Now or Later? Stress-Induced Increase and Decrease in Choice Impulsivity Are Both Associated with Elevated Affective and Endocrine Responses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. General Procedure
2.3. Measures
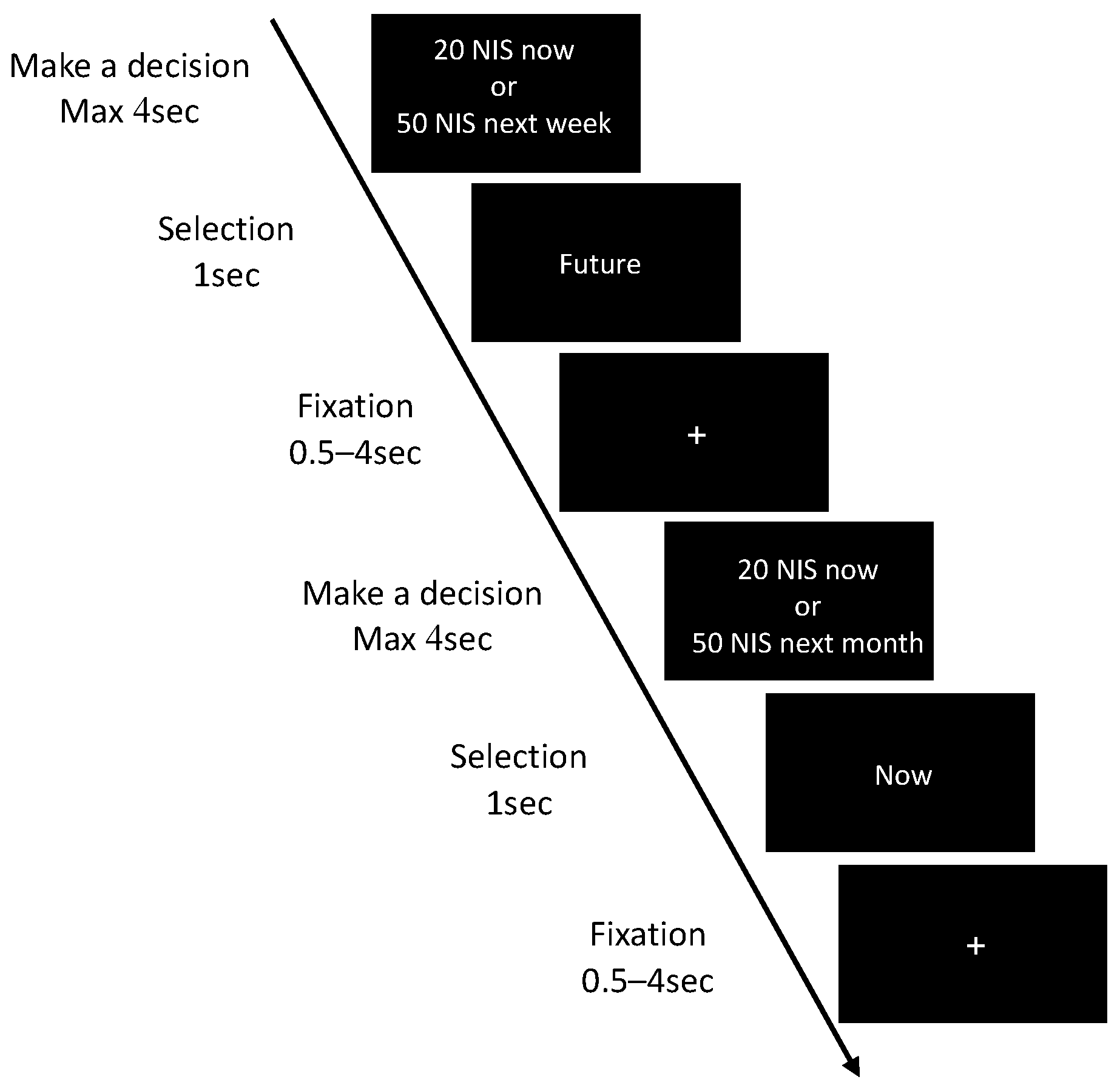
2.3.1. Delay Discounting (DD)
2.3.2. Maastricht Acute Stress Test (MAST)
2.3.3. Cortisol Saliva Samples
2.3.4. Affect and Mood
2.4. Statistical Approach
3. Results
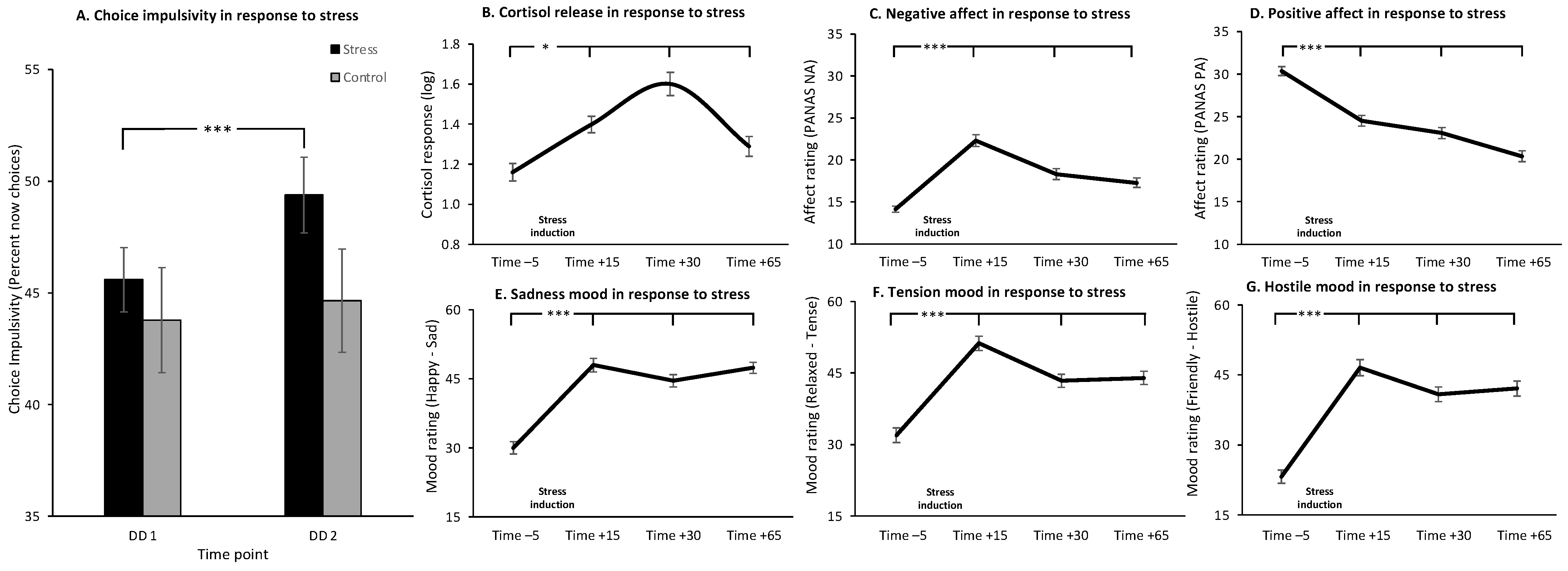
3.1. Main Effects of Stress
3.2. Individual Differences in the Effect of Stress on CI and Stress-Induced Changes in Cortisol, Affect and Mood
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bolger, N.; DeLongis, A.; Kessler, R.C.; Schilling, E.A. Effects of Daily Stress on Negative Mood. J. Personal. Soc. Psychol. 1989, 575, 808–818. [Google Scholar] [CrossRef]
- Holroyd, K.A.; Lazarus, R.S. Stress, coping and somatic adaptation. In Handbook of Stress: Theoretical and Clinical Aspects; The Free Press: New York, NY, USA, 1982; pp. 21–35. [Google Scholar]
- Keinan, G.; Friedland, N.; Benporath, Y. Decision-Making under Stress—Scanning of Alternatives under Physical Threat. Acta Psychol. 1987, 64, 219–228. [Google Scholar] [CrossRef]
- Childs, E.; de Wit, H. Effects of acute psychosocial stress on cigarette craving and smoking. Nicotine Tob. Res. 2010, 12, 449–453. [Google Scholar] [CrossRef] [Green Version]
- Magrys, S.A.; Olmstead, M.C. Acute Stress Increases Voluntary Consumption of Alcohol in Undergraduates. Alcohol Alcohol. 2015, 50, 213–218. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, M.; al’Absi, M. Predictors of Risk for Smoking Relapse in Men and Women: A Prospective Examination. Psychol. Addict. Behav. 2012, 26, 633–637. [Google Scholar] [CrossRef] [Green Version]
- Putman, P.; Antypa, N.; Crysovergi, P.; van der Does, W.A. Exogenous cortisol acutely influences motivated decision making in healthy young men. Psychopharmacology 2010, 208, 257–263. [Google Scholar] [CrossRef] [Green Version]
- Fineberg, N.A.; Chamberlain, S.R.; Goudriaan, A.E.; Stein, D.J.; Vanderschuren, L.J.; Gillan, C.M.; Shekar, S.; Gorwood, P.A.; Voon, V.; Morein-Zamir, S.; et al. New developments in human neurocognition: Clinical, genetic, and brain imaging correlates of impulsivity and compulsivity. CNS Spectr. 2014, 19, 69–89. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, K.R.; Mitchell, M.R.; Wing, V.C.; Balodis, I.M.; Bickel, W.K.; Fillmore, M.; Lane, S.D.; Lejuez, C.W.; Littlefield, A.K.; Luijten, M.; et al. Choice Impulsivity: Definitions, Measurement Issues, and Clinical Implications. Personal. Disord. Theory Res. Treat. 