Comparing Pathological Risk Factors for Dementia between Cognitively Normal Japanese and Americans
Abstract
:1. Introduction
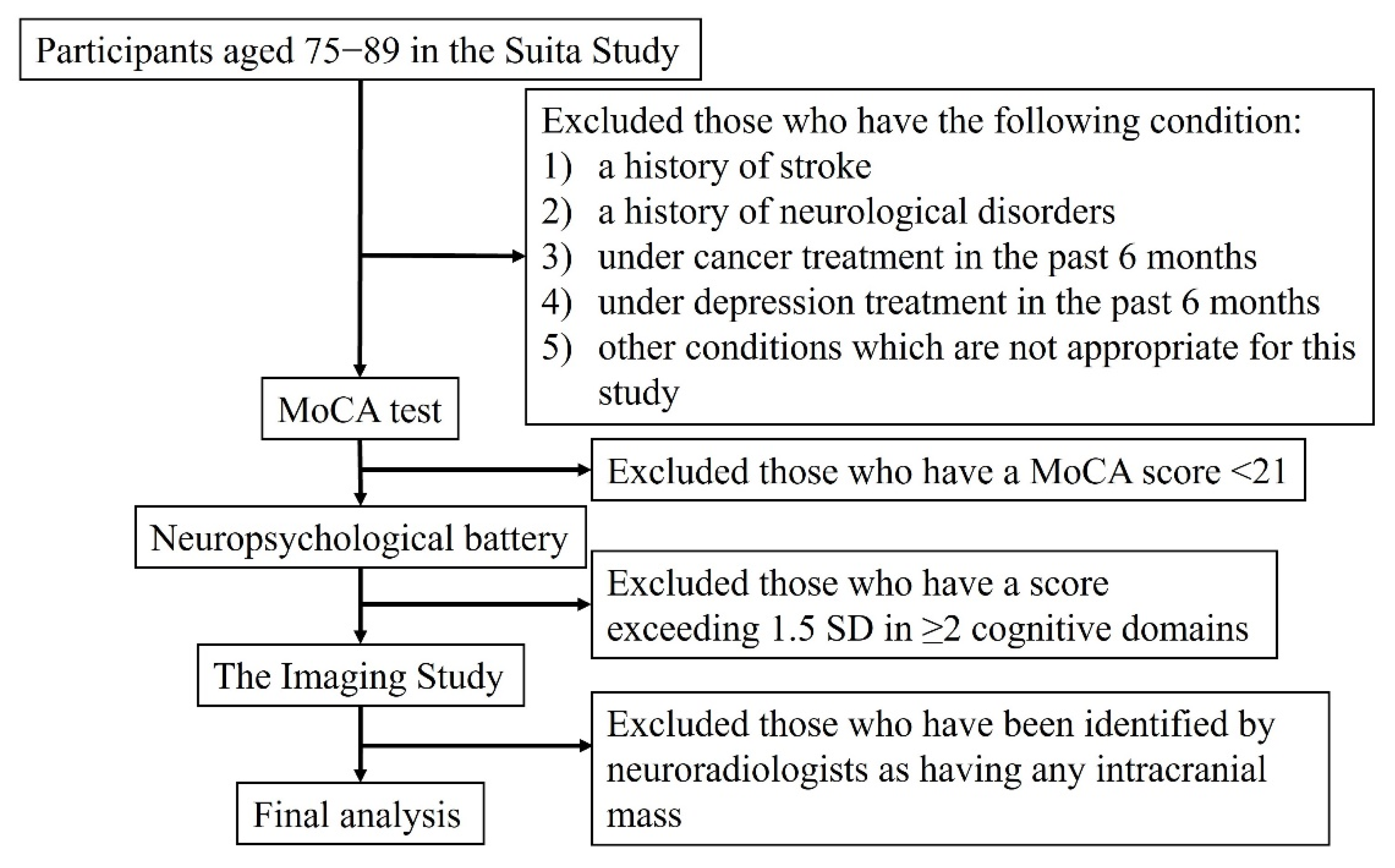
2. Materials and Methods
2.1. Study Population
2.2. Normal Cognition
2.3. Other Variables
2.4. Magnetic Resonance Imaging (MRI)
2.5. Positron-Emission Tomography Imaging
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Aβ | amyloid beta |
| ADAS-Cog | Alzheimer’s Disease Assessment Scale-Cognitive subscale |
| ADNI | Alzheimer’s Disease Neuroimaging Initiative |
| APOE | Apolipoprotein E |
| BMI | Body mass index |
| DHA | docosahexaenoic acid |
| FWHM | full-width-at-half-maximum |
| IQR | interquartile range |
| MMSE | Mini-Mental State Examination |
| MoCA | Montreal Cognitive Assessment |
| MRI | Magnetic resonance imaging |
| NCVC | National Cerebral and Cardiovascular Center |
| PET | Positron emission tomography |
| PiB | Pittsburgh Compound B |
| RCTs | randomized controlled trials |
| SD | standard deviation |
| SUVR | standardized uptake value ratio |
| WAIS-III | Wechsler Adult Intelligence Scale-III |
| WMLs | white matter lesions |
References
- Lopez, O.L.; Becker, J.T.; Chang, Y.; Klunk, W.E.; Mathis, C.; Price, J.; Aizenstein, H.J.; Snitz, B.; Cohen, A.D.; DeKosky, S.T.; et al. Amyloid deposition and brain structure as long-term predictors of MCI, dementia, and mortality. Neurology 2018, 90, e1920–e1928. [Google Scholar] [CrossRef]
- Lopez, O.L.; Klunk, W.E.; Mathis, C.; Coleman, R.L.; Price, J.; Becker, J.T.; Aizenstein, H.J.; Snitz, B.; Cohen, A.; Ikonomovic, M.; et al. Amyloid, neurodegeneration, and small vessel disease as predictors of dementia in the oldest-old. Neurology 2014, 83, 1804–1811. [Google Scholar] [CrossRef] [Green Version]
- Dodge, H.H.; Buracchio, T.J.; Fisher, G.G.; Kiyohara, Y.; Meguro, K.; Tanizaki, Y.; Kaye, J.A. Trends in the Prevalence of Dementia in Japan. Int. J. Alzheimers Dis. 2012, 2012, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Forouzanfar, M.H.; Liu, P.; Roth, G.A.; Ng, M.; Biryukov, S.; Marczak, L.; Alexander, L.; Estep, K.; Abate, K.H.; Akinyemiju, T.F.; et al. Global Burden of Hypertension and Systolic Blood Pressure of at Least 110 to 115 mm Hg, 1990–2015. JAMA 2017, 317, 165–182. [Google Scholar] [CrossRef] [Green Version]
- Danaei, G.; Finucane, M.M.; Lin, J.K.; Singh, G.M.; Paciorek, C.J.; Cowan, M.J.; Farzadfar, F.; Stevens, G.A.; Lim, S.S.; Riley, L.M.; et al. National, regional, and global trends in systolic blood pressure since 1980: Systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 5·4 million participants. Lancet 2011, 377, 568–577. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics—2018 Update: A Report From the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef]
