Against the Resilience of High-Grade Gliomas: Gene Therapies (Part II)
Abstract
1. Introduction
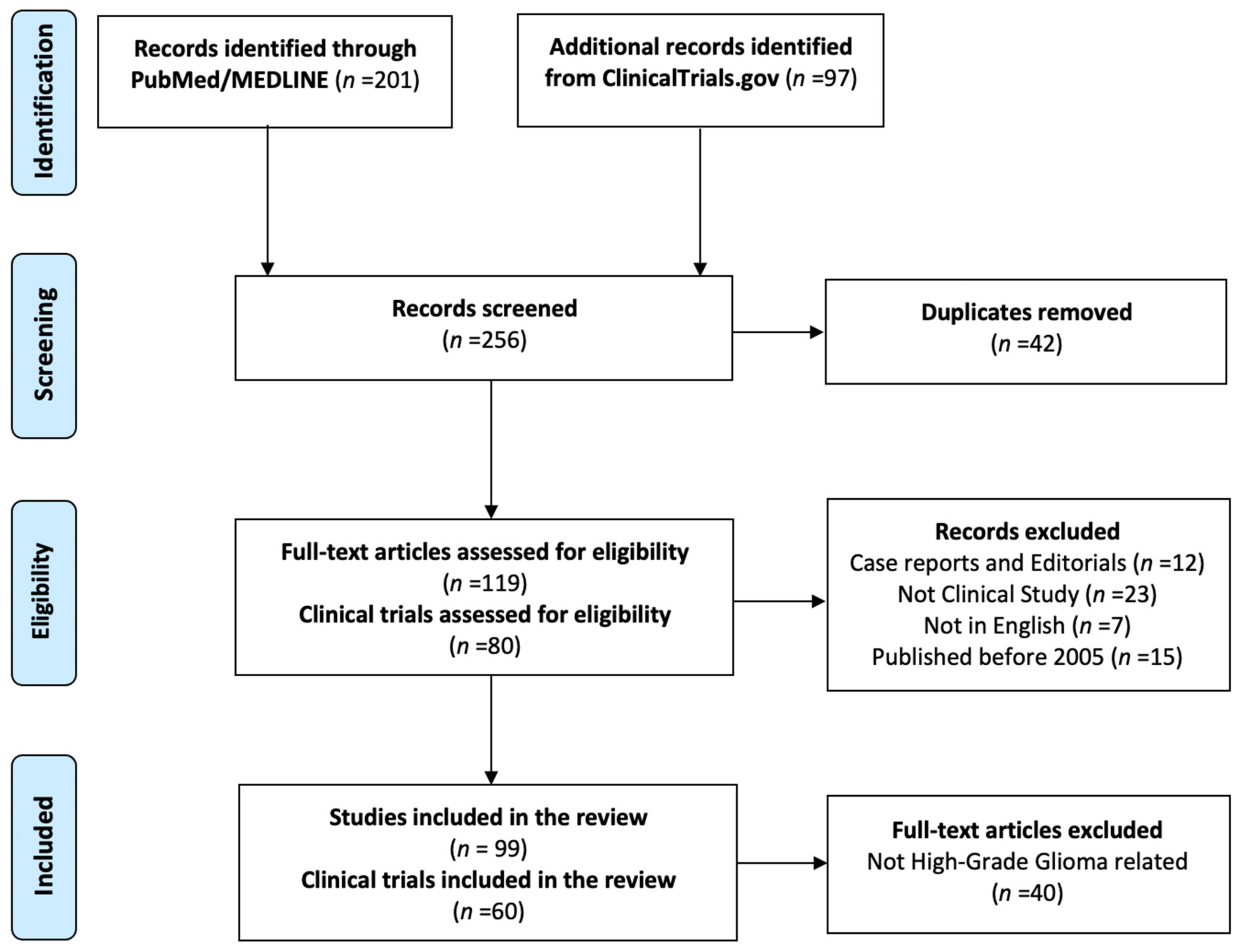
2. Methods
3. Results
3.1. Vectors
3.2. Classification of Gene Therapies for High-Grade Glioma
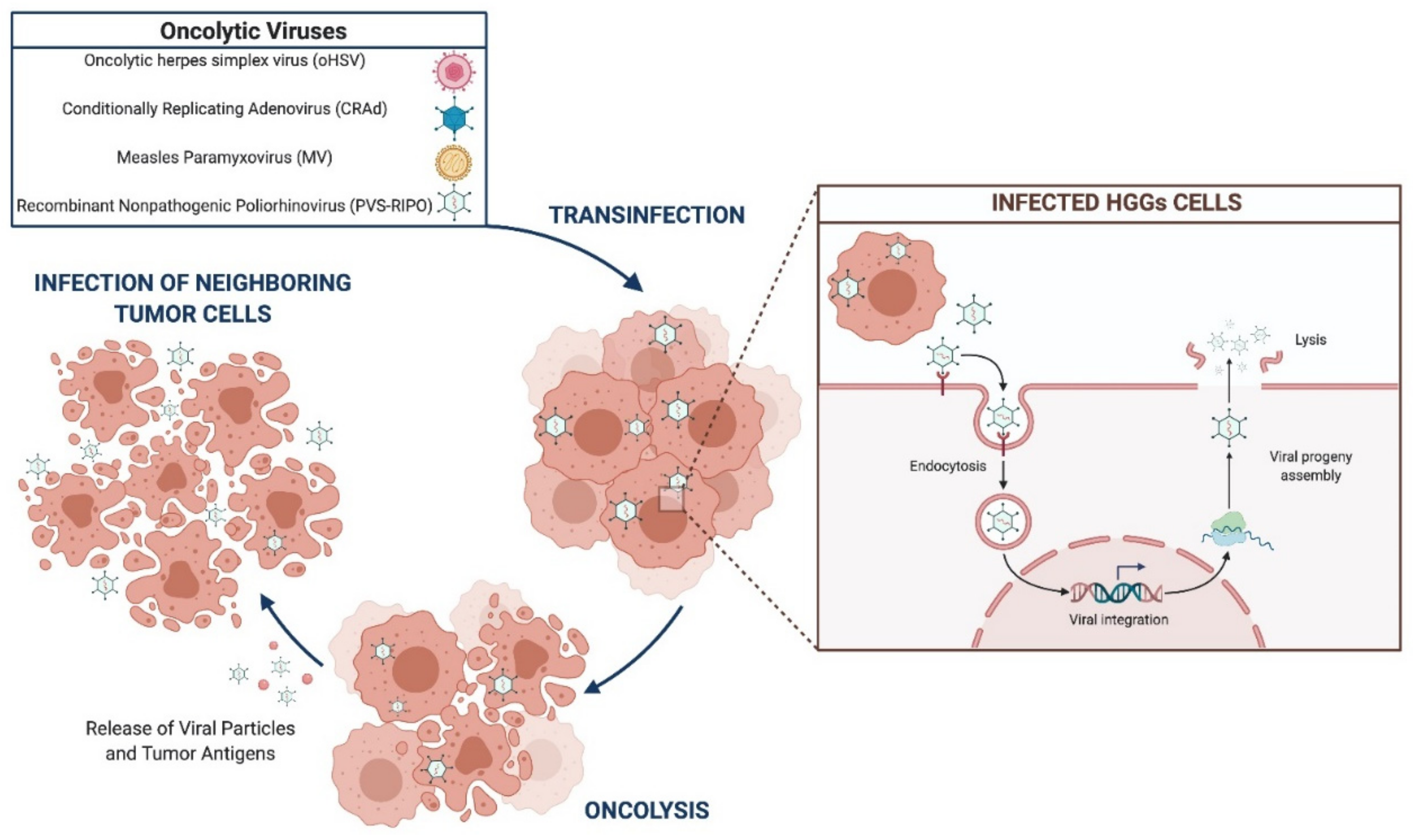
3.2.1. Oncolytic Virotherapy
oHSVs
CRAd
MV
PVS-RIPO
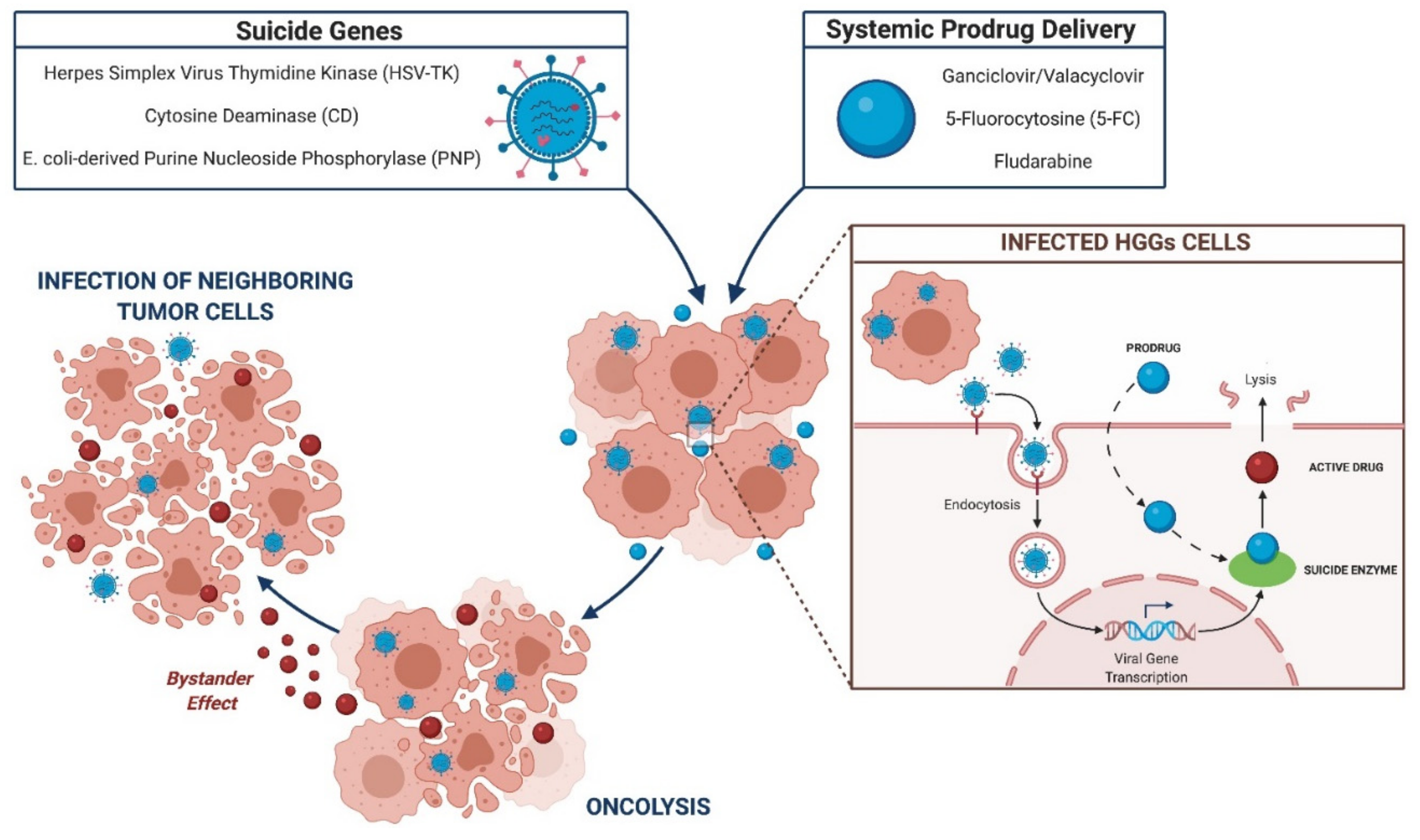
3.2.2. Suicide Gene Therapies
HSV-TK
CD
PNP
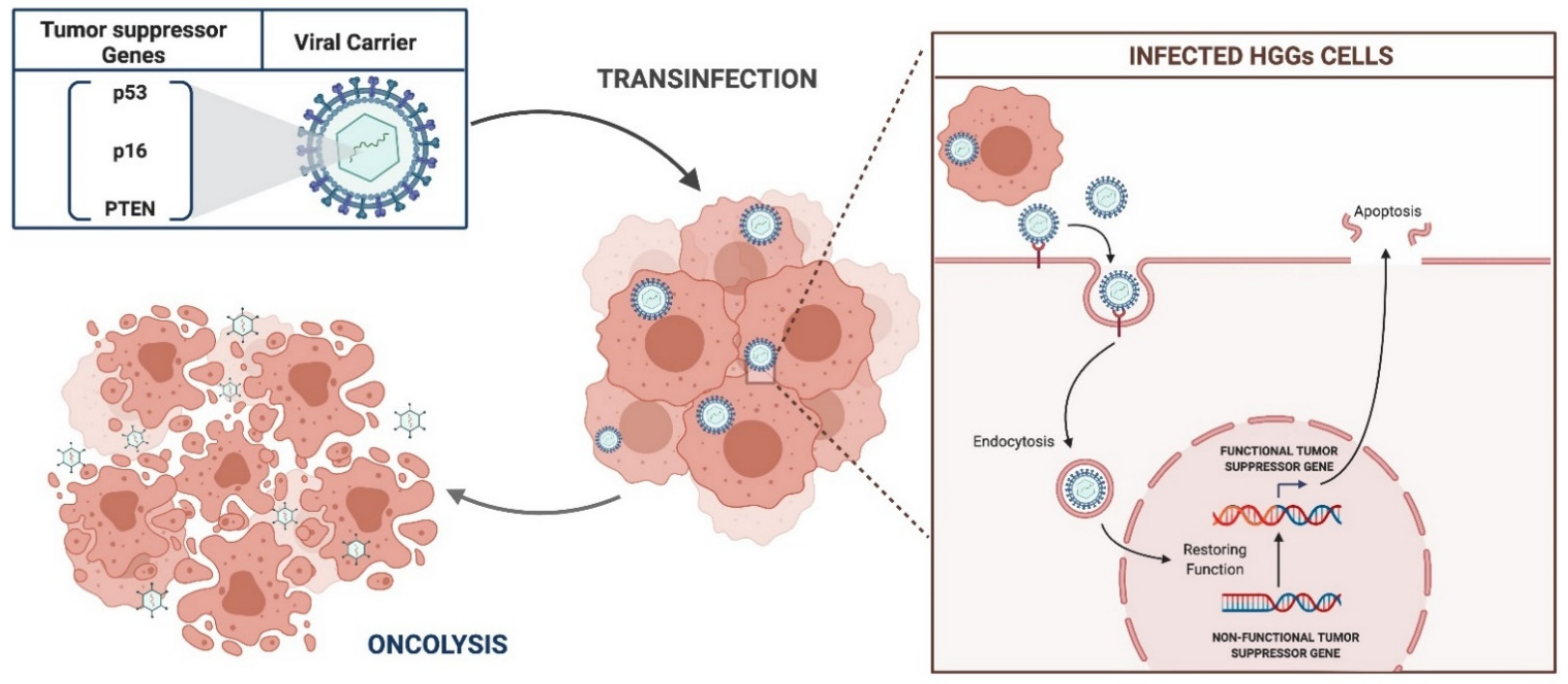
3.2.3. Tumor Suppressor Gene Therapies
p53
p16
PTEN
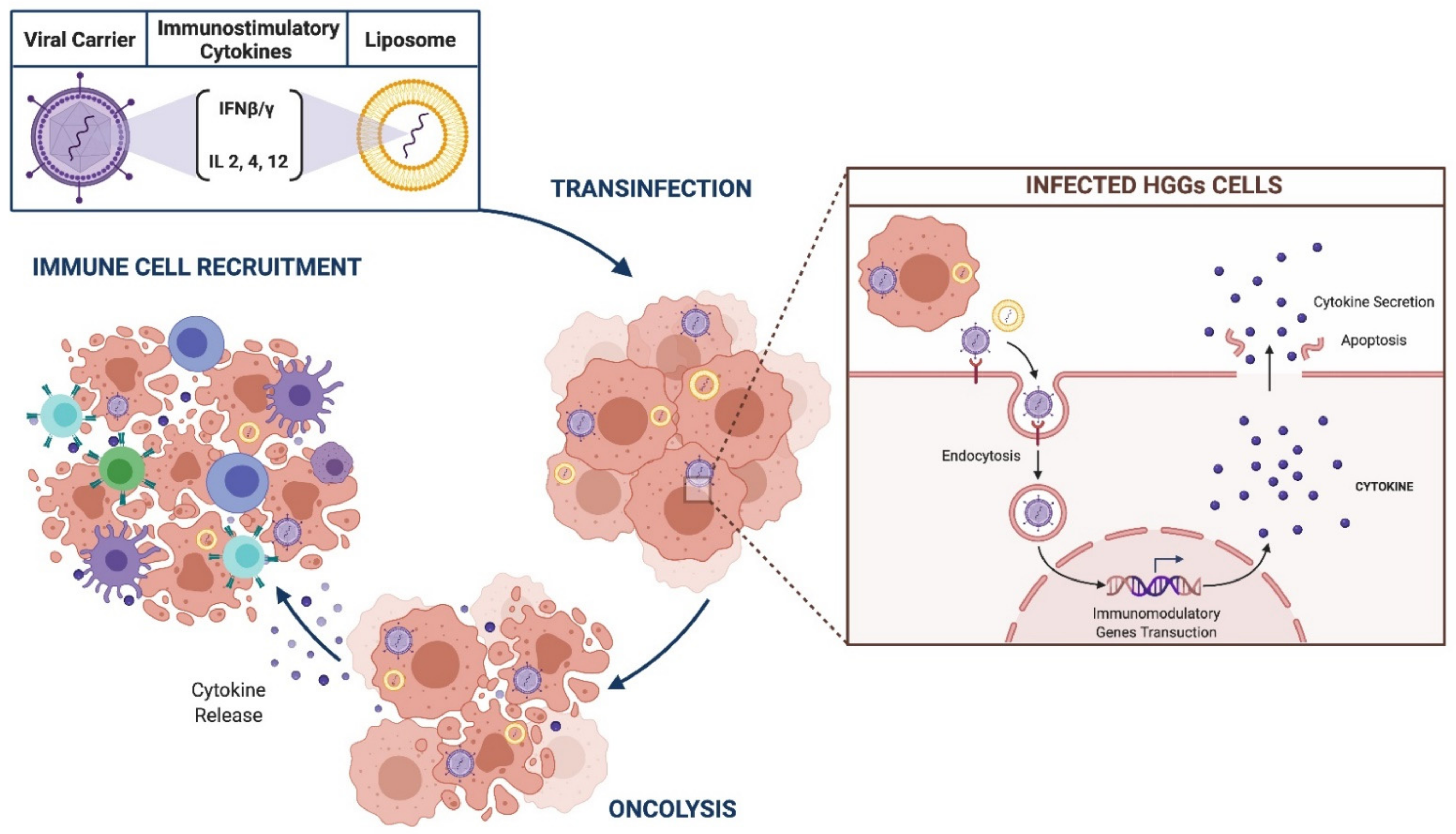
3.2.4. Immunomodulatory Gene Therapies
IFN-β/γ
IL12/4/2
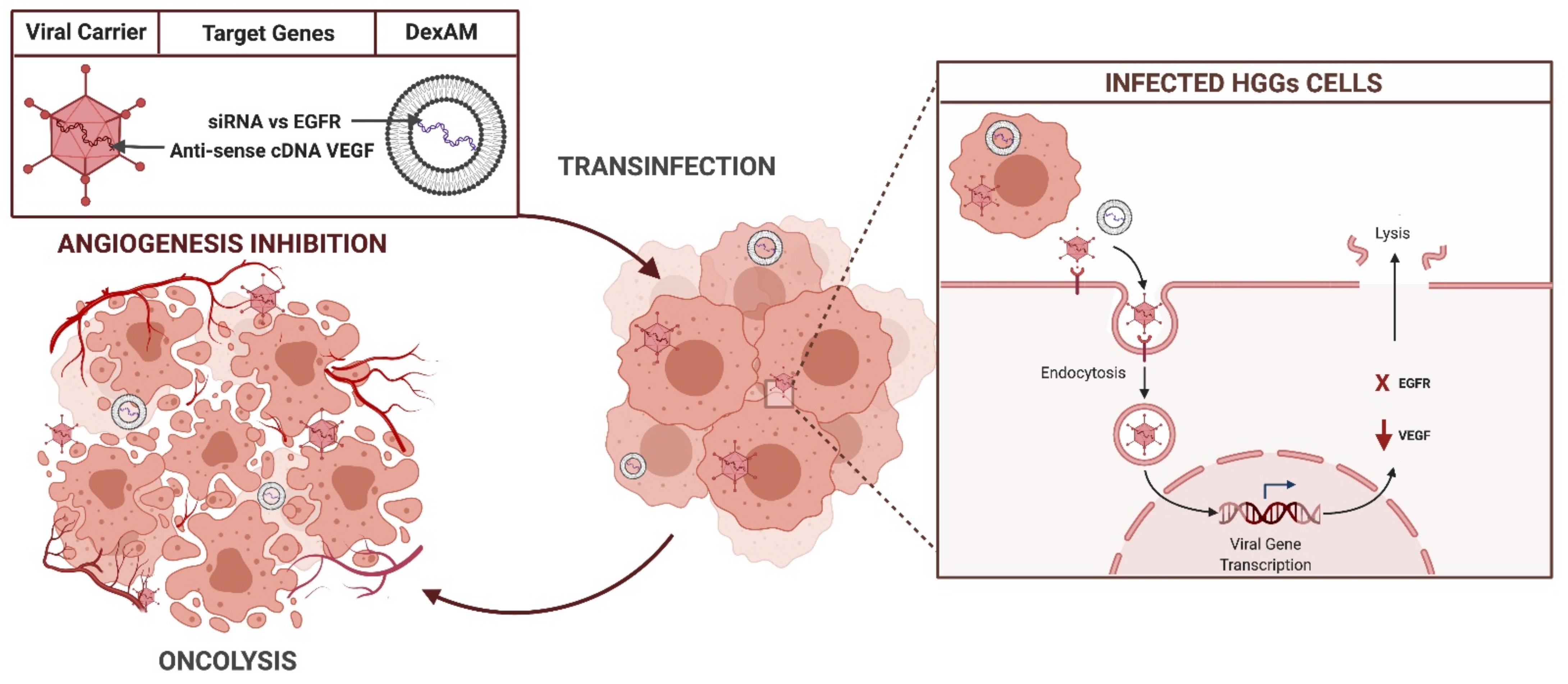
3.2.5. Gene Target Therapies
EGFRvIII
VEGF/VEGFR
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; Gittleman, H.; Liao, P.; Rouse, C.; Chen, Y.; Dowling, J.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2014, 16 (Suppl. 4), iv1–iv63. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; Stetson, L.; Virk, S.M.; Barnholtz-Sloan, J.S. Epidemiology of gliomas. Cancer Treat. Res. 2015, 163, 1–14. [Google Scholar] [CrossRef]
- Soerjomataram, I.; Lortet-Tieulent, J.; Parkin, D.M.; Ferlay, J.; Mathers, C.; Forman, D.; Bray, F. Global burden of cancer in 2008: A systematic analysis of disability-adjusted life-years in 12 world regions. Lancet 2012, 380, 1840–1850. [Google Scholar] [CrossRef]
- Eagan, R.T.; Scott, M. Evaluation of prognostic factors in chemotherapy of recurrent brain tumors. J. Clin. Oncol. 1983, 1, 38–44. [Google Scholar] [CrossRef]
- Wen, P.Y.; Kesari, S. Malignant gliomas in adults. N. Engl. J. Med. 2008, 359, 492–507. [Google Scholar] [CrossRef] [PubMed]
- Raysi Dehcordi, S.; Ricci, A.; di Vitantonio, H.; de Paulis, D.; Luzzi, S.; Palumbo, P.; Cinque, B.; Tempesta, D.; Coletti, G.; Cipolloni, G.; et al. Stemness marker detection in the periphery of glioblastoma and ability of glioblastoma to generate glioma stem cells: Clinical correlations. World Neurosurg. 2017, 105, 895–905. [Google Scholar] [CrossRef]
- Palumbo, P.; Lombardi, F.; Augello, F.R.; Giusti, I.; Luzzi, S.; Dolo, V.; Cifone, M.G.; Cinque, B. NOS2 inhibitor 1400 W induces autophagic flux and influences extracellular vesicle profile in human glioblastoma U87MG cell line. Int. J. Mol. Sci. 2019, 20, 3010. [Google Scholar] [CrossRef] [PubMed]
- De Tommasi, A.; Luzzi, S.; D’Urso, P.I.; de Tommasi, C.; Resta, N.; Ciappetta, P. Molecular genetic analysis in a case of ganglioglioma: Identification of a new mutation. Neurosurgery 2008, 63, 976–980. [Google Scholar] [CrossRef]
- Jackson, C.M.; Choi, J.; Lim, M. Mechanisms of immunotherapy resistance: Lessons from glioblastoma. Nat. Immunol. 2019, 20, 1100–1109. [Google Scholar] [CrossRef]
- DeCordova, S.; Shastri, A.; Tsolaki, A.G.; Yasmin, H.; Klein, L.; Singh, S.K.; Kishore, U. Molecular heterogeneity and immunosuppressive microenvironment in glioblastoma. Front. Immunol. 2020, 11, 1402. [Google Scholar] [CrossRef] [PubMed]
- Rolle, C.E.; Sengupta, S.; Lesniak, M.S. Mechanisms of immune evasion by gliomas. Adv. Exp. Med. Biol. 2012, 746, 53–76. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.R.; O’Neill, B.P. Glioblastoma survival in the United States before and during the temozolomide era. J. Neurooncol. 2012, 107, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, J.A.; Ramakrishna, R.; Magge, R. Immunotherapy in glioblastoma. World Neurosurg. 2018, 116, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Everson, R.G.; Antonios, J.P.; Liau, L.M. Cell-based immunotherapy of gliomas. Prog. Neurol. Surg. 2018, 32, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Zygourakis, C.; Lim, M.; Parsa, A.T. Immunotherapy for glioma: Promises and challenges. Neurosurg. Clin. N. Am. 2012, 23, 357–370. [Google Scholar] [CrossRef]
- Luzzi, S.; Crovace, A.M.; del Maestro, M.; Giotta Lucifero, A.; Elbabaa, S.K.; Cinque, B.; Palumbo, P.; Lombardi, F.; Cimini, A.; Cifone, M.G.; et al. The cell-based approach in neurosurgery: Ongoing trends and future perspectives. Heliyon 2019, 5, e02818. [Google Scholar] [CrossRef]
- Giotta Lucifero, A.; Luzzi, S. Against the resilience of high-grade gliomas: The immunotherapeutic approach (Part I). Brain Sci. 2021, 11, 386. [Google Scholar] [CrossRef]
- Giotta Lucifero, A.; Luzzi, S.; Brambilla, I.; Trabatti, C.; Mosconi, M.; Savasta, S.; Foiadelli, T. Innovative therapies for malignant brain tumors: The road to a tailored cure. Acta Biomed. 2020, 91, 5–17. [Google Scholar] [CrossRef]
- Xiong, L.; Wang, F.; Qi Xie, X. Advanced treatment in high-grade gliomas. J. BUON 2019, 24, 424–430. [Google Scholar]
- Lim, M.; Xia, Y.; Bettegowda, C.; Weller, M. Current state of immunotherapy for glioblastoma. Nat. Rev. Clin. Oncol. 2018, 15, 422–442. [Google Scholar] [CrossRef] [PubMed]
- Luzzi, S.; Giotta Lucifero, A.; Brambilla, I.; Magistrali, M.; Mosconi, M.; Savasta, S.; Foiadelli, T. Adoptive immunotherapies in neuro-oncology: Classification, recent advances, and translational challenges. Acta Biomed. 2020, 91, 18–31. [Google Scholar] [CrossRef]
- Campanella, R.; Guarnaccia, L.; Caroli, M.; Zarino, B.; Carrabba, G.; la Verde, N.; Gaudino, C.; Rampini, A.; Luzzi, S.; Riboni, L.; et al. Personalized and translational approach for malignant brain tumors in the era of precision medicine: The strategic contribution of an experienced neurosurgery laboratory in a modern neurosurgery and neuro-oncology department. J. Neurol. Sci. 2020, 417, 117083. [Google Scholar] [CrossRef]
- Luzzi, S.; Giotta Lucifero, A.; Brambilla, I.; Trabatti, C.; Mosconi, M.; Savasta, S.; Foiadelli, T. The impact of stem cells in neuro-oncology: Applications, evidence, limitations and challenges. Acta Biomed. 2020, 91, 51–60. [Google Scholar] [CrossRef]
- Luzzi, S.; Giotta Lucifero, A.; Brambilla, I.; Semeria Mantelli, S.; Mosconi, M.; Foiadelli, T.; Savasta, S. Targeting the medulloblastoma: A molecular-based approach. Acta Biomed. 2020, 91, 79–100. [Google Scholar] [CrossRef] [PubMed]
- Maguire, C.A.; Ramirez, S.H.; Merkel, S.F.; Sena-Esteves, M.; Breakefield, X.O. Gene therapy for the nervous system: Challenges and new strategies. Neurotherapeutics 2014, 11, 817–839. [Google Scholar] [CrossRef]
- Okura, H.; Smith, C.A.; Rutka, J.T. Gene therapy for malignant glioma. Mol. Cell Ther. 2014, 2, 21. [Google Scholar] [CrossRef]
- Wirth, T.; Yla-Herttuala, S. Gene therapy used in cancer treatment. Biomedicines 2014, 2, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska, A.; Nandhu, M.S.; Behera, P.; Chiocca, E.A.; Viapiano, M.S. Strategies in gene therapy for glioblastoma. Cancers 2013, 5, 1271–1305. [Google Scholar] [CrossRef] [PubMed]
- Kane, J.R.; Miska, J.; Young, J.S.; Kanojia, D.; Kim, J.W.; Lesniak, M.S. Sui generis: Gene therapy and delivery systems for the treatment of glioblastoma. Neuro Oncol. 2015, 17 (Suppl. 2), ii24–ii36. [Google Scholar] [CrossRef]
- Giotta Lucifero, A.; Luzzi, S.; Brambilla, I.; Guarracino, C.; Mosconi, M.; Foiadelli, T.; Savasta, S. Gene therapies for high-grade gliomas: From the bench to the bedside. Acta Biomed. 2020, 91, 32–50. [Google Scholar] [CrossRef]
- Campanella, R.; Guarnaccia, L.; Cordiglieri, C.; Trombetta, E.; Caroli, M.; Carrabba, G.; la Verde, N.; Rampini, P.; Gaudino, C.; Costa, A.; et al. Tumor-educated platelets and angiogenesis in glioblastoma: AnoTher. brick in the wall for novel prognostic and targetable biomarkers, changing the vision from a localized tumor to a systemic pathology. Cells 2020, 9, 294. [Google Scholar] [CrossRef]