2015, 6, 182–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirby, K.N.; Petry, N.M.; Bickel, W.K. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J. Exp. Psychol. Gen. 1999, 128, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Berns, G.S.; Laibson, D.; Loewenstein, G. Intertemporal choice—Toward an integrative framework. Trends Cogn. Sci. 2007, 11, 482–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, J.; Buchel, C. The neural mechanisms of inter-temporal decision-making: Understanding variability. Trends Cogn. Sci. 2011, 15, 227–239. [Google Scholar] [CrossRef]
- Kirby, K.N.; Petry, N.M. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction 2004, 99, 461–471. [Google Scholar] [CrossRef]
- Audrain-McGovern, J.; Rodriguez, D.; Tercyak, K.P.; Epstein, L.H.; Goldman, P.; Wileyto, E. Applying a behavioral economic framework to understanding adolescent smoking. Psychol. Addict. Behav. 2004, 18, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Petry, N.M. Pathological gamblers, with and without substance use disorders, discount delayed rewards at high rates. J Abnorm. Psychol. 2001, 110, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Malesza, M. Stress and delay discounting: The mediating role of difficulties in emotion regulation. Personal. Individ. Differ. 2019, 144, 56–60. [Google Scholar] [CrossRef]
- Lu, Q.; Tao, F.; Hou, F.; Zhang, Z.; Sun, Y.; Xu, Y.; Xu, S.; Zhao, Y. Cortisol reactivity, delay discounting and percent body fat in Chinese urban young adolescents. Appetite 2014, 72, 13–20. [Google Scholar] [CrossRef]
- Riis-Vestergaard, M.I.; van Ast, V.; Cornelisse, S.; Joëls, M.; Haushofer, J. The effect of hydrocortisone administration on intertemporal choice. Psychoneuroendocrinology 2018, 88, 173–182. [Google Scholar] [CrossRef]
- Kimura, K.; Izawa, S.; Sugaya, N.; Ogawa, N.; Yamada, K.C.; Shirotsuki, K.; Mikami, I.; Hirata, K.; Nagano, Y.; Hasegawa, T. The biological effects of acute psychosocial stress on delay discounting. Psychoneuroendocrinology 2013, 38, 2300–2308. [Google Scholar] [CrossRef]
- Diller, J.W.; Patros, C.H.G.; Prentice, P.R. Temporal discounting and heart rate reactivity to stress. Behav. Process. 2011, 87, 306–309. [Google Scholar] [CrossRef]
