Memory-Guided Saccades in Psychosis: Effects of Medication and Stimulus Location
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participant Selection
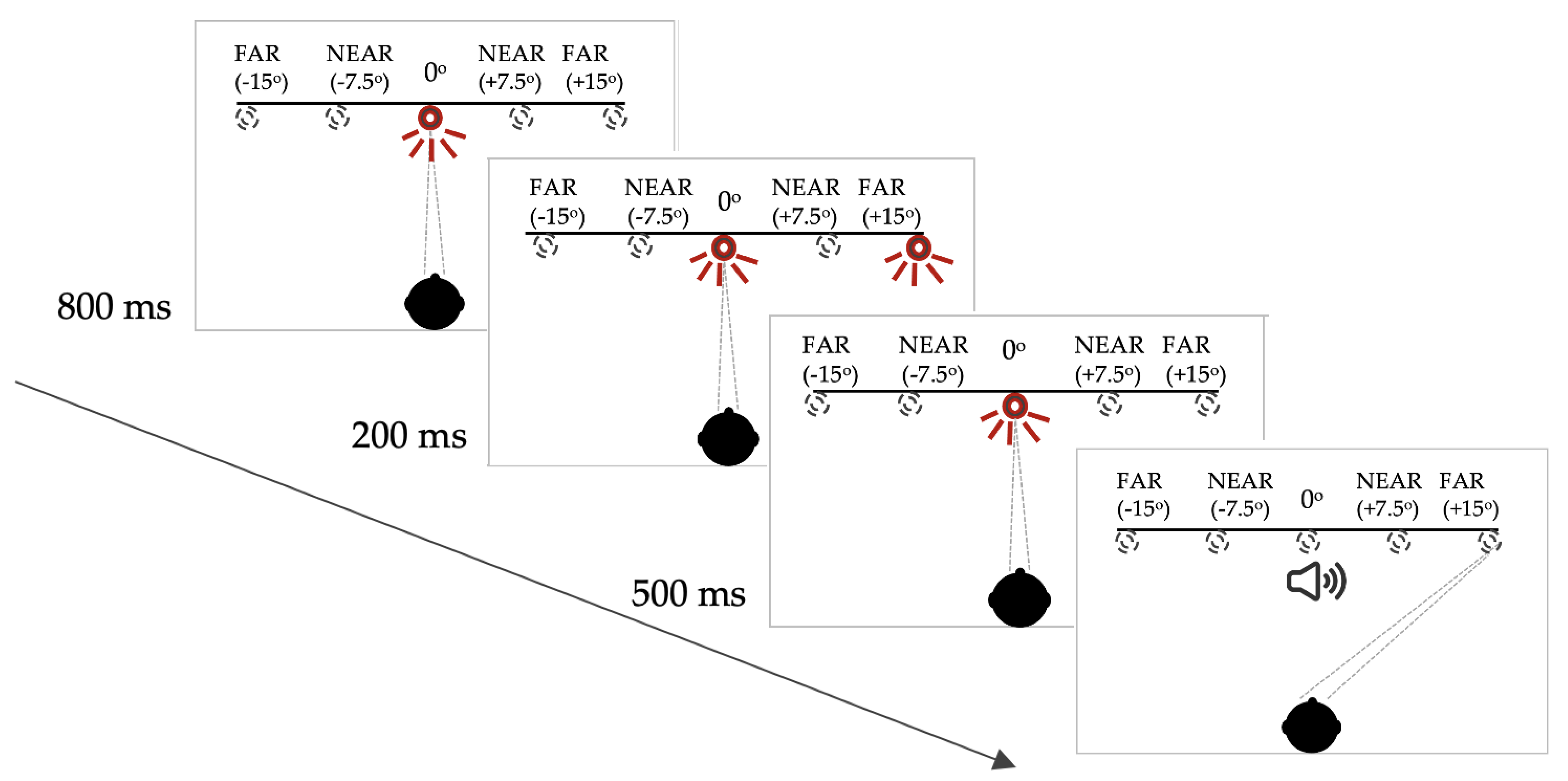
2.2. Measurement of Saccades