- Caffery, B.; Lee, J.S.; Alexander-Bryant, A.A. Vectors for glioblastoma gene therapy: Viral & non-viral delivery strategies. Nanomaterials 2019, 9, 105. [Google Scholar] [CrossRef]
- Anguela, X.M.; High, K.A. Entering the modern era of gene therapy. Annu. Rev. Med. 2019, 70, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Kariyawasam, D.; Alexander, I.E.; Kurian, M.; Farrar, M.A. Great expectations: Virus-mediated gene therapy in neurological disorders. J. Neurol. Neurosurg. Psychiatry 2020, 91, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Su, W.; Wang, S.; Wang, X.; Liao, Z.; Kang, C.; Han, L.; Chang, J.; Wang, G.; Pu, P. Smart multifunctional core-shell nanospheres with drug and gene co-loaded for enhancing the therapeutic effect in a rat intracranial tumor model. Nanoscale 2012, 4, 6501–6508. [Google Scholar] [CrossRef] [PubMed]
- Godbey, W.T.; Wu, K.K.; Mikos, A.G. Poly(ethylenimine) and its role in gene delivery. J. Control Release 1999, 60, 149–160. [Google Scholar] [CrossRef]
- Lu, X.; Ping, Y.; Xu, F.J.; Li, Z.H.; Wang, Q.Q.; Chen, J.H.; Yang, W.T.; Tang, G.P. Bifunctional conjugates comprising β-cyclodextrin, polyethylenimine, and 5-fluoro-2’- deoxyuridine for drug delivery and gene transfer. Bioconjug. Chem. 2010, 21, 1855–1863. [Google Scholar] [CrossRef]
- Li, J.; Gu, B.; Meng, Q.; Yan, Z.; Gao, H.; Chen, X.; Yang, X.; Lu, W. The use of myristic acid as a ligand of polyethylenimine/DNA nanoparticles for targeted gene therapy of glioblastoma. Nanotechnology 2011, 22, 435101. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.; Meng, Q.; Li, Q.; Feng, L.; Zhu, J.; Lu, W. Cyclic RGD-polyethylene glycol-polyethylenimine for intracranial glioblastoma-targeted gene delivery. Chem. Asian J. 2012, 7, 91–96. [Google Scholar] [CrossRef]
- Richardson, S.C.; Pattrick, N.G.; Man, Y.K.; Ferruti, P.; Duncan, R. Poly(amidoamine)s as potential nonviral vectors: Ability to form interpolyelectrolyte complexes and to mediate transfection in vitro. Biomacromolecules 2001, 2, 1023–1028. [Google Scholar] [CrossRef]
- Bai, C.Z.; Choi, S.; Nam, K.; An, S.; Park, J.S. Arginine modified PAMAM dendrimer for interferon beta gene delivery to malignant glioma. Int. J. Pharm. 2013, 445, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Wankhede, M.; Bouras, A.; Kaluzova, M.; Hadjipanayis, C.G. Magnetic nanoparticles: An emerging technology for malignant brain tumor imaging and therapy. Expert Rev. Clin. Pharmacol. 2012, 5, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Sonabend, A.M.; Ulasov, I.V.; Kim, D.H.; Rozhkova, E.A.; Novosad, V.; Dashnaw, S.; Brown, T.; Canoll, P.; Bruce, J.N.; et al. A novel adenoviral vector labeled with superparamagnetic iron oxide nanoparticles for real-time tracking of viral delivery. J. Clin. Neurosci. 2012, 19, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, J.K.; Chiocca, E.A. Glioma virus therapies between bench and bedside. Neuro Oncol. 2014, 16, 334–351. [Google Scholar] [CrossRef]
- Ikeda, K.; Ichikawa, T.; Wakimoto, H.; Silver, J.S.; Deisboeck, T.S.; Finkelstein, D.; Harsh, G.R.T.; Louis, D.N.; Bartus, R.T.; Hochberg, F.H.; et al. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat. Med. 1999, 5, 881–887. [Google Scholar] [CrossRef]
- Natsume, A.; Yoshida, J. Gene therapy for high-grade glioma: Current approaches and future directions. Cell Adh. Migr. 2008, 2, 186–191. [Google Scholar] [CrossRef]
- Terada, K.; Wakimoto, H.; Tyminski, E.; Chiocca, E.A.; Saeki, Y. Development of a rapid method to generate multiple oncolytic HSV vectors and their in vivo evaluation using syngeneic mouse tumor models. Gene Ther. 2006, 13, 705–714. [Google Scholar] [CrossRef]
- Aghi, M.; Visted, T.; Depinho, R.A.; Chiocca, E.A. Oncolytic herpes virus with defective ICP6 specifically replicates in quiescent cells with homozygous genetic mutations in p16. Oncogene 2008, 27, 4249–4254. [Google Scholar] [CrossRef]
- Rampling, R.; Cruickshank, G.; Papanastassiou, V.; Nicoll, J.; Hadley, D.; Brennan, D.; Petty, R.; MacLean, A.; Harland, J.; McKie, E.; et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000, 7, 859–866. [Google Scholar] [CrossRef]
- Papanastassiou, V.; Rampling, R.; Fraser, M.; Petty, R.; Hadley, D.; Nicoll, J.; Harland, J.; Mabbs, R.; Brown, M. The potential for efficacy of the modified (ICP 34.5(-)) herpes simplex virus HSV1716 following intratumoural injection into human malignant glioma: A proof of principle study. Gene Ther. 2002, 9, 398–406. [Google Scholar] [CrossRef]
- Kanai, R.; Zaupa, C.; Sgubin, D.; Antoszczyk, S.J.; Martuza, R.L.; Wakimoto, H.; Rabkin, S.D. Effect of γ34.5 deletions on oncolytic herpes simplex virus activity in brain tumors. J. Virol. 2012, 86, 4420–4431. [Google Scholar] [CrossRef]
- Markert, J.M.; Medlock, M.D.; Rabkin, S.D.; Gillespie, G.Y.; Todo, T.; Hunter, W.D.; Palmer, C.A.; Feigenbaum, F.; Tornatore, C.; Tufaro, F.; et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: Results of a phase I trial. Gene Ther. 2000, 7, 867–874. [Google Scholar] [CrossRef]
- Markert, J.M.; Liechty, P.G.; Wang, W.; Gaston, S.; Braz, E.; Karrasch, M.; Nabors, L.B.; Markiewicz, M.; Lakeman, A.D.; Palmer, C.A.; et al. Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM. Mol. Ther. 2009, 17, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, J.R.; Kirn, D.H.; Williams, A.; Heise, C.; Horn, S.; Muna, M.; Ng, L.; Nye, J.A.; Sampson-Johannes, A.; Fattaey, A.; et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science 1996, 274, 373–376. [Google Scholar] [CrossRef]
- Chiocca, E.A.; Abbed, K.M.; Tatter, S.; Louis, D.N.; Hochberg, F.H.; Barker, F.; Kracher, J.; Grossman, S.A.; Fisher, J.D.; Carson, K.; et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-Attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol. Ther. 2004, 10, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Fueyo, J.; Gomez-Manzano, C.; Alemany, R.; Lee, P.S.; McDonnell, T.J.; Mitlianga, P.; Shi, Y.X.; Levin, V.A.; Yung, W.K.; Kyritsis, A.P. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene 2000, 19, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.F.; Conrad, C.; Gomez-Manzano, C.; Yung, W.K.A.; Sawaya, R.; Weinberg, J.S.; Prabhu, S.S.; Rao, G.; Fuller, G.N.; Aldape, K.D.; et al. Phase I study of DNX-2401 (Delta-24-RGD) oncolytic adenovirus: Replication and immunotherapeutic effects in recurrent malignant glioma. J. Clin. Oncol. 2018, 36, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Tran, N.; Puduvalli, V.; Elder, J.; Fink, K.; Conrad, C.; Yung, W.; Penas-Prado, M.; Gomez-Manzano, C.; Peterkin, J.; et al. Phase 1b open-label randomized study of the oncolytic adenovirus DNX-2401 administered with or without interferon gamma for recurrent glioblastoma. J. Clin. Oncol. 2017, 35, 2002. [Google Scholar] [CrossRef]
- Dörig, R.E.; Marcil, A.; Chopra, A.; Richardson, C.D. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 1993, 75, 295–305. [Google Scholar] [CrossRef]
- Anderson, B.D.; Nakamura, T.; Russell, S.J.; Peng, K.W. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res. 2004, 64, 4919–4926. [Google Scholar] [CrossRef] [PubMed]
- Phuong, L.K.; Allen, C.; Peng, K.W.; Giannini, C.; Greiner, S.; TenEyck, C.J.; Mishra, P.K.; Macura, S.I.; Russell, S.J.; Galanis, E.C. Use of a vaccine strain of measles virus genetically engineered to produce carcinoembryonic antigen as a novel therapeutic agent against glioblastoma multiforme. Cancer Res. 2003, 63, 2462–2469. [Google Scholar] [PubMed]