- Lempert, K.M.; Porcelli, A.J.; Delgado, M.R.; Tricomi, E. Individual differences in delay discounting under acute stress: The role of trait perceived stress. Front. Psychol. 2012, 3, 251. [Google Scholar] [CrossRef] [Green Version]
- Krause-Utz, A.; Cackowski, S.; Daffner, S.; Sobanski, E.; Plichta, M.M.; Bohus, M.; Ende, G.; Schmahl, C. Delay discounting and response disinhibition under acute experimental stress in women with borderline personality disorder and adult attention deficit hyperactivity disorder. Psychol. Med. 2016, 46, 3137–3149. [Google Scholar] [CrossRef] [Green Version]
- Robinson, O.J.; Bond, R.L.; Roiser, J. The impact of threat of shock on the framing effect and temporal discounting: Executive functions unperturbed by acute stress? Front. Psychol. 2015, 6, 1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haushofer, J.; Cornelisse, S.; Seinstra, M.; Fehr, E.; Joëls, M.; Kalenscher, T. No Effects of Psychosocial Stress on Intertemporal Choice. PLoS ONE 2013, 8, e78597. [Google Scholar] [CrossRef] [Green Version]
- Rab, S.L.; Admon, R. Parsing inter- and intra-individual variability in key nervous system mechanisms of stress responsivity and across functional domains. Neurosci. Biobehav. Rev. 2021, 120, 550–564. [Google Scholar] [CrossRef] [PubMed]
- Carroll, D.; Ginty, A.T.; Whittaker, A.C.; Lovallo, W.R.; De Rooij, S.R. The behavioural, cognitive, and neural corollaries of blunted cardiovascular and cortisol reactions to acute psychological stress. Neurosci. Biobehav. Rev. 2017, 77, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Chida, Y.; Hamer, M. Chronic psychosocial factors and acute physiological responses to laboratory-induced stress in healthy populations: A quantitative review of 30 years of investigations. Psychol. Bull. 2008, 134, 829–885. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.L.; Alloy, L.B. Atypical reactivity of heart rate variability to stress and depression across development: Systematic review of the literature and directions for future research. Clin. Psychol. Rev. 2016, 50, 67–79. [Google Scholar] [CrossRef] [Green Version]
- Admon, R.; Treadway, M.T.; Valeri, L.; Mehta, M.; Douglas, S.; Pizzagalli, D.A. Distinct Trajectories of Cortisol Response to Prolonged Acute Stress Are Linked to Affective Responses and Hippocampal Gray Matter Volume in Healthy Females. J Neurosci. 2017, 37, 7994–8002. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, T.; Shinada, M.; Inukai, K.; Tanida, S.; Takahashi, C.; Mifune, N.; Takagishi, H.; Horita, Y.; Hashimoto, H.; Yokota, K.; et al. Stress hormones predict hyperbolic time-discount rates six months later in adults. Neuro. Endocrinol. Lett. 2010, 31, 616–621. [Google Scholar]