- Vermeer, S.E.; Longstreth, W.T., Jr.; Koudstaal, P.J. Silent brain infarcts: A systematic review. Lancet Neurol. 2007, 6, 611–619. [Google Scholar] [CrossRef]
- Iwatsubo, T.; Iwata, A.; Suzuki, K.; Ihara, R.; Arai, H.; Ishii, K.; Senda, M.; Ito, K.; Ikeuchi, T.; Kuwano, R.; et al. Japanese and North American Alzheimer’s Disease Neuroimaging Initiative studies: Harmonization for international trials. Alzheimer’s Dement. J. Alzheimers Assoc. 2018, 14, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.D.; Mowrey, W.; Weissfeld, L.A.; Aizenstein, H.; McDade, E.; Mountz, J.M.; Nebes, R.D.; Saxton, J.A.; Snitz, B.; DeKosky, S.; et al. Classification of amyloid-positivity in controls: Comparison of visual read and quantitative approaches. NeuroImage 2013, 71, 207–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokubo, Y.; Okamura, T.; Yoshimasa, Y.; Miyamoto, Y.; Kawanishi, K.; Kotani, Y.; Okayama, A.; Tomoike, H. Impact of Metabolic Syndrome Components on the Incidence of Cardiovascular Disease in a General Urban Japanese Population: The Suita Study. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2008, 31, 2027–2035. [Google Scholar] [CrossRef]
- Jansen, W.J.; Ossenkoppele, R.; Knol, D.L.; Tijms, B.M.; Scheltens, P.; Verhey, F.R.; Visser, P.J.; Aalten, P.; Aarsland, D.; Alcolea, D.; et al. Prevalence of cerebral amyloid pathology in persons without dementia: A meta-analysis. JAMA 2015, 313, 1924–1938. [Google Scholar] [CrossRef] [PubMed]
- DeKosky, S.T.; Fitzpatrick, A.; Ives, D.G.; Saxton, J.; Williamson, J.; Lopez, O.L.; Burke, G.; Fried, L.; Kuller, L.H.; Robbins, J.; et al. The Ginkgo Evaluation of Memory (GEM) study: Design and baseline data of a randomized trial of Ginkgo biloba extract in prevention of dementia. Contemp. Clin. Trials 2006, 27, 238–253. [Google Scholar] [CrossRef]
- Dodge, H.H.; Kita, Y.; Takechi, H.; Hayakawa, T.; Ganguli, M.; Ueshima, H. Healthy Cognitive Aging and Leisure Activities among the Oldest Old in Japan: Takashima Study. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2008, 63, 1193–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, O.L.; Jagust, W.J.; DeKosky, S.T.; Becker, J.T.; Fitzpatrick, A.; Dulberg, C.; Breitner, J.; Lyketsos, C.; Jones, B.; Kawas, C.; et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: Part 1. Arch. Neurol. 2003, 60, 1385–1389. [Google Scholar] [CrossRef] [PubMed]
