Evaluation of Fixational Behavior throughout Life
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Examination
2.3. Fixational Behavior Assessment
2.4. Statistical Analysis
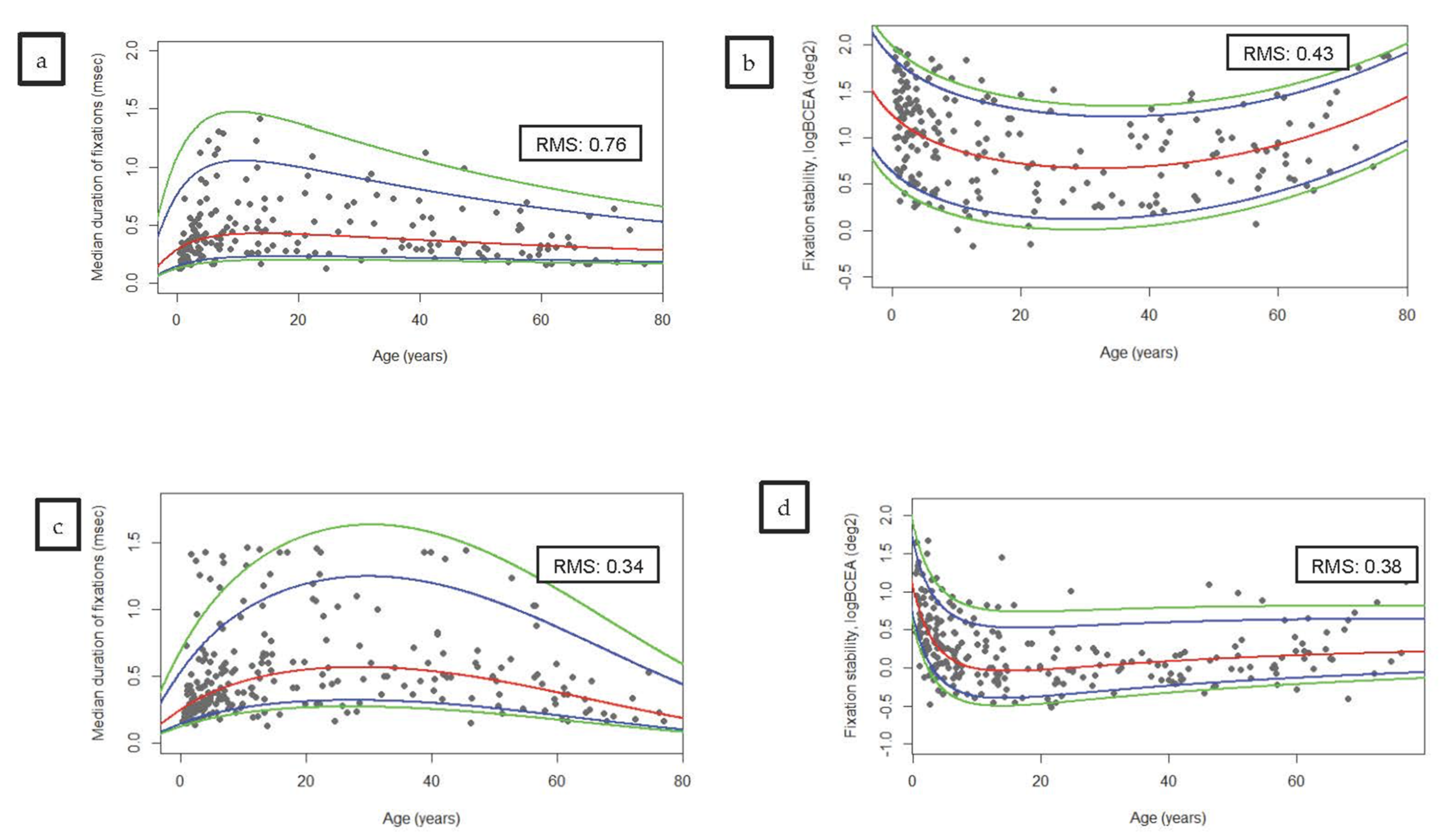
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rucci, M.; Casile, A. Decorrelation of neural activity during fixational instability: Possible implications for the refinement of V1 receptive fields. Vis. Neurosci. 2004, 21, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.K.; Morse, C.L.; Melia, M.; Sprunger, D.T.; Repka, M.X.; Lee, K.A.; Christiansen, S.P. Pediatric Eye Evaluations Preferred Practice Pattern®. Vision screening in the primary care and community setting; II. Comprehensive ophthalmic examination. Ophthalmology 2018, 125, P184–P227. [Google Scholar] [CrossRef] [PubMed]
- Committee on Practice and Ambulatory Medicine. S on OAA of COAA for PO and SAA of O. Eye examination in infants, children, and young adults by pediatricians. Pediatrics 2003, 111, 902–907. [Google Scholar] [CrossRef]
- Jung, R.; Kornhuber, H.H. Results of electronystagmography in man: The value of optokinetic, vestibular and spontaneous nystagmus for neurologic diagnosis and research. In The Oculomotor System; Bender, M.B., Ed.; Harper & Row: Manhattan, NY, USA, 1964. [Google Scholar]
- Treleaven, J.; Jull, G.; Grip, H. Head eye co-ordination and gaze stability in subjects with persistent whiplash associated disorders. Man. Ther. 2011, 16, 252–257. [Google Scholar] [CrossRef]
- Liu, H.; Bittencourt, M.G.; Sophie, R.; Sepah, Y.J.; Hanout, M.; Rentiya, Z.; Annam, R.; Scholl, H.P.N.; Nguyen, Q.D. Fixation Stability Measurement Using Two Types of Microperimetry Devices. Transl. Vis. Sci. Technol. 2015, 4, 3. [Google Scholar] [CrossRef]
- Vinuela-Navarro, V.; Erichsen, J.; Williams, C.; Woodhouse, J.M. Saccades and fixations in children with delayed reading skills. Ophthalmic Physiol. Opt. 2017, 37, 531–541. [Google Scholar] [CrossRef]
- Bedell, H.E.; Stevenson, S.B. Eye movement testing in clinical examination. Vis. Res. 2013, 90, 32–37. [Google Scholar] [CrossRef]
- McCamy, M.B.; Otero-Millan, J.; Leigh, R.J.; King, S.A.; Schneider, R.M.; Macknik, S.L.; Martinez-Conde, S. Simultaneous Recordings of Human Microsaccades and Drifts with a Contemporary Video Eye Tracker and the Search Coil Technique. PLoS ONE 2015, 10, e0128428. [Google Scholar] [CrossRef]
- Crossland, M.D.; Rubin, A.G.S. The Use of an Infrared Eyetracker to Measure Fixation Stability. Optom. Vis. Sci. 2002, 79, 735–739. [Google Scholar] [CrossRef]