- Myers, R.; Harvey, M.; Kaufmann, T.J.; Greiner, S.M.; Krempski, J.W.; Raffel, C.; Shelton, S.E.; Soeffker, D.; Zollman, P.; Federspiel, M.J.; et al. Toxicology study of repeat intracerebral administration of a measles virus derivative producing carcinoembryonic antigen in rhesus macaques in support of a phase I/II clinical trial for patients with recurrent gliomas. Hum. Gene Ther. 2008, 19, 690–698. [Google Scholar] [CrossRef]
- Allen, C.; Paraskevakou, G.; Iankov, I.; Giannini, C.; Schroeder, M.; Sarkaria, J.; Schroeder, M.; Puri, R.K.; Russell, S.J.; Galanis, E. Interleukin-13 displaying retargeted oncolytic measles virus strains have significant activity against gliomas with improved specificity. Mol. Ther. 2008, 16, 1556–1564. [Google Scholar] [CrossRef]
- Paraskevakou, G.; Allen, C.; Nakamura, T.; Zollman, P.; James, C.D.; Peng, K.W.; Schroeder, M.; Russell, S.J.; Galanis, E. Epidermal growth factor receptor (EGFR)-retargeted measles virus strains effectively target EGFR- or EGFRvIII expressing gliomas. Mol. Ther. 2007, 15, 677–686. [Google Scholar] [CrossRef]
- Allen, C.; Vongpunsawad, S.; Nakamura, T.; James, C.D.; Schroeder, M.; Cattaneo, R.; Giannini, C.; Krempski, J.; Peng, K.W.; Goble, J.M.; et al. Retargeted oncolytic measles strains entering via the EGFRvIII receptor maintain significant antitumor activity against gliomas with increased tumor specificity. Cancer Res. 2006, 66, 11840–11850. [Google Scholar] [CrossRef] [PubMed]
- Gromeier, M.; Bossert, B.; Arita, M.; Nomoto, A.; Wimmer, E. Dual stem loops within the poliovirus internal ribosomal entry site control neurovirulence. J. Virol. 1999, 73, 958–964. [Google Scholar] [CrossRef]
- Gromeier, M.; Alexander, L.; Wimmer, E. Internal ribosomal entry site substitution eliminates neurovirulence in intergeneric poliovirus recombinants. Proc. Natl. Acad. Sci. USA 1996, 93, 2370–2375. [Google Scholar] [CrossRef] [PubMed]
- Dobrikova, E.Y.; Goetz, C.; Walters, R.W.; Lawson, S.K.; Peggins, J.O.; Muszynski, K.; Ruppel, S.; Poole, K.; Giardina, S.L.; Vela, E.M.; et al. Attenuation of neurovirulence, biodistribution, and shedding of a poliovirus:rhinovirus chimera after intrathalamic inoculation in Macaca fascicularis. J. Virol. 2012, 86, 2750–2759. [Google Scholar] [CrossRef]
- Merrill, M.K.; Bernhardt, G.; Sampson, J.H.; Wikstrand, C.J.; Bigner, D.D.; Gromeier, M. Poliovirus receptor CD155-targeted oncolysis of glioma. Neuro Oncol. 2004, 6, 208–217. [Google Scholar] [CrossRef]
- Sloan, K.E.; Stewart, J.K.; Treloar, A.F.; Matthews, R.T.; Jay, D.G. CD155/PVR enhances glioma cell dispersal by regulating adhesion signaling and focal adhesion dynamics. Cancer Res. 2005, 65, 10930–10937. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.; Carle, G.; Faneca, H.; de Lima, M.C.; Pierrefite-Carle, V. Suicide gene therapy in cancer: Where do we stand now? Cancer Lett. 2012, 324, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Tiberghien, P. Use of suicide genes in gene therapy. J. Leukoc. Biol. 1994, 56, 203–209. [Google Scholar] [CrossRef]
- Karjoo, Z.; Chen, X.; Hatefi, A. Progress and problems with the use of suicide genes for targeted cancer therapy. Adv. Drug Deliv. Rev. 2016, 99, 113–128. [Google Scholar] [CrossRef]
- Immonen, A.; Vapalahti, M.; Tyynelä, K.; Hurskainen, H.; Sandmair, A.; Vanninen, R.; Langford, G.; Murray, N.; Ylä-Herttuala, S. AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: A randomised, controlled study. Mol. Ther. 2004, 10, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Rainov, N.G.; Heidecke, V. Clinical development of experimental virus-mediated gene therapy for malignant glioma. Anticancer Agents Med. Chem. 2011, 11, 739–747. [Google Scholar] [CrossRef]
- Halloran, P.J.; Fenton, R.G. Irreversible G2-M arrest and cytoskeletal reorganization induced by cytotoxic nucleoside analogues. Cancer Res. 1998, 58, 3855–3865. [Google Scholar]
- Wei, S.J.; Chao, Y.; Hung, Y.M.; Lin, W.C.; Yang, D.M.; Shih, Y.L.; Ch’ang, L.Y.; Whang-Peng, J.; Yang, W.K. S- and G2-phase cell cycle arrests and apoptosis induced by ganciclovir in murine melanoma cells transduced with herpes simplex virus thymidine kinase. Exp. Cell Res. 1998, 241, 66–75. [Google Scholar] [CrossRef]
- Rainov, N.G. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum. Gene Ther. 2000, 11, 2389–2401. [Google Scholar] [CrossRef]
- Sandmair, A.M.; Loimas, S.; Puranen, P.; Immonen, A.; Kossila, M.; Puranen, M.; Hurskainen, H.; Tyynelä, K.; Turunen, M.; Vanninen, R.; et al. Thymidine kinase gene therapy for human malignant glioma, using replication-deficient retroviruses or adenoviruses. Hum. Gene Ther. 2000, 11, 2197–2205. [Google Scholar] [CrossRef]
- Germano, I.M.; Fable, J.; Gultekin, S.H.; Silvers, A. Adenovirus/herpes simplex-thymidine kinase/ganciclovir complex: Preliminary results of a phase I trial in patients with recurrent malignant gliomas. J. Neuro Oncol. 2003, 65, 279–289. [Google Scholar] [CrossRef]
- Westphal, M.; Ylä-Herttuala, S.; Martin, J.; Warnke, P.; Menei, P.; Eckland, D.; Kinley, J.; Kay, R.; Ram, Z. Adenovirus-mediated gene therapy with sitimagene ceradenovec followed by intravenous ganciclovir for patients with operable high-grade glioma (ASPECT): A randomised, open-label, phase 3 trial. Lancet Oncol. 2013, 14, 823–833. [Google Scholar] [CrossRef]
- Takahashi, M.; Valdes, G.; Hiraoka, K.; Inagaki, A.; Kamijima, S.; Micewicz, E.; Gruber, H.E.; Robbins, J.M.; Jolly, D.J.; McBride, W.H.; et al. Radiosensitization of gliomas by intracellular generation of 5-fluorouracil potentiates prodrug activator gene therapy with a retroviral replicating vector. Cancer Gene Ther. 2014, 21, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Tobias, A.; Ahmed, A.; Moon, K.S.; Lesniak, M.S. The art of gene therapy for glioma: A review of the challenging road to the bedside. J. Neurol. Neurosurg. Psychiatry 2013, 84, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Cloughesy, T.F.; Landolfi, J.; Vogelbaum, M.A.; Ostertag, D.; Elder, J.B.; Bloomfield, S.; Carter, B.; Chen, C.C.; Kalkanis, S.N.; Kesari, S.; et al. Durable complete responses in some recurrent high-grade glioma patients treated with Toca 511 + Toca FC. Neuro Oncol. 2018, 20, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Cloughesy, T.F.; Landolfi, J.; Hogan, D.J.; Bloomfield, S.; Carter, B.; Chen, C.C.; Elder, J.B.; Kalkanis, S.N.; Kesari, S.; Lai, A.; et al. Phase 1 trial of vocimagene amiretrorepvec and 5-fluorocytosine for recurrent high-grade glioma. Sci. Transl. Med. 2016, 8, 341ra375. [Google Scholar] [CrossRef]
- Ardiani, A.; Johnson, A.J.; Ruan, H.; Sanchez-Bonilla, M.; Serve, K.; Black, M.E. Enzymes to die for: Exploiting nucleotide metabolizing enzymes for cancer gene therapy. Curr. Gene Ther. 2012, 12, 77–91. [Google Scholar] [CrossRef]
- Tai, C.K.; Wang, W.; Lai, Y.H.; Logg, C.R.; Parker, W.B.; Li, Y.F.; Hong, J.S.; Sorscher, E.J.; Chen, T.C.; Kasahara, N. Enhanced efficiency of prodrug activation therapy by tumor-selective replicating retrovirus vectors armed with the Escherichia coli purine nucleoside phosphorylase gene. Cancer Gene Ther. 2010, 17, 614–623. [Google Scholar] [CrossRef]