- Roalf, D.R.; Moberg, P.J.; Xie, S.X.; Wolk, D.A.; Moelter, S.T.; Arnold, S.E. Comparative accuracies of two common screening instruments for classification of Alzheimer’s disease, mild cognitive impairment, and healthy aging. Alzheimers Dement. J. Alzheimers Assoc. 2013, 9, 529–537. [Google Scholar] [CrossRef] [Green Version]
- Ohno, Y.; Kitahara, H.; Fujii, K.; Kohno, Y.; Ariyoshi, N.; Nishi, T.; Fujimoto, Y.; Kobayashi, Y. High residual platelet reactivity after switching from clopidogrel to low-dose prasugrel in Japanese patients with end-stage renal disease on hemodialysis. J. Cardiol. 2018, 73, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Rosano, C.; Butters, M.; Whyte, E.; Nable, M.; Crooks, R.; Meltzer, C.C.; Reynolds, C.F., 3rd; Aizenstein, H.J. A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Res. 2006, 148, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Tustison, N.J.; Cook, P.A.; Klein, A.; Song, G.; Das, S.R.; Duda, J.T.; Kandel, B.M.; van Strien, N.; Stone, J.R.; Gee, J.C.; et al. Large-scale evaluation of ANTs and FreeSurfer cortical thickness measurements. NeuroImage 2014, 99, 166–179. [Google Scholar] [CrossRef]
- Jack, C.R.; Wiste, H.J.; Weigand, S.D.; Knopman, D.S.; Mielke, M.M.; Vemuri, P.; Lowe, V.; Senjem, M.L.; Gunter, J.L.; Reyes, D.; et al. Different definitions of neurodegeneration produce similar amyloid/neurodegeneration biomarker group findings. Brain J. Neurol. 2015, 138 Pt 12, 3747–3759. [Google Scholar] [CrossRef] [Green Version]
- Mathis, C.A.; Kuller, L.H.; Klunk, W.E.; Snitz, B.E.; Price, J.C.; Weissfeld, L.A.; Rosario, B.L.; Bs, B.J.L.; Saxton, J.A.; Aizenstein, H.J.; et al. In vivo assessment of amyloid-β deposition in nondemented very elderly subjects. Ann. Neurol. 2013, 73, 751–761. [Google Scholar] [CrossRef]
- McNamee, R.L.; Yee, S.-H.; Price, J.C.; Klunk, W.E.; Rosario, B.; Weissfeld, L.; Ziolko, S.; Berginc, M.; Lopresti, B.; DeKosky, S.; et al. Consideration of Optimal Time Window for Pittsburgh Compound B PET Summed Uptake Measurements. J. Nuclear Med. Off. Publ. Soc. Nucl. Med. 2009, 50, 348–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischl, B.; Salat, D.H.; Busa, E.; Albert, M.; Dieterich, M.; Haselgrove, C.; van der Kouwe, A.; Killiany, R.; Kennedy, D.; Klaveness, S.; et al. Whole Brain Segmentation: Automated Labeling of Neuroanatomical Structures in the Human Brain. Neuron 2002, 33, 341–355. [Google Scholar] [CrossRef] [Green Version]
