 2012, 59, 2142–2154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Power, J.D.; Mitra, A.; Laumann, T.O.; Snyder, A.Z.; Schlaggar, B.L.; Petersen, S.E. Methods to Detect, Characterize, and Remove Motion Artifact in Resting State FMRI. NeuroImage 2014, 84, 320–341. [Google Scholar] [CrossRef]
- Behzadi, Y.; Restom, K.; Liau, J.; Liu, T.T. A Component Based Noise Correction Method (CompCor) for BOLD and Perfusion Based FMRI. NeuroImage 2007, 37, 90–101. [Google Scholar] [CrossRef] [Green Version]
- Dickie, E.W.; Anticevic, A.; Smith, D.E.; Coalson, T.S.; Manogaran, M.; Calarco, N.; Viviano, J.D.; Glasser, M.F.; Van Essen, D.C.; Voineskos, A.N. Ciftify: A Framework for Surface-Based Analysis of Legacy MR Acquisitions. NeuroImage 2019, 197, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.L.; Spronk, M.; Kulkarni, K.; Repovš, G.; Anticevic, A.; Cole, M.W. Mapping the Human Brain’s Cortical-Subcortical Functional Network Organization. NeuroImage 2019, 185, 35–57. [Google Scholar] [CrossRef]
- Glasser, M.F.; Coalson, T.S.; Robinson, E.C.; Hacker, C.D.; Harwell, J.; Yacoub, E.; Ugurbil, K.; Andersson, J.; Beckmann, C.F.; Jenkinson, M.; et al. A Multi-Modal Parcellation of Human Cerebral Cortex. Nature 2016, 536, 171–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cieslak, M.; Cook, P.A.; He, X.; Yeh, F.-C.; Dhollander, T.; Adebimpe, A.; Aguirre, G.K.; Bassett, D.S.; Betzel, R.F.; Bourque, J.; et al. QSIPrep: An Integrative Platform for Preprocessing and Reconstructing Diffusion MRI. bioRxiv 2020. [Google Scholar] [CrossRef]
- Veraart, J.; Novikov, D.S.; Christiaens, D.; Ades-aron, B.; Sijbers, J.; Fieremans, E. Denoising of Diffusion MRI Using Random Matrix Theory. NeuroImage 2016, 142, 394–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellner, E.; Dhital, B.; Kiselev, V.G.; Reisert, M. Gibbs-Ringing Artifact Removal Based on Local Subvoxel-Shifts. Magn. Reson. Med. 2016, 76, 1574–1581. [Google Scholar] [CrossRef] [Green Version]
- Tustison, N.J.; Avants, B.B.; Cook, P.A.; Zheng, Y.; Egan, A.; Yushkevich, P.A.; Gee, J.C. N4ITK: Improved N3 Bias Correction. IEEE Trans. Med. Imaging 2010, 29, 1310–1320. [Google Scholar] [CrossRef] [Green Version]
- Merlet, S.L.; Deriche, R. Continuous Diffusion Signal, EAP and ODF Estimation via Compressive Sensing in Diffusion MRI. Med. Image Anal. 2013, 17, 556–572. [Google Scholar] [CrossRef]
- Huang, W.; Bolton, T.A.W.; Medaglia, J.D.; Bassett, D.S.; Ribeiro, A.; Ville, D.V.D. A Graph Signal Processing Perspective on Functional Brain Imaging. Proc. IEEE 2018, 106, 868–885. [Google Scholar] [CrossRef]
- Medaglia, J.D.; Huang, W.; Karuza, E.A.; Kelkar, A.; Thompson-Schill, S.L.; Ribeiro, A.; Bassett, D.S. Functional Alignment with Anatomical Networks Is Associated with Cognitive Flexibility. Nat. Hum. Behav. 2018, 2, 156–164. [Google Scholar] [CrossRef]
- Sandryhaila, A.; Moura, J.M.F. Discrete Signal Processing on Graphs. IEEE Trans. Signal Process. 2013, 61, 1644–1656. [Google Scholar] [CrossRef] [Green Version]