- Parker, W.B.; King, S.A.; Allan, P.W.; Bennett, L.L., Jr.; Secrist, J.A., 3rd; Montgomery, J.A.; Gilbert, K.S.; Waud, W.R.; Wells, A.H.; Gillespie, G.Y.; et al. In vivo gene therapy of cancer with E. coli purine nucleoside phosphorylase. Hum. Gene Ther. 1997, 8, 1637–1644. [Google Scholar] [CrossRef]
- Zhang, Y.; Parker, W.B.; Sorscher, E.J.; Ealick, S.E. PNP anticancer gene therapy. Curr. Top. Med. Chem. 2005, 5, 1259–1274. [Google Scholar] [CrossRef]
- Sorscher, E.J.; Hong, J.S.; Allan, P.W.; Waud, W.R.; Parker, W.B. In vivo antitumor activity of intratumoral fludarabine phosphate in refractory tumors expressing E. coli purine nucleoside phosphorylase. Cancer Chemo Ther. Pharmacol. 2012, 70, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Curlee, K.V.; Parker, W.B.; Sorscher, E.J. Tumor sensitization to purine analogs by E. coli PNP. Methods Mol. Med. 2004, 90, 223–245. [Google Scholar] [CrossRef] [PubMed]
- Secrist, J.A., 3rd; Parker, W.B.; Allan, P.W.; Bennett, L.L., Jr.; Waud, W.R.; Truss, J.W.; Fowler, A.T.; Montgomery, J.A.; Ealick, S.E.; Wells, A.H.; et al. Gene therapy of cancer: Activation of nucleoside prodrugs with E. coli purine nucleoside phosphorylase. Nucleosides Nucleotides 1999, 18, 745–757. [Google Scholar] [CrossRef]
- Hong, J.S.; Waud, W.R.; Levasseur, D.N.; Townes, T.M.; Wen, H.; McPherson, S.A.; Moore, B.A.; Bebok, Z.; Allan, P.W.; Secrist, J.A., 3rd; et al. Excellent in vivo bystander activity of fludarabine phosphate against human glioma xenografts that express the Escherichia coli purine nucleoside phosphorylase gene. Cancer Res. 2004, 64, 6610–6615. [Google Scholar] [CrossRef] [PubMed]
- Bharara, S.; Sorscher, E.J.; Gillespie, G.Y.; Lindsey, J.R.; Hong, J.S.; Curlee, K.V.; Allan, P.W.; Gadi, V.K.; Alexander, S.A.; Secrist, J.A., 3rd; et al. Antibiotic-mediated chemoprotection enhances adaptation of E. coli PNP for herpes simplex virus-based glioma therapy. Hum. Gene Ther. 2005, 16, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008, 455, 1061–1068. [Google Scholar] [CrossRef]
- Bogler, O.; Huang, H.J.; Kleihues, P.; Cavenee, W.K. The p53 gene and its role in human brain tumors. Glia 1995, 15, 308–327. [Google Scholar] [CrossRef]
- England, B.; Huang, T.; Karsy, M. Current understanding of the role and targeting of tumor suppressor p53 in glioblastoma multiforme. Tumor Biol 2013, 34, 2063–2074. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.F.; Yung, W.K.; Sawaya, R.; Tofilon, P.J. Adenovirus-mediated p53 gene therapy for human gliomas. Neurosurgery 1999, 45, 1093–1104. [Google Scholar] [CrossRef]
- Li, H.; Alonso-Vanegas, M.; Colicos, M.A.; Jung, S.S.; Lochmuller, H.; Sadikot, A.F.; Snipes, G.J.; Seth, P.; Karpati, G.; Nalbantoglu, J. Intracerebral adenovirus-mediated p53 tumor suppressor gene therapy for experimental human glioma. Clin. Cancer Res. 1999, 5, 637–642. [Google Scholar]
- Van Meir, E.G.; Polverini, P.J.; Chazin, V.R.; Su Huang, H.J.; de Tribolet, N.; Cavenee, W.K. Release of an inhibitor of angiogenesis upon induction of wild type p53 expression in glioblastoma cells. Nat. Genet. 1994, 8, 171–176. [Google Scholar] [CrossRef]
- Cirielli, C.; Inyaku, K.; Capogrossi, M.C.; Yuan, X.; Williams, J.A. Adenovirus-mediated wild-type p53 expression induces apoptosis and suppresses tumorigenesis of experimental intracranial human malignant glioma. J. Neuro Oncol. 1999, 43, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Shono, T.; Tofilon, P.J.; Schaefer, T.S.; Parikh, D.; Liu, T.J.; Lang, F.F. Apoptosis induced by adenovirus-mediated p53 gene transfer in human glioma correlates with site-specific phosphorylation. Cancer Res. 2002, 62, 1069–1076. [Google Scholar] [PubMed]
- Lang, F.F.; Bruner, J.M.; Fuller, G.N.; Aldape, K.; Prados, M.D.; Chang, S.; Berger, M.S.; McDermott, M.W.; Kunwar, S.M.; Junck, L.R.; et al. Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: Biological and clinical results. J. Clin. Oncol. 2003, 21, 2508–2518. [Google Scholar] [CrossRef] [PubMed]
- Biroccio, A.; Bufalo, D.D.; Ricca, A.; D’Angelo, C.; D’Orazi, G.; Sacchi, A.; Soddu, S.; Zupi, G. Increase of BCNU sensitivity by wt-p53 gene therapy in glioblastoma lines depends on the administration schedule. Gene Ther. 1999, 6, 1064–1072. [Google Scholar] [CrossRef]
- Badie, B.; Kramar, M.H.; Lau, R.; Boothman, D.A.; Economou, J.S.; Black, K.L. Adenovirus-mediated p53 gene delivery potentiates the radiation-induced growth inhibition of experimental brain tumors. J. Neuro Oncol. 1998, 37, 217–222. [Google Scholar] [CrossRef]
- Kim, S.S.; Rait, A.; Kim, E.; Pirollo, K.F.; Nishida, M.; Farkas, N.; Dagata, J.A.; Chang, E.H. A nanoparticle carrying the p53 gene targets tumors including cancer stem cells, sensitizes glioblastoma to chemotherapy and improves survival. ACS Nano 2014, 8, 5494–5514. [Google Scholar] [CrossRef]
- Kanu, O.O.; Hughes, B.; Di, C.; Lin, N.; Fu, J.; Bigner, D.D.; Yan, H.; Adamson, C. Glioblastoma multiforme oncogenomics and signaling pathways. Clin. Med. Oncol. 2009, 3, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Chintala, S.K.; Fueyo, J.; Gomez-Manzano, C.; Venkaiah, B.; Bjerkvig, R.; Yung, W.K.; Sawaya, R.; Kyritsis, A.P.; Rao, J.S. Adenovirus-mediated p16/CDKN2 gene transfer suppresses glioma invasion in vitro. Oncogene 1997, 15, 2049–2057. [Google Scholar] [CrossRef]
- Hung, K.S.; Hong, C.Y.; Lee, J.; Lin, S.K.; Huang, S.C.; Wang, T.M.; Tse, V.; Sliverberg, G.D.; Weng, S.C.; Hsiao, M. Expression of p16(INK4A) induces dominant suppression of glioblastoma growth in situ through necrosis and cell cycle arrest. Biochem. Biophys. Res. Commun. 2000, 269, 718–725. [Google Scholar] [CrossRef]
- Hama, S.; Matsuura, S.; Tauchi, H.; Yamasaki, F.; Kajiwara, Y.; Arita, K.; Yoshioka, H.; Heike, Y.; Mandai, K.; Kurisu, K. p16 gene transfer increases cell killing with abnormal nucleation after ionising radiation in glioma cells. Br. J. Cancer 2003, 89, 1802–1811. [Google Scholar] [CrossRef][Green Version]
- Xande, J.G.; Dias, A.P.; Tamura, R.E.; Cruz, M.C.; Brito, B.; Ferreira, R.A.; Strauss, B.E.; Costanzi-Strauss, E. Bicistronic transfer of CDKN2A and p53 culminates in collaborative killing of human lung cancer cells in vitro and in vivo. Gene Ther. 2020, 27, 51–61. [Google Scholar] [CrossRef]
- Ohgaki, H.; Kleihues, P. The definition of primary and secondary glioblastoma. Clin. Cancer Res. 2013, 19, 764–772. [Google Scholar] [CrossRef]
- Dunn, G.P.; Rinne, M.L.; Wykosky, J.; Genovese, G.; Quayle, S.N.; Dunn, I.F.; Agarwalla, P.K.; Chheda, M.G.; Campos, B.; Wang, A.; et al. Emerging insights into the molecular and cellular basis of glioblastoma. Genes Dev. 2012, 26, 756–784. [Google Scholar] [CrossRef]
- Davies, M.A.; Lu, Y.; Sano, T.; Fang, X.; Tang, P.; LaPushin, R.; Koul, D.; Bookstein, R.; Stokoe, D.; Yung, W.K.; et al. Adenoviral transgene expression of MMAC/PTEN in human glioma cells inhibits Akt activation and induces anoikis. Cancer Res. 1998, 58, 5285–5290. [Google Scholar] [PubMed]
- Cheney, I.W.; Johnson, D.E.; Vaillancourt, M.T.; Avanzini, J.; Morimoto, A.; Demers, G.W.; Wills, K.N.; Shabram, P.W.; Bolen, J.B.; Tavtigian, S.V.; et al. Suppression of tumorigenicity of glioblastoma cells by adenovirus-mediated MMAC1/PTEN gene transfer. Cancer Res. 1998, 58, 2331–2334. [Google Scholar] [PubMed]
- Lu, W.; Zhou, X.; Hong, B.; Liu, J.; Yue, Z. Suppression of invasion in human U87 glioma cells by adenovirus-mediated co-transfer of TIMP-2 and PTEN gene. Cancer Lett. 2004, 214, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Terada, K.; Wakimoto, H.; Inoue, R.; Tyminski, E.; Bookstein, R.; Basilion, J.P.; Chiocca, E.A. PTEN decreases in vivo vascularization of experimental gliomas in spite of proangiogenic stimuli. Cancer Res. 2003, 63, 2300–2305. [Google Scholar] [PubMed]