- Birch, E.E.; Wang, J.; Felius, J.; Stager, D.R.; Hertle, R.W. Fixation control and eye alignment in children treated for dense congenital or developmental cataracts. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2012, 16, 156–160. [Google Scholar] [CrossRef]
- Abadi, R.V.; Forster, J.E.; Lloyd, I.C. Ocular motor outcomes after bilateral and unilateral infantile cataracts. Vis. Res. 2006, 46, 940–952. [Google Scholar] [CrossRef]
- Kelly, K.R.; Cheng-Patel, C.S.; Jost, R.M.; Wang, Y.-Z.; Birch, E.E. Fixation instability during binocular viewing in anisometropic and strabismic children. Exp. Eye Res. 2019, 183, 29–37. [Google Scholar] [CrossRef]
- Chung, S.T.; Kumar, G.; Li, R.W.; Levi, D.M. Characteristics of fixational eye movements in amblyopia: Limitations on fixation stability and acuity? Vis. Res. 2015, 114, 87–99. [Google Scholar] [CrossRef]
- Salati, R.; Borgatti, R.; Giammari, G.; Jacobson, L. Oculomotor dysfunction in cerebral visual impairment following perinatal hypoxia. Dev. Med. Child Neurol. 2002, 44, 542–550. [Google Scholar] [CrossRef]
- Razuk, M.; Barela, J.A.; Peyre, H.; Gerard, C.L.; Bucci, M.P. Eye movements and postural control in dyslexic children performing different visual tasks. PLoS ONE 2018, 13, e0198001. [Google Scholar] [CrossRef]
- Subramanian, V.; Jost, R.M.; Birch, E.E. A Quantitative Study of Fixation Stability in Amblyopia. Investig. Opthalmol. Vis. Sci. 2013, 54, 1998–2003. [Google Scholar] [CrossRef]
- Sivaprasad, S.; Pearce, E.; Chong, V. Quality of fixation in eyes with neovascular age-related macular degeneration treated with ranibizumab. Eye 2011, 25, 1612–1616. [Google Scholar] [CrossRef]
- Shakespeare, T.J.; Kaski, D.; Yong, K.X.X.; Paterson, R.W.; Slattery, C.F.; Ryan, N.S.; Schott, J.; Crutch, S. Abnormalities of fixation, saccade and pursuit in posterior cortical atrophy. Brain 2015, 138, 1976–1991. [Google Scholar] [CrossRef]
- Wan, Y.; Yang, J.; Ren, X.; Yu, Z.; Zhang, R.; Li, X. Evaluation of eye movements and visual performance in patients with cataract. Sci. Rep. 2020, 10, 9875. [Google Scholar] [CrossRef]
- Apaev, A.; Tarutta, E. Comparative assessment of the parameters of visual fixation in amblyopia of different origin. Vestnik Oftalmologii 2020, 136, 26–31. [Google Scholar] [CrossRef]
- González, E.G.; Wong, A.M.F.; Niechwiej-Szwedo, E.; Tarita-Nistor, L.; Steinbach, M.J. Eye Position Stability in Amblyopia and in Normal Binocular Vision. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5386–5394. [Google Scholar] [CrossRef]
- Abadi, R.V.; Gowen, E. Characteristics of saccadic intrusions. Vis. Res. 2004, 44, 2675–2765. [Google Scholar] [CrossRef]