- Yeh, F.-C.; Tseng, W.-Y.I. NTU-90: A High Angular Resolution Brain Atlas Constructed by q-Space Diffeomorphic Reconstruction. NeuroImage 2011, 58, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Yeh, F.-C.; Liu, L.; Hitchens, T.K.; Wu, Y.L. Mapping Immune Cell Infiltration Using Restricted Diffusion MRI. Magn. Reson. Med. 2017, 77, 603–612. [Google Scholar] [CrossRef]
- Yeh, F.; Wedeen, V.J.; Tseng, W.I. Generalized Q-Sampling Imaging. IEEE Trans. Med. Imaging 2010, 29, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Yeh, F.-C.; Verstynen, T.D.; Wang, Y.; Fernández-Miranda, J.C.; Tseng, W.-Y.I. Deterministic Diffusion Fiber Tracking Improved by Quantitative Anisotropy. PLoS ONE 2013, 8, e80713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, F.-C.; Panesar, S.; Fernandes, D.; Meola, A.; Yoshino, M.; Fernandez-Miranda, J.C.; Vettel, J.M.; Verstynen, T. Population-Averaged Atlas of the Macroscale Human Structural Connectome and Its Network Topology. NeuroImage 2018, 178, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Yeh, F.-C. Shape Analysis of the Human Association Pathways. NeuroImage 2020, 223, 117329. [Google Scholar] [CrossRef]
- Preti, M.G.; Ville, D.V.D. Decoupling of Brain Function from Structure Reveals Regional Behavioral Specialization in Humans. Nat. Commun. 2019, 10, 1–7. [Google Scholar] [CrossRef]
- McIntosh, A.R.; Lobaugh, N.J. Partial Least Squares Analysis of Neuroimaging Data: Applications and Advances. NeuroImage 2004, 23, S250–S263. [Google Scholar] [CrossRef]
- Krishnan, A.; Williams, L.J.; McIntosh, A.R.; Abdi, H. Partial Least Squares (PLS) Methods for Neuroimaging: A Tutorial and Review. NeuroImage 2011, 56, 455–475. [Google Scholar] [CrossRef]
- Luo, N.; Sui, J.; Abrol, A.; Lin, D.; Chen, J.; Vergara, V.M.; Fu, Z.; Du, Y.; Damaraju, E.; Xu, Y.; et al. Age-Related Structural and Functional Variations in 5967 Individuals across the Adult Lifespan. Hum. Brain Mapp. 2020, 41, 1725–1737. [Google Scholar] [CrossRef] [Green Version]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Betzel, R.F.; Byrge, L.; He, Y.; Goñi, J.; Zuo, X.-N.; Sporns, O. Changes in Structural and Functional Connectivity among Resting-State Networks across the Human Lifespan. NeuroImage 2014, 102, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; van den Brink, R.L.; Pfeffer, T.; Voytek, B. Neuronal Timescales Are Functionally Dynamic and Shaped by Cortical Microarchitecture. eLife 2020, 9, e61277. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.W.; Dennis, N.A.; Daselaar, S.M.; Fleck, M.S.; Cabeza, R. Que PASA? The Posterior-Anterior Shift in Aging. Cereb. Cortex 2008, 18, 1201–1209. [Google Scholar] [CrossRef]
- Reuter-Lorenz, P.A.; Cappell, K.A. Neurocognitive Aging and the Compensation Hypothesis. Curr. Dir. Psychol. Sci. 2008, 17, 177–182. [Google Scholar] [CrossRef]
- Millar, P.R.; Petersen, S.E.; Ances, B.M.; Gordon, B.A.; Benzinger, T.L.S.; Morris, J.C.; Balota, D.A. Evaluating the Sensitivity of Resting-State BOLD Variability to Age and Cognition after Controlling for Motion and Cardiovascular Influences: A Network-Based Approach. Cereb. Cortex 2020, 30, 5686–5701. [Google Scholar] [CrossRef]