- Inaba, N.; Kimura, M.; Fujioka, K.; Ikeda, K.; Somura, H.; Akiyoshi, K.; Inoue, Y.; Nomura, M.; Saito, Y.; Saito, H.; et al. The effect of PTEN on proliferation and drug-, and radiosensitivity in malignant glioma cells. Anticancer Res. 2011, 31, 1653–1658. [Google Scholar]
- Iwami, K.; Natsume, A.; Wakabayashi, T. Gene therapy for high-grade glioma. Neurol. Med. Chir. 2010, 50, 727–736. [Google Scholar] [CrossRef]
- Assi, H.; Candolfi, M.; Baker, G.; Mineharu, Y.; Lowenstein, P.R.; Castro, M.G. Gene therapy for brain tumors: Basic developments and clinical implementation. Neurosci. Lett. 2012, 527, 71–77. [Google Scholar] [CrossRef]
- Qin, X.Q.; Beckham, C.; Brown, J.L.; Lukashev, M.; Barsoum, J. Human and mouse IFN-beta gene therapy exhibits different anti-tumor mechanisms in mouse models. Mol. Ther. 2001, 4, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Natsume, A.; Mizuno, M.; Ryuke, Y.; Yoshida, J. Antitumor effect and cellular immunity activation by murine interferon-beta gene transfer against intracerebral glioma in mouse. Gene Ther. 1999, 6, 1626–1633. [Google Scholar] [CrossRef]
- Natsume, A.; Tsujimura, K.; Mizuno, M.; Takahashi, T.; Yoshida, J. IFN-beta gene therapy induces systemic antitumor immunity against malignant glioma. J. Neuro Oncol. 2000, 47, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, J.; Mizuno, M.; Fujii, M.; Kajita, Y.; Nakahara, N.; Hatano, M.; Saito, R.; Nobayashi, M.; Wakabayashi, T. Human gene therapy for malignant gliomas (glioblastoma multiforme and anaplastic astrocytoma) by in vivo transduction with human interferon beta gene using cationic liposomes. Hum. Gene Ther. 2004, 15, 77–86. [Google Scholar] [CrossRef]
- Wakabayashi, T.; Natsume, A.; Hashizume, Y.; Fujii, M.; Mizuno, M.; Yoshida, J. A phase I clinical trial of interferon-beta gene therapy for high-grade glioma: Novel findings from gene expression profiling and autopsy. J. Gene Med. 2008, 10, 329–339. [Google Scholar] [CrossRef]
- Knüpfer, M.M.; Poppenborg, H.; Van Gool, S.; Domula, M.; Wolff, J.E. Interferon-gamma inhibits proliferation and adhesion of T98G human malignant glioma cells in vitro. Klin. Padiatr. 1997, 209, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Borden, E.C.; Lindner, D.; Dreicer, R.; Hussein, M.; Peereboom, D. Second-generation interferons for cancer: Clinical targets. Semin. Cancer Biol. 2000, 10, 125–144. [Google Scholar] [CrossRef] [PubMed]
- Ehtesham, M.; Samoto, K.; Kabos, P.; Acosta, F.L.; Gutierrez, M.A.; Black, K.L.; Yu, J.S. Treatment of intracranial glioma with in situ interferon-gamma and tumor necrosis factor-alpha gene transfer. Cancer Gene Ther. 2002, 9, 925–934. [Google Scholar] [CrossRef]
- Enderlin, M.; Kleinmann, E.V.; Struyf, S.; Buracchi, C.; Vecchi, A.; Kinscherf, R.; Kiessling, F.; Paschek, S.; Sozzani, S.; Rommelaere, J.; et al. TNF-alpha and the IFN-gamma-inducible protein 10 (IP-10/CXCL-10) delivered by parvoviral vectors act in synergy to induce antitumor effects in mouse glioblastoma. Cancer Gene Ther. 2009, 16, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.P.; Trinchieri, G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002, 13, 155–168. [Google Scholar] [CrossRef]
- Ma, X.; Chow, J.M.; Gri, G.; Carra, G.; Gerosa, F.; Wolf, S.F.; Dzialo, R.; Trinchieri, G. The interleukin 12 p40 gene promoter is primed by interferon gamma in monocytic cells. J. Exp. Med. 1996, 183, 147–157. [Google Scholar] [CrossRef]
- Chen, B.; Timiryasova, T.M.; Andres, M.L.; Kajioka, E.H.; Dutta-Roy, R.; Gridley, D.S.; Fodor, I. Evaluation of combined vaccinia virus-mediated antitumor gene therapy with p53, IL-2, and IL-12 in a glioma model. Cancer Gene Ther. 2000, 7, 1437–1447. [Google Scholar] [CrossRef]
- Chiu, T.L.; Wang, M.J.; Su, C.C. The treatment of glioblastoma multiforme through activation of microglia and TRAIL induced by rAAV2-mediated IL-12 in a syngeneic rat model. J. Biomed. Sci. 2012, 19, 45. [Google Scholar] [CrossRef]
- Hellums, E.K.; Markert, J.M.; Parker, J.N.; He, B.; Perbal, B.; Roizman, B.; Whitley, R.J.; Langford, C.P.; Bharara, S.; Gillespie, G.Y. Increased efficacy of an interleukin-12-secreting herpes simplex virus in a syngeneic intracranial murine glioma model. Neuro Oncol. 2005, 7, 213–224. [Google Scholar] [CrossRef]
- Markert, J.M.; Cody, J.J.; Parker, J.N.; Coleman, J.M.; Price, K.H.; Kern, E.R.; Quenelle, D.C.; Lakeman, A.D.; Schoeb, T.R.; Palmer, C.A.; et al. Preclinical evaluation of a genetically engineered herpes simplex virus expressing interleukin-12. J. Virol. 2012, 86, 5304–5313. [Google Scholar] [CrossRef]
- Yu, J.S.; Wei, M.X.; Chiocca, E.A.; Martuza, R.L.; Tepper, R.I. Treatment of glioma by engineered interleukin 4-secreting cells. Cancer Res. 1993, 53, 3125–3128. [Google Scholar]
- Okada, H.; Giezeman-Smits, K.M.; Tahara, H.; Attanucci, J.; Fellows, W.K.; Lotze, M.T.; Chambers, W.H.; Bozik, M.E. Effective cytokine gene therapy against an intracranial glioma using a retrovirally transduced IL-4 plus HSVtk tumor vaccine. Gene Ther. 1999, 6, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Okada, H.; Pollack, I.F.; Lotze, M.T.; Lunsford, L.D.; Kondziolka, D.; Lieberman, F.; Schiff, D.; Attanucci, J.; Edington, H.; Chambers, W.; et al. Gene therapy of malignant gliomas: A phase I study of IL-4-HSV-TK gene-modified autologous tumor to elicit an immune response. Hum. Gene Ther. 2000, 11, 637–653. [Google Scholar] [CrossRef] [PubMed]
- Colombo, F.; Barzon, L.; Franchin, E.; Pacenti, M.; Pinna, V.; Danieli, D.; Zanusso, M.; Palu, G. Combined HSV-TK/IL-2 gene therapy in patients with recurrent glioblastoma multiforme: Biological and clinical results. Cancer Gene Ther. 2005, 12, 835–848. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, C.A.; Li, G.; Wong, A.J. Targeting EGF receptor variant III: Tumor-specific peptide vaccination for malignant gliomas. Expert Rev. Vaccines 2012, 11, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Gan, H.K.; Cvrljevic, A.N.; Johns, T.G. The epidermal growth factor receptor variant III (EGFRvIII): Where wild things are altered. FEBS J. 2013, 280, 5350–5370. [Google Scholar] [CrossRef]
- Shir, A.; Levitzki, A. Inhibition of glioma growth by tumor-specific activation of double-stranded RNA-dependent protein kinase PKR. Nat. Biotechnol. 2002, 20, 895–900. [Google Scholar] [CrossRef]
- Kang, C.S.; Zhang, Z.Y.; Jia, Z.F.; Wang, G.X.; Qiu, M.Z.; Zhou, H.X.; Yu, S.Z.; Chang, J.; Jiang, H.; Pu, P.Y. Suppression of EGFR expression by antisense or small interference RNA inhibits U251 glioma cell growth in vitro and in vivo. Cancer Gene Ther. 2006, 13, 530–538. [Google Scholar] [CrossRef]
- Wang, K.; Park, J.O.; Zhang, M. Treatment of glioblastoma multiforme using a combination of small interfering RNA targeting epidermal growth factor receptor and β-catenin. J. Gene Med. 2013, 15, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Karpel-Massler, G.; Schmidt, U.; Unterberg, A.; Halatsch, M.E. Therapeutic inhibition of the epidermal growth factor receptor in high-grade gliomas: Where do we stand? Mol. Cancer Res. 2009, 7, 1000–1012. [Google Scholar] [CrossRef] [PubMed]