- Sharpe, J.A.; Herishanu, Y.O.; White, O.B. Cerebral square wave jerks. Neurology 1982, 32, 57. [Google Scholar] [CrossRef]
- Sharpe, J.A.; Sylvester, T.O. Effect of aging on horizontal smooth pursuit. Investig. Ophthalmol. Vis. Sci. 1978, 17, 465–468. [Google Scholar]
- Chamberlain, W. Restriction in Upward Gaze with Advancing Age. Am. J. Ophthalmol. 1971, 71, 341–346. [Google Scholar] [CrossRef][Green Version]
- Kosnik, W.; Fikre, J.; Sekuler, R. Visual fixation stability in older adults. Investig. Ophthalmol. Vis. Sci. 1986, 27, 1720–1725. [Google Scholar]
- Aring, E.; Grönlund, M.A.; Hellström, A.; Ygge, J. Visual fixation development in children. Graefe’s Arch. Clin. Exp. Ophthalmol. 2007, 245, 1659–1665. [Google Scholar] [CrossRef]
- Ygge, J.; Aring, E.; Han, Y.; Bolzani, R.; Hellstrom, A. Fixation Stability in Normal Children. Ann. N. Y. Acad. Sci. 2005, 1039, 480–483. [Google Scholar] [CrossRef]
- Morales, M.U.; Saker, S.; Wilde, C.; Pellizzari, C.; Pallikaris, A.; Notaroberto, N.; Rubinstein, M.; Rui, C.; Limoli, P.; Smolek, M.K.; et al. Reference Clinical Database for Fixation Stability Metrics in Normal Subjects Measured with the MAIA Microperimeter. Transl. Vis. Sci. Technol. 2016, 5, 6. [Google Scholar] [CrossRef]
- Molina-Martín, A.; Piñero, D.P.; Pérez-Cambrodí, R.J. Normal Values for Microperimetry with the MAIA Microperimeter: Sensitivity and Fixation Analysis in Healthy Adults and Children. Eur. J. Ophthalmol. 2017, 27, 607–613. [Google Scholar] [CrossRef]
- Fragiotta, S.; Carnevale, C.; Cutini, A.; Rigoni, E.; Grenga, P.L.; Vingolo, E.M. Factors Influencing Fixation Stability Area: A Comparison of Two Methods of Recording. Optom. Vis. Sci. 2018, 95, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, H.M.P.; Crossland, M.D.; Rubin, G.S. Fixation Stability: A Comparison between the Nidek MP-1 and the Rodenstock Scanning Laser Ophthalmoscope in Persons with and without Diabetic Maculopathy. Investig. Opthalmol. Vis. Sci. 2010, 51, 4346–4350. [Google Scholar] [CrossRef] [PubMed]
- Galambos, A.; Turcsán, B.; Oláh, K. Visual Fixation Patterns During Viewing of Half-Face Stimuli in Adults: An Eye-Tracking Study. Front. Psychol. 2018, 9, 2478. [Google Scholar] [CrossRef] [PubMed]
- Ooms, K.; Krassanakis, V. Measuring the Spatial Noise of a Low-Cost Eye Tracker to Enhance Fixation Detection. J. Imaging 2018, 4, 96. [Google Scholar] [CrossRef]
- Available online: https://f.hubspotusercontent30.net/hubfs/1995689/is5/tobii_is5_evaluation-kit_data-sheet_v3_20190925.pdf (accessed on 27 November 2021).