- Snitz, B.E.; Tudorascu, D.L.; Yu, Z.; Campbell, E.; Lopresti, B.J.; Laymon, C.M.; Minhas, D.S.; Nadkarni, N.K.; Aizenstein, H.J.; Klunk, W.E.; et al. Associations between NIH Toolbox Cognition Battery and in vivo brain amyloid and tau pathology in non-demented older adults. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2020, 12, e12018. [Google Scholar] [CrossRef]
- Lopresti, B.J.; Klunk, W.E.; Mathis, C.A.; Hoge, J.A.; Ziolko, S.K.; Lu, X.; Meltzer, C.C.; Schimmel, K.; Tsopelas, N.D.; DeKosky, S.T.; et al. Simplified quantification of Pittsburgh Compound B amyloid imaging PET studies: A comparative analysis. J. Nuclear Med. Off. Publ. Soc. Nucl. Med. 2005, 46, 1959–1972. [Google Scholar]
- Trzepacz, P.T.; Hochstetler, H.; Yu, P.; Castelluccio, P.; Witte, M.M.; Dell’Agnello, G.; Degenhardt, E.K.; Initiative, F.T.A.D.N. Relationship of Hippocampal Volume to Amyloid Burden across Diagnostic Stages of Alzheimer’s Disease. Dement. Geriatr. Cogn. Disord. 2015, 41, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Roseborough, A.; Ramirez, J.; Black, S.E.; Edwards, J.D. Associations between amyloid β and white matter hyperintensities: A systematic review. Alzheimers Dement. J. Alzheimers Assoc. 2017, 13, 1154–1167. [Google Scholar] [CrossRef]
- Legdeur, N.; Visser, P.J.; Woodworth, D.C.; Muller, M.; Fletcher, E.; Maillard, P.; Scheltens, P.; DeCarli, C.; Kawas, C.H.; Corrada, M. White Matter Hyperintensities and Hippocampal Atrophy in Relation to Cognition: The 90+ Study. J. Am. Geriatr. Soc. 2019, 67, 1827–1834. [Google Scholar] [CrossRef]
- Dickie, D.A.; Gardner, K.; Wagener, A.; Wyss, A.; Arba, F.; Wardlaw, J.M.; Dawson, J.; Collaborators, V.-P. Cortical thickness, white matter hyperintensities, and cognition after stroke. Int. J. Stroke Off. J. Int. Stroke Soc. 2020, 15, 46–54. [Google Scholar] [CrossRef] [Green Version]
- Becker, J.A.; Hedden, T.; Ba, J.C.; Bs, J.M.; Rentz, D.M.; Putcha, D.; Fischl, B.; Greve, D.N.; Marshall, G.A.; Salloway, S.; et al. Amyloid-β associated cortical thinning in clinically normal elderly. Ann. Neurol. 2010, 69, 1032–1042. [Google Scholar] [CrossRef]
- Gabriel, A.S.; Ninomiya, K.; Uneyama, H. The Role of the Japanese Traditional Diet in Healthy and Sustainable Dietary Patterns around the World. Nutrients 2018, 10, 173. [Google Scholar] [CrossRef] [Green Version]
- Resch, J.A.; Okabe, N.; Kimoto, K. Stroke: U.S. and Japan. Cerebral atherosclerosis. Geriatrics 1969, 24, 111–123. [Google Scholar]
- Sekikawa, A.; Ueshima, H.; Kadowaki, T.; El-Saed, A.; Okamura, T.; Takamiya, T.; Kashiwagi, A.; Edmundowicz, D.; Murata, K.; Sutton-Tyrrell, K.; et al. Less Subclinical Atherosclerosis in Japanese Men in Japan than in White Men in the United States in the Post-World War II Birth Cohort. Am. J. Epidemiol. 2007, 165, 617–624. [Google Scholar] [CrossRef] [Green Version]