- Padfield, E.; Ellis, H.P.; Kurian, K.M. Current therapeutic advances targeting EGFR and EGFRvIII in glioblastoma. Front. Oncol. 2015, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Shah, B.P.; Subramaniam, P.; Lee, K.B. Synergistic induction of apoptosis in brain cancer cells by targeted codelivery of siRNA and anticancer drugs. Mol. Pharm. 2011, 8, 1955–1961. [Google Scholar] [CrossRef][Green Version]
- Im, S.A.; Gomez-Manzano, C.; Fueyo, J.; Liu, T.J.; Ke, L.D.; Kim, J.S.; Lee, H.Y.; Steck, P.A.; Kyritsis, A.P.; Yung, W.K. Antiangiogenesis treatment for gliomas: Transfer of antisense-vascular endothelial growth factor inhibits tumor growth in vivo. Cancer Res. 1999, 59, 895–900. [Google Scholar]
- Niola, F.; Evangelisti, C.; Campagnolo, L.; Massalini, S.; Buè, M.C.; Mangiola, A.; Masotti, A.; Maira, G.; Farace, M.G.; Ciafrè, S.A. A plasmid-encoded VEGF siRNA reduces glioblastoma angiogenesis and its combination with interleukin-4 blocks tumor growth in a xenograft mouse model. Cancer Biol. Ther. 2006, 5, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Kim, J.H.; Kwon, Y.G.; Kim, E.C.; Kim, N.K.; Choi, H.J.; Yun, C.O. VEGF-specific short hairpin RNA-expressing oncolytic adenovirus elicits potent inhibition of angiogenesis and tumor growth. Mol. Ther. 2007, 15, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.A.; Shin, H.C.; Yoo, J.Y.; Kim, J.H.; Kim, J.S.; Yun, C.O. Novel cancer antiangiotherapy using the VEGF promoter-targeted artificial zinc-finger protein and oncolytic adenovirus. Mol. Ther. 2008, 16, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, R.; Machein, M.; Nicolaus, A.; Hilbig, A.; Wild, C.; Clauss, M.; Plate, K.H.; Breier, G. Inhibition of solid tumor growth by gene transfer of VEGF receptor-1 mutants. Int. J. Cancer 2004, 111, 348–357. [Google Scholar] [CrossRef]
- Thorne, S.H.; Tam, B.Y.; Kirn, D.H.; Contag, C.H.; Kuo, C.J. Selective intratumoral amplification of an antiangiogenic vector by an oncolytic virus produces enhanced antivascular and anti-tumor efficacy. Mol. Ther. 2006, 13, 938–946. [Google Scholar] [CrossRef]
- Lachmann, R. Herpes simplex virus-based vectors. Int. J. Exp. Pathol. 2004, 85, 177–190. [Google Scholar] [CrossRef]
- Chiocca, E.A.; Aghi, M.; Fulci, G. Viral therapy for glioblastoma. Cancer J. 2003, 9, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Kurozumi, K.; Hardcastle, J.; Thakur, R.; Yang, M.; Christoforidis, G.; Fulci, G.; Hochberg, F.H.; Weissleder, R.; Carson, W.; Chiocca, E.A.; et al. Effect of tumor microenvironment modulation on the efficacy of oncolytic virus therapy. J. Natl. Cancer Inst. 2007, 99, 1768–1781. [Google Scholar] [CrossRef]
- Chiocca, E.A. Oncolytic viruses. Nat. Rev. Cancer 2002, 2, 938–950. [Google Scholar] [CrossRef]
- Selznick, L.A.; Shamji, M.F.; Fecci, P.; Gromeier, M.; Friedman, A.H.; Sampson, J. Molecular strategies for the treatment of malignant glioma—Genes, viruses, and vaccines. Neurosurg. Rev. 2008, 31, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Marelli, G.; Howells, A.; Lemoine, N.R.; Wang, Y. Oncolytic viral therapy and the immune system: A double-edged sword against cancer. Front. Immunol. 2018, 9, 866. [Google Scholar] [CrossRef] [PubMed]
- Kaliberov, S.A.; Market, J.M.; Gillespie, G.Y.; Krendelchtchikova, V.; della Manna, D.; Sellers, J.C.; Kaliberova, L.N.; Black, M.E.; Buchsbaum, D.J. Mutation of Escherichia coli cytosine deaminase significantly enhances molecular chemotherapy of human glioma. Gene Ther. 2007, 14, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Touraine, R.L.; Vahanian, N.; Ramsey, W.J.; Blaese, R.M. Enhancement of the herpes simplex virus thymidine kinase/ganciclovir bystander effect and its antitumor efficacy in vivo by pharmacologic manipulation of gap junctions. Hum. Gene Ther. 1998, 9, 2385–2391. [Google Scholar] [CrossRef] [PubMed]
- Touraine, R.L.; Ishii-Morita, H.; Ramsey, W.J.; Blaese, R.M. The bystander effect in the HSVtk/ganciclovir system and its relationship to gap junctional communication. Gene Ther. 1998, 5, 1705–1711. [Google Scholar] [CrossRef]
- Fick, J.; Barker, F.G., 2nd; Dazin, P.; Westphale, E.M.; Beyer, E.C.; Israel, M.A. The extent of heterocellular communication mediated by gap junctions is predictive of bystander tumor cytotoxicity in vitro. Proc. Natl. Acad. Sci. USA 1995, 92, 11071–11075. [Google Scholar] [CrossRef]
- Lang, F.F.; Yung, W.K.; Raju, U.; Libunao, F.; Terry, N.H.; Tofilon, P.J. Enhancement of radiosensitivity of wild-type p53 human glioma cells by adenovirus-mediated delivery of the p53 gene. J. Neurosurg. 1998, 89, 125–132. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Reviews, Peer-Reviews, Editorials | Case Reports, Abstracts, and Dissertations |
| Clinical, Pre-clinical Trials | Abandoned Clinical Trials |
| English language, or translated | Non-English language |
| Publications in 2005–2020 decade | Studies prior to 2005 |
| Studies on Human, or Human Products | Animal Studies |
| Neuro-oncology relevance | Publications not related to neuro-oncology |
| Publications about High-Grade Glioma treatment | Publications not related to High-Grade Glioma |
| Vectors | |||||
|---|---|---|---|---|---|
| Viral | Non-Viral | ||||
| AD | HSV | RT | Cationic Liposomes | Polymers (PEI, PAMAM) | |
| Diameter (nm) | 150–200 | 100–300 | 100 | 20–200 | 50–250 |
| Genetic Payload | dsDNA | dsDNA | RNA | dsDNA/RNA | dsDNA/RNA |
| Transduction Efficiency | High | Very High | Medium | High | High |
| Immunogenicity | Very High | Very High | Medium | None | None |
| Mutagenesis Risk | None | None | Yes | None | None |
| Gene Therapies | ||
|---|---|---|
| Oncolytic Virotherapy | Oncolytic viruses | oHSV |
| CRAd | ||
| MV | ||
| PVS-RIPO | ||
| Suicide Gene Therapy | Suicide Genes | TK |
| CD | ||
| PNP | ||
| Tumor Suppressor Gene Therapy | Tumor Suppressor Genes | p53 |
| p16 | ||
| PTEN | ||
| Immunomodulatory Gene Therapy | Immunomodulatory Genes | IFNβ/γ |
| IL-4, IL-12 | ||
| Gene Target Therapy | Target Genes | EGFRvIII |
| VEGF | ||
| # | ClinicalTrials.gov Identifier | Title | Status | Phase | Diseases | # of Pts. Enrolled | Treatment | Locations |
|---|---|---|---|---|---|---|---|---|
| 1 | NCT00028158 | Safety and Effectiveness Study of G207, a Tumor-Killing Virus, in Patients with Recurrent Brain Cancer | Completed | I/II | Glioma Astrocytoma Glioblastoma | 65 | Drug: G207, an oncolytic virus | NA |
| 2 | NCT00157703 | G207 Followed by Radiation Therapy in Malignant Glioma | Completed | I | Malignant Glioma | 9 | Drug: G207, an oncolytic virus | USA |
| 3 | NCT02031965 | Oncolytic HSV-1716 in Treating Younger Patients with Refractory or Recurrent High-Grade Glioma That Can Be Removed by Surgery | Terminated | I | Brain and Central Nervous System Tumors | 2 | Biological: oncolytic HSV-1716; Drug: dexamethasone Procedure: therapeutic conventional surgery | USA |
| 4 | NCT03152318 | A Study of the Treatment of Recurrent Malignant Glioma With rQNestin34.5 v.2 | Recruiting | I | Brain and Central Nervous System Tumors | 108 | Drug: rQNestin, Cyclophosphamide; Procedure: Stereotactic biopsy | USA |
| 5 | NCT02197169 | DNX-2401 With Interferon Gamma (IFN-γ) for Recurrent Glioblastoma or Gliosarcoma Brain Tumors | Completed | I | Glioblastoma Gliosarcoma | 37 | Single intratumoral injection of DNX-2401; Drug: Interferon-gamma | USA |