- Chan, M.Y.; Park, D.C.; Savalia, N.K.; Petersen, S.E.; Wig, G.S. Decreased Segregation of Brain Systems across the Healthy Adult Lifespan. Proc. Natl. Acad. Sci. USA 2014, 111, E4997–E5006. [Google Scholar] [CrossRef] [Green Version]
- Lindenberger, U.; Baltes, P.B. Sensory Functioning and Intelligence in Old Age: A Strong Connection. Psychol. Aging 1994, 9, 339–355. [Google Scholar] [CrossRef]
- Manza, P.; Wiers, C.E.; Shokri-Kojori, E.; Kroll, D.; Feldman, D.; Schwandt, M.; Wang, G.-J.; Tomasi, D.; Volkow, N.D. Brain Network Segregation and Glucose Energy Utilization: Relevance for Age-Related Differences in Cognitive Function. Cereb. Cortex 2020, 30, 5930–5942. [Google Scholar] [CrossRef]
- Kim, E.J.; Cho, S.S.; Jeong, Y.; Park, K.C.; Kang, S.J.; Kang, E.; Kim, S.E.; Lee, K.H.; Na, D.L. Glucose Metabolism in Early Onset versus Late Onset Alzheimer’s Disease: An SPM Analysis of 120 Patients. Brain 2005, 128, 1790–1801. [Google Scholar] [CrossRef] [Green Version]
- Silverman, D.H.S.; Small, G.W.; Chang, C.Y.; Lu, C.S.; de Aburto, M.A.K.; Chen, W.; Czernin, J.; Rapoport, S.I.; Pietrini, P.; Alexander, G.E.; et al. Positron Emission Tomography in Evaluation of DementiaRegional Brain Metabolism and Long-Term Outcome. JAMA 2001, 286, 2120–2127. [Google Scholar] [CrossRef] [Green Version]
- Peskind, E.R.; Petrie, E.C.; Cross, D.J.; Pagulayan, K.; McCraw, K.; Hoff, D.; Hart, K.; Yu, C.-E.; Raskind, M.A.; Cook, D.G.; et al. Cerebrocerebellar Hypometabolism Associated with Repetitive Blast Exposure Mild Traumatic Brain Injury in 12 Iraq War Veterans with Persistent Post-Concussive Symptoms. NeuroImage 2011, 54, S76–S82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Provenzano, F.A.; Jordan, B.; Tikofsky, R.S.; Saxena, C.; Van Heertum, R.L.; Ichise, M. F-18 FDG PET Imaging of Chronic Traumatic Brain Injury in Boxers: A Statistical Parametric Analysis. Nucl. Med. Commun. 2010, 31, 952–957. [Google Scholar] [CrossRef]
- Giza, C.C.; Hovda, D.A. The New Neurometabolic Cascade of Concussion. Neurosurgery 2014, 75, S24–S33. [Google Scholar] [CrossRef] [Green Version]
- Chaudhuri, R.; Knoblauch, K.; Gariel, M.-A.; Kennedy, H.; Wang, X.-J. A Large-Scale Circuit Mechanism for Hierarchical Dynamical Processing in the Primate Cortex. Neuron 2015, 88, 419–431. [Google Scholar] [CrossRef] [Green Version]
- Paquola, C.; Wael, R.V.D.; Wagstyl, K.; Bethlehem, R.A.I.; Hong, S.-J.; Seidlitz, J.; Bullmore, E.T.; Evans, A.C.; Misic, B.; Margulies, D.S.; et al. Microstructural and Functional Gradients Are Increasingly Dissociated in Transmodal Cortices. PLoS Biol. 2019, 17, e3000284. [Google Scholar] [CrossRef] [Green Version]
- Grydeland, H.; Walhovd, K.B.; Tamnes, C.K.; Westlye, L.T.; Fjell, A.M. Intracortical Myelin Links with Performance Variability across the Human Lifespan: Results from T1- and T2-Weighted MRI Myelin Mapping and Diffusion Tensor Imaging. J. Neurosci. 2013, 33, 18618–18630. [Google Scholar] [CrossRef] [Green Version]