- Sekikawa, A.; Miyamoto, Y.; Miura, K.; Nishimura, K.; Willcox, B.J.; Masaki, K.H.; Rodriguez, B.; Tracy, R.P.; Okamura, T.; Kuller, L.H. Continuous decline in mortality from coronary heart disease in Japan despite a continuous and marked rise in total cholesterol: Japanese experience after the Seven Countries Study. Int. J. Epidemiol. 2015, 44, 1614–1624. [Google Scholar] [CrossRef] [Green Version]
- Langbaum, J.B.; Chen, K.; Launer, L.J.; Fleisher, A.S.; Lee, W.; Liu, X.; Protas, H.D.; Reeder, S.A.; Bandy, D.; Yu, M.; et al. Blood pressure is associated with higher brain amyloid burden and lower glucose metabolism in healthy late middle-age persons. Neurobiol. Aging 2012, 33, 827.e11–827.e19. [Google Scholar] [CrossRef] [Green Version]
- Palmqvist, S.; Scholl, M.; Strandberg, O.; Mattsson, N.; Stomrud, E.; Zetterberg, H.; Blennow, K.; Landau, S.; Jagust, W.; Hansson, O. Earliest accumulation of beta-amyloid occurs within the default-mode network and concurrently affects brain connectivity. Nat. Commun. 2017, 8, 1214. [Google Scholar] [CrossRef] [Green Version]
- Cohen, A.D.; McDade, E.; Christian, B.; Price, J.; Mathis, C.; Klunk, W.; Handen, B.L. Early striatal amyloid deposition distinguishes Down syndrome and autosomal dominant Alzheimer’s disease from late-onset amyloid deposition. Alzheimer’s Dement. J. Alzheimers Assoc. 2018, 14, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.M.; Kuller, L.H.; Barinas-Mitchell, E.J.; McDade, E.M.; Klunk, W.E.; Cohen, A.D.; Mathis, C.A.; Dekosky, S.T.; Price, J.C.; Lopez, O.L. Arterial stiffness and beta-amyloid progression in nondemented elderly adults. JAMA Neurol. 2014, 71, 562–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, N.; Saito, E.; Kondo, N.; Inoue, M.; Ikeda, S.; Satoh, T.; Wada, K.; Stickley, A.; Katanoda, K.; Mizoue, T.; et al. What has made the population of Japan healthy? Lancet 2011, 378, 1094–1105. [Google Scholar] [CrossRef]
- Sekikawa, A.; Steingrimsdottir, L.; Ueshima, H.; Shin, C.; Curb, J.D.; Evans, R.W.; Hauksdottir, A.M.; Kadota, A.; Choo, J.; Masaki, K.; et al. Serum levels of marine-derived n-3 fatty acids in Icelanders, Japanese, Koreans, and Americans—A descriptive epidemiologic study. Prostaglandins Leukot. Essent. Fat. Acids 2012, 87, 11–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, M.A.; Nahin, R.L.; Messina, M.J.; Rader, J.I.; Thompson, L.U.; Badger, T.M.; Dwyer, J.T.; Kim, Y.S.; Pontzer, C.H.; Starke-Reed, P.E.; et al. Guidance from an NIH Workshop on Designing, Implementing, and Reporting Clinical Studies of Soy Interventions. J. Nutr. 2010, 140, 1192S–1204S. [Google Scholar] [CrossRef]