- Kudielka, B.M.; Kirschbaum, C. Sex differences in HPA axis responses to stress: A review. Biol. Psychol. 2005, 69, 113–132. [Google Scholar] [CrossRef]
- van den Bos, R.; Harteveld, M.; Stoop, H. Stress and decision-making in humans: Performance is related to cortisol reactivity, albeit differently in men and women. Psychoneuroendocrinology 2009, 34, 1449–1458. [Google Scholar] [CrossRef]
- Lighthall, N.R.; Mather, M.; Gorlick, M.A. Acute stress increases sex differences in risk seeking in the balloon analogue risk task. PLoS ONE 2009, 4, e6002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izawa, S.; Miki, K.; Liu, X.; Ogawa, N. The diurnal patterns of salivary interleukin-6 and C-reactive protein in healthy young adults. Brain Behav. Immun. 2013, 27, 38–41. [Google Scholar] [CrossRef]
- Spielberger, C.D.; Gorsuch, R.; Lushene, R.E.; Vagg, P.R.; Jacobs, G.A. (Eds.) Manual for the State-Trait Anxiety Inventory; Consulting Psychologists Press: Palo Alto, CA, USA, 1983. [Google Scholar]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Barratt, E.E. Anxiety and impulsiveness related to psychomotor efficiency. Percept. Mot. Ski. 1959, 9, 191–198. [Google Scholar] [CrossRef]
- Richards, J.B.; Zhang, L.; Mitchell, S.H.; De Wit, H. Delay or probability discounting in a model of impulsive behavior: Effect of alcohol. J. Exp. Anal. Behav. 1999, 71, 121–143. [Google Scholar] [CrossRef] [Green Version]
- Smeets, T.; Cornelisse, S.; Quaedflieg, C.W.; Meyer, T.; Jelicic, M.; Merckelbach, H. Introducing the Maastricht Acute Stress Test (MAST): A quick and non-invasive approach to elicit robust autonomic and glucocorticoid stress responses. Psychoneuroendocrinology 2012, 37, 1998–2008. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Pers. Soc. Psychol. 1988, 54, 1063–1070. [Google Scholar] [CrossRef]
- Stern, R.A.; Arruda, J.E.; Hooper, C.R.; Wolfner, G.D.; Morey, C.E. Visual analogue mood scales to measure internal mood state in neurologically impaired patients: Description and initial validity evidence. Aphasiology 1997, 11, 59–71. [Google Scholar] [CrossRef]
- Pruessner, J.C.; Kirschbaum, C.; Meinlschmid, G.; Hellhammer, D.H. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 2003, 28, 916–931. [Google Scholar] [CrossRef]
- Kirschbaum, C.; Kudielka, B.M.; Gaab, J.; Schommer, N.C.; Hellhammer, D.H. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom. Med. 1999, 61, 154–162. [Google Scholar] [CrossRef]