- Available online: https://www.vive.com/us/product/vive-pro-eye/specs/ (accessed on 27 November 2021).
- Andersson, R.; Nyström, M.; Holmqvist, K. Sampling frequency and eye-tracking measures: How speed affects durations, latencies, and more. J. Eye Mov. Res. 2010, 3, 1–12. [Google Scholar] [CrossRef]
- Gibaldi, A.; Vanegas, M.; Bex, P.J.; Maiello, G. Evaluation of the Tobii EyeX Eye tracking controller and Matlab toolkit for research. Behav. Res. Methods 2017, 49, 923–946. [Google Scholar] [CrossRef]
- Bond, R.; Zhu, T.; Finlay, D.; Drew, B.; Kligfield, P.; Guldenring, D.; Breen, C.; Gallagher, A.G.; Daly, M.; Clifford, G. Assessing computerized eye tracking technology for gaining insight into expert interpretation of the 12-lead electrocardiogram: An objective quantitative approach. J. Electrocardiol. 2014, 47, 895–906. [Google Scholar] [CrossRef]
- Papageorgiou, K.; Smith, T.; Wu, R.; Johnson, M.; Kirkham, N.; Ronald, A. Individual Differences in Infant Fixation Duration Relate to Attention and Behavioral Control in Childhood. Psychol. Sci. 2014, 25, 1371–1379. [Google Scholar] [CrossRef]
- Chiang, W.-Y.; Lee, J.-J.; Chen, Y.-H.; Chen, C.-H.; Chen, Y.-J.; Wu, P.-C.; Fang, P.-C.; Kuo, H.-K. Fixation behavior in macular dystrophy assessed by microperimetry. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 1403–1410. [Google Scholar] [CrossRef]
- Miller, J.E. Aging changes in extraocular muscles. In Basic Mechanisms of Ocular Motility and Their Clinical Implications; Lennerstrand, G., Bach-y-Rita, P., Eds.; Pergamon Press: Oxford, UK, 1975; pp. 47–62. [Google Scholar]
- Collins, C.C. The human oculomotor control system. In Basic Mechanisms of Ocular Motility and Their Clinical Implications; Lennerstrand, G., Bach-y-Rita, P., Eds.; Pergamon Press: Oxford, UK, 1975; pp. 145–180. [Google Scholar]
- Seemiller, E.S.; Port, N.L.; Candy, T.R. The gaze stability of 4- to 10-week-old human infants. J. Vis. 2018, 18, 15. [Google Scholar] [CrossRef]