| 6 | NCT01174537 | New Castle Disease Virus (NDV) in Glioblastoma Multiforme (GBM), Sarcoma and Neuroblastoma | Withdrawn | I/II | Glioblastoma Sarcoma Neuroblastoma | 0 | Biological: New Castle Disease Virus | IL |
| 7 | NCT00390299 | Viral Therapy in Treating Patients With Recurrent Glioblastoma Multiforme | Completed | I | Anaplastic Astrocytoma Anaplastic Oligodendroglioma Mixed Glioma Recurrent Glioblastoma | 23 | Biological: Carcinoembryonic Antigen-Expressing Measles Virus; Therapeutic Conventional Surgery | USA |
| 8 | NCT01301430 | Parvovirus H-1 (ParvOryx) in Patients with Progressive Primary or Recurrent Glioblastoma Multiforme. | Completed | I/II | Glioblastoma Multiforme | 18 | Drug: H-1PV | DE |
| 9 | NCT01582516 | Safety Study of Replication-competent Adenovirus (Delta-24-rgd) in Patients with Recurrent Glioblastoma | Completed | I/II | Brain Tumor Recurring Glioblastoma | 20 | Biological: delta-24-RGD adenovirus | NL |
| 10 | NCT02062827 | Genetically Engineered HSV-1 Phase 1 Study for the Treatment of Recurrent Malignant Glioma | Recruiting | I | Recurrent Glioblastoma Multiforme Progressive Glioblastoma Multiforme Anaplastic Astrocytoma or Gliosarcoma | 36 | Biological: M032 (NSC 733972) | USA |
| 11 | NCT03911388 | HSV G207 in Children with Recurrent or Refractory Cerebellar Brain Tumors | Recruiting | I | Brain and Central Nervous System Tumors | 15 | Biological: G207 | USA |
| 12 | NCT00805376 | DNX-2401 (Formerly Known as Delta-24-RGD-4C) for Recurrent Malignant Gliomas | Completed | I | Brain Cancer Central Nervous System Diseases | 37 | Drug: DNX-2401 Procedure: Tumor Removal | USA |
| 13 | NCT03896568 | Oncolytic Adenovirus DNX-2401 in Treating Patients with Recurrent High-Grade Glioma | Recruiting | I | Brain and Central Nervous System Tumors | 36 | Oncolytic Adenovirus Ad5-DNX-2401 Therapeutic Conventional Surgery | USA |
| 14 | NCT01956734 | Virus DNX2401 and Temozolomide in Recurrent Glioblastoma | Completed | I | Glioblastoma Multiforme Recurrent Tumor | 31 | Procedure: DNX2401 and Temozolomide | ES |
| 15 | NCT02986178 | PVSRIPO in Recurrent Malignant Glioma | Active, not recruiting | II | Malignant Glioma | 122 | PVSRIPO | USA |
| 16 | NCT03973879 | Combination of PVSRIPO and Atezolizumab for Adults with Recurrent Malignant Glioma | Withdrawn | I/II | Malignant Glioma | 0 | Biological: PVSRIPO Drug: Atezolizumab | NA |
| 17 | NCT03043391 | Phase 1b Study PVSRIPO for Recurrent Malignant Glioma in Children | Recruiting | I | Brain and Central Nervous System Tumors | 12 | Biological: Polio/Rhinovirus Recombinant (PVSRIPO) | USA |
| 18 | NCT01491893 | PVSRIPO for Recurrent Glioblastoma (GBM) | Active, not recruiting | I | Glioma Malignant Glioma | 61 | Recombinant nonpathogenic polio-rhinovirus chimera (PVSRIPO) | USA |
| 19 | NCT03072134 | Neural Stem Cell Based Virotherapy of Newly Diagnosed Malignant Glioma | Active, not recruiting | I | Brain and Central Nervous System Tumors | NA | Neural stem cells loaded with an oncolytic adenovirus | NA |
| 20 | NCT03657576 | Trial of C134 in Patients with Recurrent GBM | Active, not recruiting | I | Glioblastoma Multiforme of Brain Anaplastic Astrocytoma of Brain Gliosarcoma of Brain | 24 | Biological: C134 | USA |
| 21 | NCT02798406 | Combination Adenovirus + Pembrolizumab to Trigger Immune Virus Effects | Active, not recruiting | II | Brain and Central Nervous System Tumors | 49 | Biological: DNX-2401 Biological: pembrolizumab | USA |
| 22 | NCT03714334 | DNX-2440 Oncolytic Adenovirus for Recurrent Glioblastoma | Recruiting | I | Glioblastoma Glioblastoma, Adult | 24 | Drug: DNX-2440 injection | ES |
| 23 | NCT03294486 | Safety and Efficacy of the Oncolytic Virus Armed for Local Chemotherapy, TG6002/5-FC, in Recurrent Glioblastoma Patients | Recruiting | I/II | Glioblastoma Brain Cancer | 78 | Drug: Combination of TG6002 and 5-flucytosine (5-FC, Ancotil®) | FR |
| 24 | NCT02457845 | HSV G207 Alone or With a Single Radiation Dose in Children With Progressive or Recurrent Supratentorial Brain Tumors | Active, not recruiting | I | Brain and Central Nervous System Tumors | 12 | Biological: G207 | USA |
| 25 | NCT00006106 | ONYX-015 With Cisplatin and Fluorouracil in Treating Patients with Advanced Head and Neck Cancer | Withdrawn | I | Lip and Oral Cavity Cancer Head and Neck Cancer Oropharyngeal Cancer | 0 | Drug: Cisplatin, Fluorouracil Drug: ONYX-015 | USA |
| 26 | NCT00528684 | Safety and Efficacy Study of REOLYSIN® in the Treatment of Recurrent Malignant Gliomas | Completed | I | Malignant Glioma | 18 | Biological: REOLYSIN® | USA |
| # | ClinicalTrials.gov Identifier | Title | Status | Phase | Diseases | # of Pts. Enrolled | Treatment | Locations |
|---|---|---|---|---|---|---|---|---|
| 1 | NCT00870181 | ADV-TK Improves Outcome of Recurrent High-Grade Glioma | Completed | II | Malignant Glioma of Brain Glioblastoma | 47 | Biological: ADV-TK/GCV Procedure: Surgery Drug: systemic chemotherapy | CHN |
| 2 | NCT00002824 | Gene Therapy in Treating Patients with Primary Brain Tumors | Completed | I | Brain and Central Nervous System Tumors | NA | Biological: gene therapy Drug: chemotherapy, ganciclovir Procedure: conventional surgery | USA |
| 3 | NCT00751270 | Phase 1b Study of AdV-tk + Valacyclovir CombinedWith Radiation Therapy for Malignant Gliomas | Completed | I | Malignant Glioma Glioblastoma Multiforme Anaplastic Astrocytoma | 15 | Biological: AdV-tk Drug: Valacyclovir | USA |
| 4 | NCT03596086 | HSV-tk + Valacyclovir + SBRT + Chemotherapy for Recurrent GBM | Recruiting | I/II | Glioblastoma Multiforme Astrocytoma, Grade III | 62 | Drug: ADV/HSV-tk (gene therapy) | USA |
| 5 | NCT00634231 | A Phase I Study of AdV-tk + Prodrug Therapy in Combination with Radiation Therapy for Pediatric Brain Tumors | Active, not recruiting | I | Malignant Glioma Recurrent Ependymoma | 12 | Biological: AdV-tk Drug: valacyclovir Radiation: Radiation | USA |
| 6 | NCT00589875 | Phase 2a Study of AdV-tk with Standard Radiation Therapy for Malignant Glioma (BrTK02) | Completed | II | Malignant Glioma Glioblastoma Multiforme Anaplastic Astrocytoma | 52 | Biological: AdV-tk Drug: Valacyclovir | USA |
| 7 | NCT03603405 | HSV-tk and XRT and Chemotherapy for Newly Diagnosed GBM | Recruiting | I/II | Glioblastoma Anaplastic Astrocytoma | 62 | Drug: ADV/HSV-tk (gene therapy) | USA |
| 8 | NCT00001328 | Gene Therapy for the Treatment of Brain Tumors | Completed | I | Brain Neoplasm Neoplasm Metastasis | 15 | Drug: Cytovene (Ganciclovir Sodium) Device: G1TKSVNa.53 Producer Cell Line | USA |
| 9 | NCT03576612 | GMCI, Nivolumab, and Radiation Therapy in Treating Patients with Newly Diagnosed High-Grade Gliomas | Active, not recruiting | I | Glioma, Malignant | 36 | Biological: AdV-tk, Nivolumab Drug: Valacyclovir, Temozolomide; Radiation | USA |
| 10 | NCT01985256 | Study of a Retroviral Replicating Vector Given Intravenously to Patients Undergoing Surgery for Recurrent Brain Tumor | Completed | I | Glioblastoma Multiforme Anaplastic Astrocytoma Anaplastic OligodendrogliomaAnaplastic Oligoastrocytoma | 17 | Biological: Toca 511 Drug: Toca FC | USA |
| 11 | NCT01156584 | A Study of a Retroviral Replicating Vector Combined with a Prodrug Administered to Patients with Recurrent Malignant Glioma | Completed | I | Glioblastoma Anaplastic Astrocytoma Anaplastic OligodendrogliomaAnaplastic Oligoastrocytoma | 54 | Biological: Toca 511Drug: Toca FC | USA |