- Businelle, M.S.; McVay, M.A.; Kendzor, D.; Copeland, A. A comparison of delay discounting among smokers, substance abusers, and non-dependent controls. Drug Alcohol. Depend. 2010, 112, 247–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirschbaum, C.; Wust, S.; Strasburger, C.J. ‘Normal’ cigarette smoking increases free cortisol in habitual smokers. Life Sci. 1992, 50, 435–442. [Google Scholar] [CrossRef]
- Shilton, A.L.; Laycock, R.; Crewther, S.G. The Maastricht Acute Stress Test (MAST): Physiological and Subjective Responses in Anticipation, and Post-stress. Front. Psychol. 2017, 8, 567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitton, A.E.; Van’t Veer, A.; Kakani, P.; Dillon, D.G.; Ironside, M.L.; Haile, A.; Crowley, D.J.; Pizzagalli, D.A. Acute stress impairs frontocingulate activation during error monitoring in remitted depression. Psychoneuroendocrinology 2017, 75, 164–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- al’Absi, M.; Hatsukami, D.; Davis, G.L. Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacology 2005, 181, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D.; McClure, J. In-patient treatment of alcohol problems—predicting and preventing relapse. Alcohol Alcohol. 1992, 27, 449–456. [Google Scholar] [PubMed]
- Lempert, K.M.; Steinglass, J.E.; Pinto, A.; Kable, J.W.; Simpson, H.B. Can delay discounting deliver on the promise of RDoC? Psychol. Med. 2019, 49, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Sapolsky, R.M. Stress and the brain: Individual variability and the inverted-U. Nat. Neurosci. 2015, 18, 1344–1346. [Google Scholar] [CrossRef]
- McEwen, B.S. Protection and damage from acute and chronic stress: Allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann. N. Y. Acad. Sci. 2004, 1032, 1–7. [Google Scholar] [CrossRef]
- Kalisch, R.; Muller, M.B.; Tuscher, O. A conceptual framework for the neurobiological study of resilience. Behav. Brain Sci. 2015, 38, e92. [Google Scholar] [CrossRef]
- Andrade, L.F.; Alessi, S.M.; Petry, N.M. The effects of alcohol problems and smoking on delay discounting in individuals with gambling problems. J. Psychoact. Drugs 2013, 45, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Honkanen, P.; Olsen, S.O.; Verplanken, B.; Tuu, H.H. Reflective and impulsive influences on unhealthy snacking. The moderating effects of food related self-control. Appetite 2012, 58, 616–622. [Google Scholar] [CrossRef]