- Siepmann, K.; Reinhard, J.; Herzau, V. The locus of fixation in strabismic amblyopia changes with increasing effort of recognition as assessed by scanning laser ophthalmoscope. Acta Ophthalmol. Scand. 2006, 84, 124–129. [Google Scholar] [CrossRef]
- Castet, E.; Crossland, M. Quantifying Eye Stability During a Fixation Task: A Review of Definitions and Methods. Seeing Perceiving 2012, 25, 449–469. [Google Scholar] [CrossRef]
- Crossland, M.D.; Kabanarou, S.A.; Rubin, G.S. An unusual strategy for fixation in a patient with bilateral advanced age related macular disease. Br. J. Ophthalmol. 2004, 88, 1479–1480. [Google Scholar] [CrossRef][Green Version]
- Vingolo, E.M.; Salvatore, S.; Cavarretta, S. Low-Vision Rehabilitation by Means of MP-1 Biofeedback Examination in Patients with Different Macular Diseases: A Pilot Study. Appl. Psychophysiol. Biofeedback 2009, 34, 127–133. [Google Scholar] [CrossRef]
- Rohrschneider, K.; Becker, M.; Kruse, F.E.; Fendrich, T.; Volcker, H.E. Stability of fixation: Results of fundus-controlled examination using the scanning laser ophthalmoscope. Ger. J. Ophthalmol. 1995, 4, 197–202. [Google Scholar]
- Tarita-Nistor, L.; Gonzalez, E.G.; Mandelcorn, M.S.; Lillakas, L.; Steinbach, M.J. Fixation Stability, Fixation Location, and Visual Acuity after Successful Macular Hole Surgery. Investig. Opthalmol. Vis. Sci. 2009, 50, 84–89. [Google Scholar] [CrossRef]
- Crossland, M.D.; Culham, L.E.; Rubin, G.S. Fixation stability and reading speed in patients with newly developed macular disease. Ophthalmic Physiol. Opt. 2004, 24, 327–333. [Google Scholar] [CrossRef]
- van der Geest, J.; Frens, M. Recording eye movements with video-oculography and scleral search coils: A direct comparison of two methods. J. Neurosci. Methods 2002, 114, 185–195. [Google Scholar] [CrossRef]
- Andersson, R.; Larsson, L.; Holmqvist, K.; Stridh, M.; Nyström, M. One algorithm to rule them all? An evaluation and discussion of ten eye movement event-detection algorithms. Behav. Res. Methods 2016, 49, 616–637. [Google Scholar] [CrossRef]