- Yassine, H.N.; Feng, Q.; Azizkhanian, I.; Rawat, V.; Castor, K.; Fonteh, A.N.; Harrington, M.; Zheng, L.; Reed, B.R.; DeCarli, C.; et al. Association of Serum Docosahexaenoic Acid with Cerebral Amyloidosis. JAMA Neurol. 2016, 73, 1208–1216. [Google Scholar] [CrossRef]
- Bazinet, R.P.; Layé, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014, 15, 771–785. [Google Scholar] [CrossRef]
- Ren, H.; Luo, C.; Feng, Y.; Yao, X.; Shi, Z.; Liang, F.; Kang, J.X.; Wan, J.B.; Pei, Z.; Su, H. Omega-3 polyunsaturated fatty acids promote amyloid-beta clearance from the brain through mediating the function of the glymphatic system. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2017, 31, 282–293. [Google Scholar]
- Cheng, P.-F.; Chen, J.-J.; Zhou, X.-Y.; Ren, Y.-F.; Huang, W.; Zhou, J.-J.; Xie, P. Do soy isoflavones improve cognitive function in postmenopausal women? A meta-analysis. Menopause 2015, 22, 198–206. [Google Scholar] [CrossRef]
- Cui, C.; Birru, R.; Snitz, B.E.; Ihara, M.; Lopresti, B.J.; Aizenstein, H.J.; Lopez, O.L.; Mathis, C.; Miyamoto, Y.; Kuller, L.H.; et al. Effects of Soy Isoflavones on Cognitive Function: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Alzheimers Dement. 2018, 14 (Suppl. 7), P1365–P1366. [Google Scholar] [CrossRef]
- Man, B.; Cui, C.; Zhang, X.; Sugiyama, D.; Barinas-Mitchell, E.; Sekikawa, A. The effect of soy isoflavones on arterial stiffness: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Nutr. 2020, 60, 603–614. [Google Scholar] [CrossRef]
- Hughes, T.M.; Kuller, L.H.; Barinas-Mitchell, E.J.; Mackey, R.H.; McDade, E.M.; Klunk, W.E.; Aizenstein, H.J.; Cohen, A.D.; Snitz, B.E.; Mathis, C.A.; et al. Pulse wave velocity is associated with beta-amyloid deposition in the brains of very elderly adults. Neurology 2013, 81, 1711–1718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, C.; Sekikawa, A.; Kuller, L.H.; Lopez, O.L.; Newman, A.B.; Kuipers, A.L.; Mackey, R.H. Aortic Stiffness is Associated with Increased Risk of Incident Dementia in Older Adults. J. Alzheimers Dis. 2018, 66, 297–306. [Google Scholar] [CrossRef]
- Strazzullo, P.; D’Elia, L.; Kandala, N.-B.; Cappuccio, F.P. Salt intake, stroke, and cardiovascular disease: Meta-analysis of prospective studies. BMJ 2009, 339, b4567. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, Y.; Zhao, N.; Caulfield, T.R.; Liu, C.-C.; Bu, G. Apolipoprotein E and Alzheimer disease: Pathobiology and targeting strategies. Nat. Rev. Neurol. 2019, 15, 501–518. [Google Scholar] [CrossRef]