- Horn, N.R.; Dolan, M.; Elliott, R.; Deakin, J.F.; Woodruff, P.W. Response inhibition and impulsivity: An fMRI study. Neuropsychologia 2003, 41, 1959–1966. [Google Scholar] [CrossRef]
- Juster, R.P.; McEwen, B.S.; Lupien, S.J. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav. Rev. 2010, 35, 2–16. [Google Scholar] [CrossRef]
- Reimers, S.; Maylor, E.A.; Stewart, N.; Chater, N. Associations between a one-shot delay discounting measure and age, income, education and real-world impulsive behavior. Personal. Individ. Differ. 2009, 47, 973–978. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, S.H.; Wilson, V.B.; Karalunas, S.L. Comparing hyperbolic, delay-amount sensitivity and present-bias models of delay discounting. Behav. Process. 2015, 114, 52–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baradell, J.G.; Klein, K. Relationship of Life Stress and Body Consciousness to Hypervigilant Decision-Making. J. Personal. Soc. Psychol. 1993, 64, 267–273. [Google Scholar] [CrossRef]
- Harty, S.C.; Whaley, J.E.; Halperin, J.M.; Ranaldi, R. Impulsive choice, as measured in a delay discounting paradigm, remains stable after chronic heroin administration. Pharmacol. Biochem. Behav. 2011, 98, 337–340. [Google Scholar] [CrossRef]
- Sinha, R. How does stress increase risk of drug abuse and relapse? Psychopharmacology 2001, 158, 343–359. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simon, L.; Jiryis, T.; Admon, R. Now or Later? Stress-Induced Increase and Decrease in Choice Impulsivity Are Both Associated with Elevated Affective and Endocrine Responses. Brain Sci. 2021, 11, 1148. https://doi.org/10.3390/brainsci11091148
Simon L, Jiryis T, Admon R. Now or Later? Stress-Induced Increase and Decrease in Choice Impulsivity Are Both Associated with Elevated Affective and Endocrine Responses. Brain Sciences. 2021; 11(9):1148. https://doi.org/10.3390/brainsci11091148
Chicago/Turabian StyleSimon, Lisa, Talita Jiryis, and Roee Admon. 2021. "Now or Later? Stress-Induced Increase and Decrease in Choice Impulsivity Are Both Associated with Elevated Affective and Endocrine Responses" Brain Sciences 11, no. 9: 1148. https://doi.org/10.3390/brainsci11091148
APA StyleSimon, L., Jiryis, T., & Admon, R. (2021). Now or Later? Stress-Induced Increase and Decrease in Choice Impulsivity Are Both Associated with Elevated Affective and Endocrine Responses. Brain Sciences, 11(9), 1148. https://doi.org/10.3390/brainsci11091148