- Crossland, M.; Sims, M.; Galbraith, R.; Rubin, G. Evaluation of a new quantitative technique to assess the number and extent of preferred retinal loci in macular disease. Vis. Res. 2004, 44, 1537–1546. [Google Scholar] [CrossRef]
- Shah, V.A.; Chalam, K.V. Values for macular perimetry using the MP-1 microperimeter in normal sub-jects. Ophthalmic Res. 2009, 41, 9–13. [Google Scholar] [CrossRef]

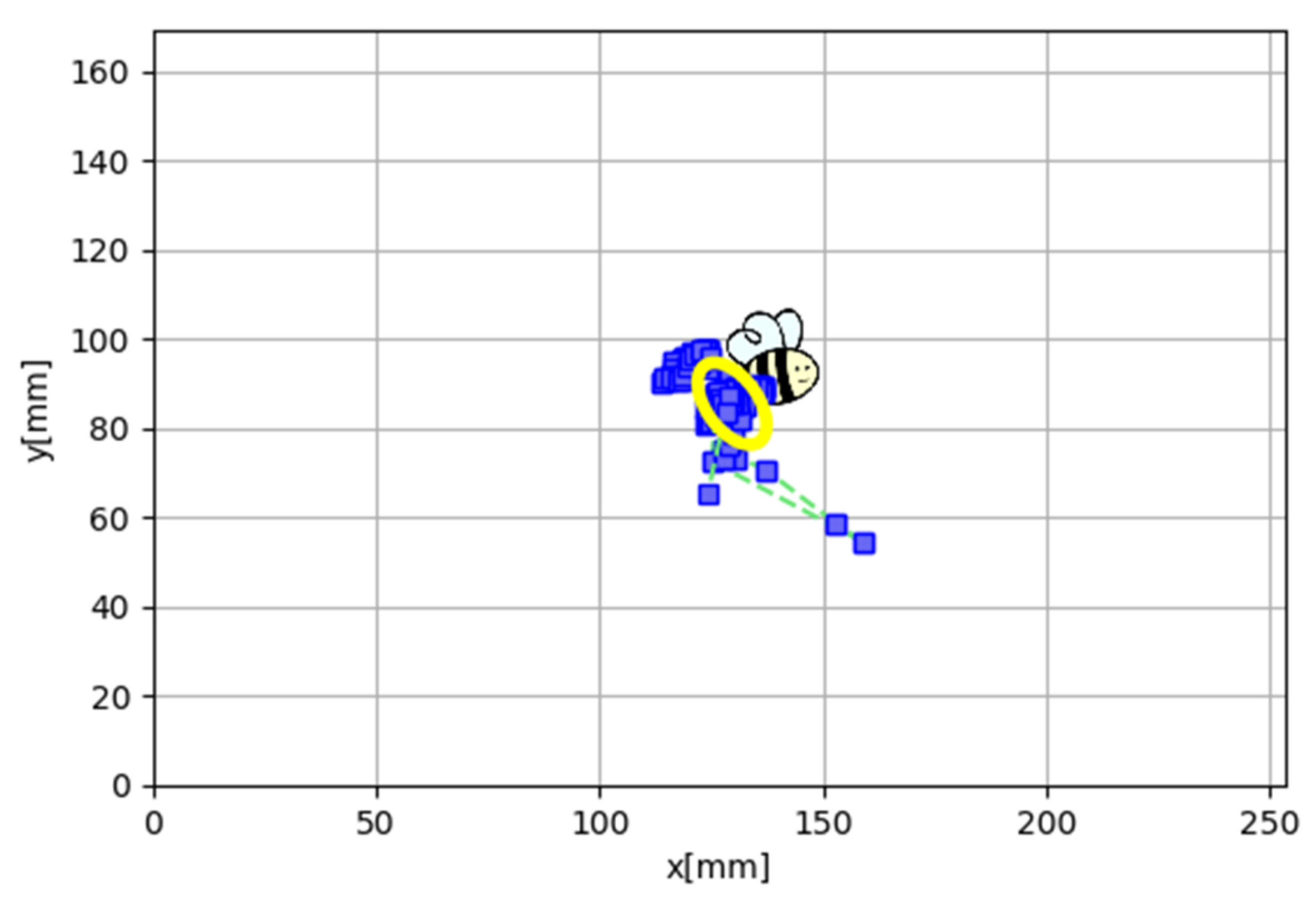
| 1 (0–2 Year) | 2 (2–5 Year) | 3 (5–10 Year) | 4 (10–20 Year) | 5 (20–30 Year) | 6 (30–40 Year) | 7 (40–50 Year) | 8 (50–60 Year) | 9 (60–70 Year) | 10 (>70 Year) | Global | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| VA (LogMar) | - | 0.03 (0.06) | −0.01 (0.04) | −0.03 (0.05) | −0.01 (0.03) | 0.0 (0.02) | 0.03 (0.07) | −0.00 (0.02) | 0.01 (0.02) | 0.03 (0.06) | −0.00 (0.05) |
| VA (cpd) | 4.85 (2.28) | 10.0 (2.83) | - | - | - | - | - | - | - | - | - |
| Sphere RE | 2.13 (0.53) | 1.03 (0.48) | 1.24 (0.99) | 0.16 (0.52) | −0.20 (0.51) | −0.15 (0.51) | −0.06 (0.49) | 0.33 (0.97) | 0.45 (0.70) | 0.69 (0.55) | 0.44 (0.87) |
| Sphere LE | 2.13 (0.53) | 1.14 (0.49) | 1.20 (0.83) | 0.30 (0.55) | −0.22 (0.56) | 0.19 (0.45) | 0.28 (0.63) | 0.20 (0.86) | 0.47 (0.59) | 1.0 (0.57) | 0.50 (0.82) |
| Cylinder RE | 0.75 (0.35) | 0.44 (0.39) | 0.40 (0.33) | 0.27 (0.33) | 0.15 (0.24) | 0.50 (0.18) | 0.52 (0.26) | 0.55 (0.20) | 0.54 (0.27) | 0.63 (0.25) | 0.42 (0.31) |
| Cylinder LE | 0.75 (0.35) | 0.44 (0.44) | 0.33 (0.29) | 0.30 (0.44) | 0.17 (0.25) | 0.35 (0.24) | 0.45 (0.26) | 0.55 (0.16) | 0.56 (0.29) | 1.00 (0.25) | 0.41 (0.34) |
| 1 (0–2 Year) | 2 (2–5 Year) | 3 (5–10 Year) | 4 (10–20 Year) | 5 (20–30 Year) | 6 (30–40 Year) | 7 (40–50 Year) | 8 (50–60 Year) | 9 (60–70 Year) | 10 (>70 Year) | p | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Long Fixational Tasks | |||||||||||
| Median duration of fixation, s | 0.25 (0.21–0.29) | 0.43 (0.36–0.49) | 0.58 (0.43–0.73) | 1.10 (0.51–1.69) | 0.81 (0.24–1.38) | 0.53 (0.36–0.63) | 0.60 (0.38–0.68) | 0.37 (0.28–0.45) | 0.29 (0.22–0.36) | 0.37 (−0.00–0.73) | 0.001 |