| 12 | NCT01174537 | New Castle Disease Virus (NDV) in Glioblastoma Multiforme (GBM), Sarcoma and Neuroblastoma | Withdrawn | I/II | Glioblastoma Sarcoma Neuroblastoma | 0 | Biological: New Castle Disease Virus | IL |
| 13 | NCT01470794 | Study of a Retroviral Replicating Vector Combined with a Prodrug to Treat Patients Undergoing Surgery for a Recurrent Malignant Brain Tumor | Completed | I | Glioblastoma Multiforme Anaplastic Astrocytoma Anaplastic OligodendrogliomaAnaplastic Oligoastrocytoma | 58 | Biological: Toca 511 Drug: Toca FC | USA |
| 14 | NCT00390299 | Viral Therapy in Treating Patients with Recurrent Glioblastoma Multiforme | Completed | I | Anaplastic Astrocytoma Anaplastic Oligodendroglioma Mixed Glioma Recurrent Glioblastoma | 23 | Biological: Carcinoembryonic Antigen-Expressing Measles Virus; Therapeutic Conventional Surgery | USA |
| 15 | NCT02414165 | The Toca 5 Trial: Toca 511 & Toca FC Versus Standard of Carec in Patients with Recurrent High-Grade Glioma | Terminated | II/III | Glioblastoma Multiforme Anaplastic Astrocytoma | 403 | Biological: Toca 511, Bevacizumab; Drug: Toca FC; Drug: Lomustine, Temozolomide | USA |
| 16 | NCT01811992 | Combined Cytotoxic and Immune-Stimulatory Therapy for Glioma | Active, not recruiting | I | Malignant Glioma Glioblastoma Multiforme | 19 | Dose Escalation of Ad-hCMV-TK and Ad-hCMV-Flt3L | USA |
| 17 | NCT02598011 | A Study of the Safety of Toca 511, a Retroviral Replicating Vector, Combined with Toca FC in Subjects with Newly Diagnosed High Grade Glioma Receiving Standard of Care | Withdrawn | I | Newly Diagnosed High Grade Glioma (HGG) | 0 | Biological: Toca 511 Drug: Toca FC | NA |
| 18 | NCT04406272 | VB-111 in Surgically Accessible Recurrent/Progressive GBM | Recruiting | II | Glioblastoma Recurrent Glioblastoma | 45 | Drug: VB11 Procedure: Surgery Drug: Bevacizumab | USA |
| # | ClinicalTrials.gov Identifier | Title | Status | Phase | Diseases | # of Pts. Enrolled | Treatment | Locations |
|---|---|---|---|---|---|---|---|---|
| 1 | NCT00004041 | Gene Therapy in Treating Patients with Recurrent Malignant Gliomas | Completed | I | Brain and Central Nervous System Tumors | NA | Biological: Ad5CMV-p53 gene; Procedure: conventional surgery | USA |
| 2 | NCT00004080 | Gene Therapy in Treating Patients with Recurrent or Progressive Brain Tumors | Completed | I | Brain and Central Nervous System Tumors | NA | Biological: recombinant adenovirus-p53 SCH-58500; Procedure: conventional surgery | NA |
| # | ClinicalTrials.gov Identifier | Title | Status | Phase | Diseases | # of Pts. Enrolled | Treatment | Locations |
|---|---|---|---|---|---|---|---|---|
| 1 | NCT00031083 | Dose Escalation Study to Determine the Safety of IFN-Beta Gene Transfer in the Treatment of Grade III & Grade IV Gliomas | Completed | I | Glioblastoma Multiforme Anaplastic Astrocytoma Oligoastrocytoma, Mixed Gliosarcoma | 12 | Genetic: Interferon-beta | USA |
| 2 | NCT02026271 | A Study of Ad-RTS-hIL-12 With Veledimex in Subjects with Glioblastoma or Malignant Glioma | Active, not recruiting | I | Glioblastoma Multiforme Anaplastic Oligoastrocytoma | 48 | Biological: Ad-RTS-hIL-12; Drug: veledimex | USA |
| 3 | NCT03679754 | Evaluation of Ad-RTS-hIL-12 + Veledimex in Subjects with Recurrent or Progressive Glioblastoma, a Substudy to ATI001-102 | Active, not recruiting | I | Glioblastoma Multiforme | 36 | Biological: Ad-RTS-hIL-12; Drug: veledimex | USA |
| 4 | NCT03636477 | A Study of Ad-RTS-hIL-12 With Veledimex in Combination With Nivolumab in Subjects with Glioblastoma; a Substudy to ATI001-102 | Active, not recruiting | I | Glioblastoma Multiforme | 21 | Biological: Ad-RTS-hIL-12 Drug: veledimexDrug: Nivolumab | USA |
| 5 | NCT03330197 | A Study of Ad-RTS-hIL-12 + Veledimex in Pediatric Subjects with Brain Tumors Including DIPG | Recruiting | I/II | Pediatric Brain Tumor Diffuse Intrinsic Pontine Glioma | 45 | Biological: Ad-RTS-hIL-12 Oral Veledimex | USA |
| 6 | NCT03866109 | A Study Evaluating Temferon in Patients with Glioblastoma & Unmethylated MGMT | Recruiting | I/II | Glioblastoma Multiforme | 21 | Temferon | IT |
| 7 | NCT03383978 | Intracranial Injection of NK-92/5.28. z Cells in Patients with Recurrent HER2-positive Glioblastoma | Recruiting | I | Glioblastoma Multiforme | 30 | Biological: NK-92/5.28.z | DE |
| 8 | NCT04165941 | Novel Gamma-Delta (γδ)T Cell Therapy for Treatment of Patients With Newly Diagnosed Glioblastoma | Recruiting | I | Brain Tumor Adult | 12 | Biological: DRI cell therapy | USA |
| 9 | NCT04214392 | Chimeric Antigen Receptor (CAR) T Cells with a Chlorotoxin Tumor- Targeting Domain for the Treatment of MPP2 + Recurrent or Progressive Glioblastoma | Recruiting | I | Recurrent Glioblastoma Recurrent Malignant Glioma Recurrent WHO Grade II Glioma Recurrent WHO Grade III Glioma | 36 | Biological: Chlorotoxin (EQ)-CD28-CD3zeta-CD19t- expressing CAR T-lymphocytes | USA |
| 10 | NCT02208362 | Genetically Modified T-cells in Treating Patients with Recurrent or Refractory Malignant Glioma | Recruiting | I | Brain and Central Nervous System Tumors | 92 | IL13Ralpha2-specific Hinge-optimized 4-1BB-co-stimulatory CAR/Truncated CD19-expressing Autologous TN/MEM Cells; IL13Ralpha2-specific Hinge-optimized 41BB-co-stimulatory CAR Truncated CD19-expressing Autologous T-Lymphocytes | USA |
| 11 | NCT00730613 | Cellular Adoptive Immunotherapy Using Genetically Modified T-Lymphocytes in Treating Patients with Recurrent or Refractory High-Grade Malignant Glioma | Completed | I | Brain and Central Nervous System Tumors | 3 | Biological: therapeutic autologous lymphocytes Genetic: gene expression analysis | NA |
| 12 | NCT00005796 | Combination Chemotherapy Plus Gene Therapy in Treating Patients with CNS Tumors | Completed | I | Bone Marrow Suppression Brain and Central Nervous System Tumors | 10 | Filgrastim, gene therapy, lomustine; procarbazine, vincristine sulfate | USA |
| 13 | NCT02444546 | Wild-Type Reovirus in Combination with Sargramostim in Treating Younger Patients with High-Grade Relapsed or Refractory Brain Tumors | Active, not recruiting | I | Brain and Central Nervous System Tumors | 6 | Biological: Sargramostim Biological: Wild-type Reovirus | USA |
| 14 | NCT01082926 | Phase I Study of Cellular Immunotherapy for Recurrent/Refractory Malignant Glioma Using Intratumoral Infusions of GRm13Z40-2, An Allogeneic CD8 + Cytolitic T-Cell Line Genetically Modified to Express the IL 13-Zetakine and HyTK and to be Resistant to Glucocorticoids, in Combination with Interleukin-2 | Completed | I | Brain and Central Nervous System Tumors | 6 | Biological: therapeutic allogeneic lymphocytes; Biological: aldesleukin | USA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giotta Lucifero, A.; Luzzi, S. Against the Resilience of High-Grade Gliomas: Gene Therapies (Part II). Brain Sci. 2021, 11, 976. https://doi.org/10.3390/brainsci11080976
Giotta Lucifero A, Luzzi S. Against the Resilience of High-Grade Gliomas: Gene Therapies (Part II). Brain Sciences. 2021; 11(8):976. https://doi.org/10.3390/brainsci11080976
Chicago/Turabian StyleGiotta Lucifero, Alice, and Sabino Luzzi. 2021. "Against the Resilience of High-Grade Gliomas: Gene Therapies (Part II)" Brain Sciences 11, no. 8: 976. https://doi.org/10.3390/brainsci11080976
APA StyleGiotta Lucifero, A., & Luzzi, S. (2021). Against the Resilience of High-Grade Gliomas: Gene Therapies (Part II). Brain Sciences, 11(8), 976. https://doi.org/10.3390/brainsci11080976