- Asken, B.M.; Rabinovici, G.D. Professional Soccer and Dementia Risk—The Ugly Side of the Beautiful Game. JAMA Neurol. 2021, 78, 1049–1051. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Wang, X.; Gao, Y.; Li, T.; Zhang, H.; Hussain, W.; Xie, Y.; Wang, J.; Wang, B.; Xiang, J. Abnormal Anatomical Rich-Club Organization and Structural–Functional Coupling in Mild Cognitive Impairment and Alzheimer’s Disease. Front. Neurol. 2020, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Lin, Q.; Li, T.; Wang, X.; Yuan, H.; Yu, X.; He, Y.; Wang, H. Disrupted Structural and Functional Brain Networks in Alzheimer’s Disease. Neurobiol. Aging 2019, 75, 71–82. [Google Scholar] [CrossRef]
- Sihag, S.; Naze, S.; Taghdiri, F.; Tator, C.; Wennberg, R.; Mikulis, D.; Green, R.; Colella, B.; Tartaglia, M.C.; Kozloski, J.R. Multimodal Dynamic Brain Connectivity Analysis Based on Graph Signal Processing for Former Athletes With History of Multiple Concussions. IEEE Trans. Signal Inf. Process. Netw. 2020, 6, 284–299. [Google Scholar] [CrossRef]
| Roller Derby (n = 19) | Controls (n = 14) | |||
|---|---|---|---|---|
| Age (Years) | Mean FD (mm) | Age (Years) | Mean FD (mm) | Sport History |
| 23 | 0.191 | 19 | 0.069 | Track, Soccer, Volleyball |
| 24 | 0.059 | 20 | 0.054 | Volleyball, Track |
| 24 | 0.054 | 21 | 0.065 | Gymnastics |
| 26 | 0.146 | 21 | 0.062 | Badminton, Swimming, Tennis |
| 26 | 0.118 | 21 | 0.055 | Tennis, Taekwondo, Soccer |
| 27 | 0.159 | 22 | 0.045 | Soccer |
| 28 | 0.061 | 22 | 0.109 | Volleyball *, Tennis |
| 29 | 0.062 | 22 | 0.066 | Track, Volleyball * |
| 30 | 0.476 | 23 | 0.07 | Cheerleading, Volleyball, Track |
| 31 | 0.066 | 25 | 0.062 | Dance, Competitive Cheer |
| 32 | 0.095 | 25 | 0.138 | No |
| 32 | 0.062 | 26 | 0.047 | Basketball |
| 35 | 0.073 | 29 | 0.065 | Tennis, Lacrosse, Softball, Cheerleading |
| 35 | 0.072 | 49 | 0.069 | Soccer, Field Hockey, Basketball, Lacrosse, Softball |
| 36 | 0.163 | |||
| 40 | 0.137 | |||
| 41 | 0.076 | |||
| 41 | 0.077 | |||
| 45 | 0.064 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monroe, D.C.; DuBois, S.L.; Rhea, C.K.; Duffy, D.M. Age-Related Trajectories of Brain Structure–Function Coupling in Female Roller Derby Athletes. Brain Sci. 2022, 12, 22. https://doi.org/10.3390/brainsci12010022
Monroe DC, DuBois SL, Rhea CK, Duffy DM. Age-Related Trajectories of Brain Structure–Function Coupling in Female Roller Derby Athletes. Brain Sciences. 2022; 12(1):22. https://doi.org/10.3390/brainsci12010022
Chicago/Turabian StyleMonroe, Derek C., Samantha L. DuBois, Christopher K. Rhea, and Donna M. Duffy. 2022. "Age-Related Trajectories of Brain Structure–Function Coupling in Female Roller Derby Athletes" Brain Sciences 12, no. 1: 22. https://doi.org/10.3390/brainsci12010022
APA StyleMonroe, D. C., DuBois, S. L., Rhea, C. K., & Duffy, D. M. (2022). Age-Related Trajectories of Brain Structure–Function Coupling in Female Roller Derby Athletes. Brain Sciences, 12(1), 22. https://doi.org/10.3390/brainsci12010022