| Ellipse area (logBCEA) deg2 | 1.27 (1.14–1.41) | 1.04 (0.90–1.18) | 1.05 (0.85–1.25) | 0.77 (0.57–0.97) | 0.57 (0.33–0.81) | 0.56 (0.35–0.77) | 0.79 (0.56–1.01) | 0.83 (0.66–1.00) | 0.91 (0.70–1.13) | 1.42 (0.70–2.14) | <0.001 |
| Short Fixational Tasks | |||||||||||
| Median duration of fixation, s | 0.41 (0.32–0.50) | 0.59 (0.51–0.68) | 0.49 (0.40–0.58) | 0.67 (0.57–0.76) | 0.82 (0.67–0.96) | 0.84 (0.66–1.00) | 0.80 (0.65–0.96) | 0.70 (0.56–0.84) | 0.51 (0.32–0.70) | 0.42 (0.55–0.79) | <0.001 |
| Ellipse area (logBCEA) deg2 | 0.73 (0.56–0.89) | 0.38( 0.25–0.50) | 0.23 (0.11–0.35) | 0.09 (−0.04–0.23) | −0.04 (−0.18–0.10) | −0.01 (−0.09–0.08) | 0.01 (−0.15–0.18) | 0.18 (0.01–0.37) | 0.28 (0.11–0.45) | 0.44 (−0.21–1.09) | <0.001 |
| Target Size | Target Movement | Target Sound | p1 | p2 | p3 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Small | Large | No Vibration | Vibration | No Sound | With Sound | ||||
| Duration of fixations, s | 0.70 | 0.64 | 0.67 | 0.67 | 0.67 | 0.66 | <0.001 | 0.608 | 0.594 |
| Fixation stability (logBCEA) deg2 | 0.32 | 0.32 | 0.34 | 0.29 | 0.31 | 0.32 | 0.958 | 0.039 | 0.606 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altemir, I.; Alejandre, A.; Fanlo-Zarazaga, A.; Ortín, M.; Pérez, T.; Masiá, B.; Pueyo, V. Evaluation of Fixational Behavior throughout Life. Brain Sci. 2022, 12, 19. https://doi.org/10.3390/brainsci12010019
Altemir I, Alejandre A, Fanlo-Zarazaga A, Ortín M, Pérez T, Masiá B, Pueyo V. Evaluation of Fixational Behavior throughout Life. Brain Sciences. 2022; 12(1):19. https://doi.org/10.3390/brainsci12010019
Chicago/Turabian StyleAltemir, Irene, Adrian Alejandre, Alvaro Fanlo-Zarazaga, Marta Ortín, Teresa Pérez, Belén Masiá, and Victoria Pueyo. 2022. "Evaluation of Fixational Behavior throughout Life" Brain Sciences 12, no. 1: 19. https://doi.org/10.3390/brainsci12010019
APA StyleAltemir, I., Alejandre, A., Fanlo-Zarazaga, A., Ortín, M., Pérez, T., Masiá, B., & Pueyo, V. (2022). Evaluation of Fixational Behavior throughout Life. Brain Sciences, 12(1), 19. https://doi.org/10.3390/brainsci12010019