- Villain, N.; Chetelat, G.; Grassiot, B.; Bourgeat, P.; Jones, G.; Ellis, K.A.; Ames, D.; Martins, R.N.; Eustache, F.; Salvado, O.; et al. Regional dynamics of amyloid-beta deposition in healthy elderly, mild cognitive impairment and Alzheimer’s disease: A voxelwise PiB-PET longitudinal study. Brain J. Neurol. 2012, 135 Pt 7, 2126–2139. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.S.; Konrath, S. Volunteering is prospectively associated with health care use among older adults. Soc. Sci. Med. 2016, 149, 122–129. [Google Scholar] [CrossRef] [Green Version]
- Roberts, R.O.; Aakre, J.A.; Kremers, W.K.; Vassilaki, M.; Knopman, D.S.; Mielke, M.; Alhurani, R.; Geda, Y.E.; Machulda, M.M.; Coloma, P.; et al. Prevalence and Outcomes of Amyloid Positivity among Persons without Dementia in a Longitudinal, Population-Based Setting. JAMA Neurol. 2018, 75, 970–979. [Google Scholar] [CrossRef] [PubMed]
| Japanese (n = 95) | Americans (n = 95) a | p-Value | |
|---|---|---|---|
| Age in years, mean (SD) | 81.7 (3.1) | 82.2 (3.8) | 0.30 |
| BMI, kg/m2, mean (SD) | 22.4 (3.0) | 26.5 (4.4) | <0.01 |
| Men, n (%) | 48 (50.5) | 48 (50.5) | 1.00 |
| Years of education, mean (SD) | 12.6 (2.3) | 14.4 (2.6) | <0.01 |
| MMSE score, median (IQR) b | 29 (28, 29) | 28 (27, 30) | 0.40 |
| APOE, n (%) | 1.00 | ||
| ε2/ε3 | 9 (9.5) | 9 (9.5) | |
| ε2/ε4 | 1 (1.1) | 1 (1.1) | |
| ε3/ε3 | 79 (83.2) | 79 (83.2) | |
| ε3/ε4 | 6 (6.3) | 6 (6.3) |
| Japanese (n = 95) | Americans (n = 95) a | p-Value | |
|---|---|---|---|
| Continuous biomarker | |||
| WMLs, median (IQR) b | 0.011 (0.006, 0.016) | 0.005 (0.002, 0.011) | <0.01 |
| Global PiB SUVR, median (IQR) b | 1.16 (1.11, 1.41) | 1.19 (1.11, 1.64) | 0.42 |
| Cortical thickness, mm, mean (SD) | 2.84 (0.15) | 2.79 (0.18) | 0.11 |
| Hippocampal volume, mm3, mean (SD) | 6680.03 (813.28) | 6586.31 (838.61) | 0.62 |
| Dichotomized biomarker c | |||
| High WMLs, n (%) | 27 (30.0) | 12 (17.1) | 0.06 |
| PiB positivity, n (%) | 28 (29.5) | 33 (34.7) | 0.44 |
| Abnormal cortical thickness, n (%) | 24 (25.3) | 23 (30.3) | 0.47 |
| Hippocampal atrophy, n (%) | 33 (34.7) | 8 (33.3) | 0.90 |
| Japanese (n = 95) | Americans (n = 95) a | p-Value b | |
|---|---|---|---|
| Regional PiB SUVR, median (IQR) | |||
| Anterior Ventral Striatum | 1.09 (1.02, 1.30) | 1.20 (1.10, 1.84) | <0.01 |
| Posterior Cingulate | 1.29 (1.20, 1.63) | 1.35 (1.25, 1.79) | 0.02 |
| Precuneus | 1.21 (1.14, 1.65) | 1.32 (1.17, 1.91) | <0.01 |
| Anterior Cingulate | 1.22 (1.14, 1.50) | 1.27 (1.15, 1.85) | 0.07 |
| Insula | 1.17 (1.11, 1.34) | 1.17 (1.10, 1.58) | 0.83 |
| Lateral Temporal | 1.15 (1.09, 1.36) | 1.16 (1.07, 1.56) | 0.64 |
| Orbitofrontal | 1.25 (1.17, 1.52) | 1.23 (1.11, 1.72) | 0.21 |
| Parietal | 1.14 (1.09, 1.40) | 1.21 (1.08, 1.66) | 0.37 |
| Superior Frontal | 1.14 (1.08, 1.46) | 1.18 (1.09, 1.62) | 0.24 |
| Japanese (n = 88) | Americans (n = 88) a | p-Value b | |
|---|---|---|---|
| Global PiB SUVR | 1.16 (1.11, 1.37) | 1.19 (1.10, 1.61) | 0.43 |
| Regional PiB SUVR, median (IQR) | |||
| Anterior Ventral Striatum | 1.08 (1.01, 1.23) | 1.19 (1.10, 1.64) | <0.01 |
| Posterior Cingulate | 1.28 (1.20, 1.47) | 1.34 (1.24, 1.75) | 0.02 |
| Precuneus | 1.19 (1.13, 1.49) | 1.28 (1.17, 1.82) | <0.01 |
| Anterior Cingulate | 1.22 (1.14, 1.46) | 1.27 (1.15, 1.78) | 0.05 |
| Insula | 1.16 (1.11, 1.31) | 1.17 (1.10, 1.49) | 0.72 |
| Lateral Temporal | 1.14 (1.09, 1.29) | 1.15 (1.07, 1.52) | 0.72 |
| Orbitofrontal | 1.24 (1.17, 1.50) | 1.21 (1.11, 1.67) | 0.22 |
| Parietal | 1.13 (1.08, 1.34) | 1.18 (1.08, 1.63) | 0.41 |
| Superior Frontal | 1.14 (1.08, 1.41) | 1.16 (1.09, 1.58) | 0.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, C.; Higashiyama, A.; Lopresti, B.J.; Ihara, M.; Aizenstein, H.J.; Watanabe, M.; Chang, Y.; Kakuta, C.; Yu, Z.; Mathis, C.A.; et al. Comparing Pathological Risk Factors for Dementia between Cognitively Normal Japanese and Americans. Brain Sci. 2021, 11, 1180. https://doi.org/10.3390/brainsci11091180
Cui C, Higashiyama A, Lopresti BJ, Ihara M, Aizenstein HJ, Watanabe M, Chang Y, Kakuta C, Yu Z, Mathis CA, et al. Comparing Pathological Risk Factors for Dementia between Cognitively Normal Japanese and Americans. Brain Sciences. 2021; 11(9):1180. https://doi.org/10.3390/brainsci11091180
Chicago/Turabian StyleCui, Chendi, Aya Higashiyama, Brian J. Lopresti, Masafumi Ihara, Howard J. Aizenstein, Makoto Watanabe, Yuefang Chang, Chikage Kakuta, Zheming Yu, Chester A. Mathis, and et al. 2021. "Comparing Pathological Risk Factors for Dementia between Cognitively Normal Japanese and Americans" Brain Sciences 11, no. 9: 1180. https://doi.org/10.3390/brainsci11091180
APA StyleCui, C., Higashiyama, A., Lopresti, B. J., Ihara, M., Aizenstein, H. J., Watanabe, M., Chang, Y., Kakuta, C., Yu, Z., Mathis, C. A., Kokubo, Y., Fukuda, T., Villemagne, V. L., Klunk, W. E., Lopez, O. L., Kuller, L. H., Miyamoto, Y., & Sekikawa, A. (2021). Comparing Pathological Risk Factors for Dementia between Cognitively Normal Japanese and Americans. Brain Sciences, 11(9), 1180. https://doi.org/10.3390/brainsci11091180