2.3. Data Processing and Statistical Analysis
3. Results
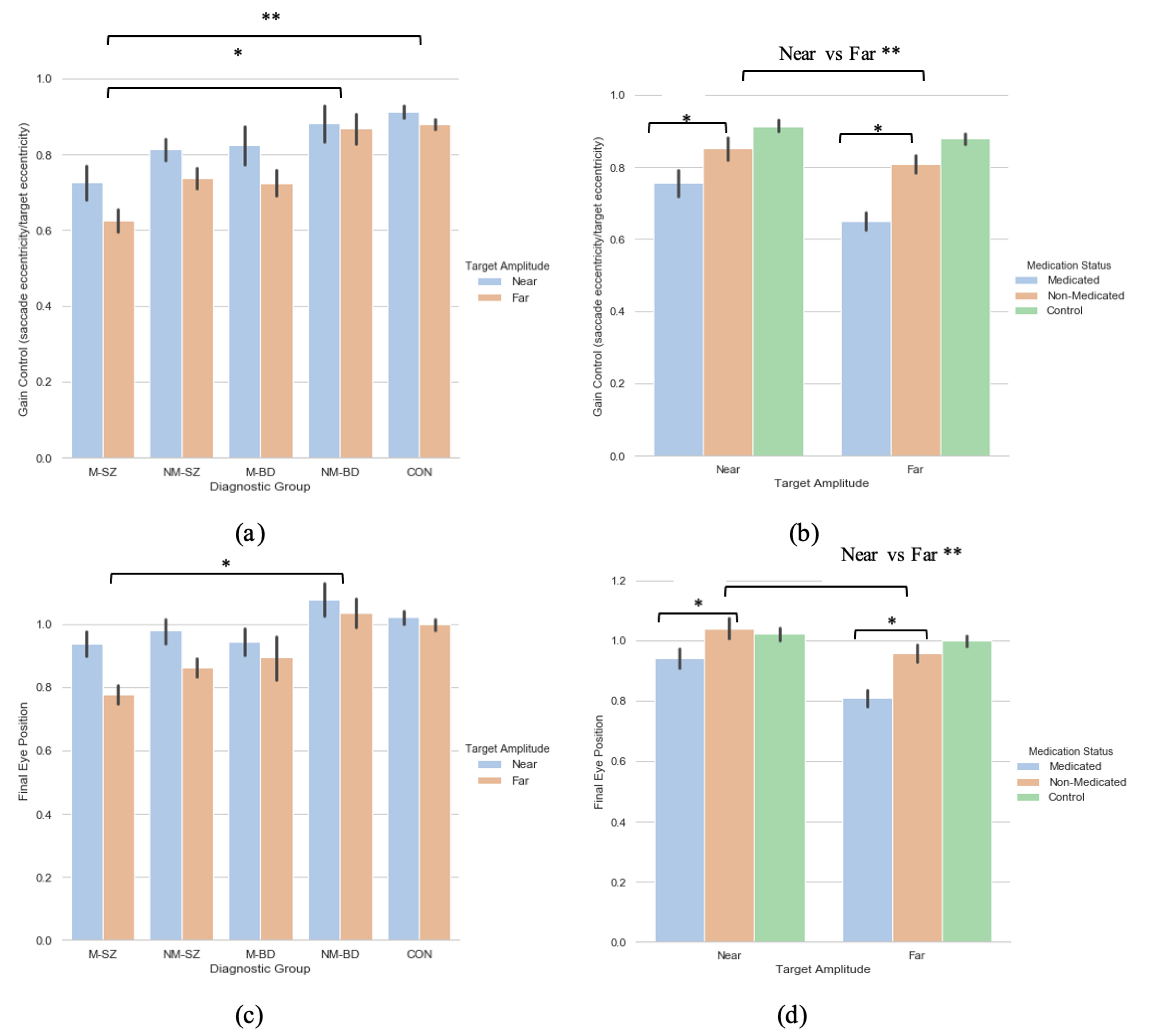
3.1. Diagnosis-Based Analysis
3.1.1. Saccade Primary Saccade Gain
3.1.2. Saccade Final Eye Position
3.1.3. Saccade Latency
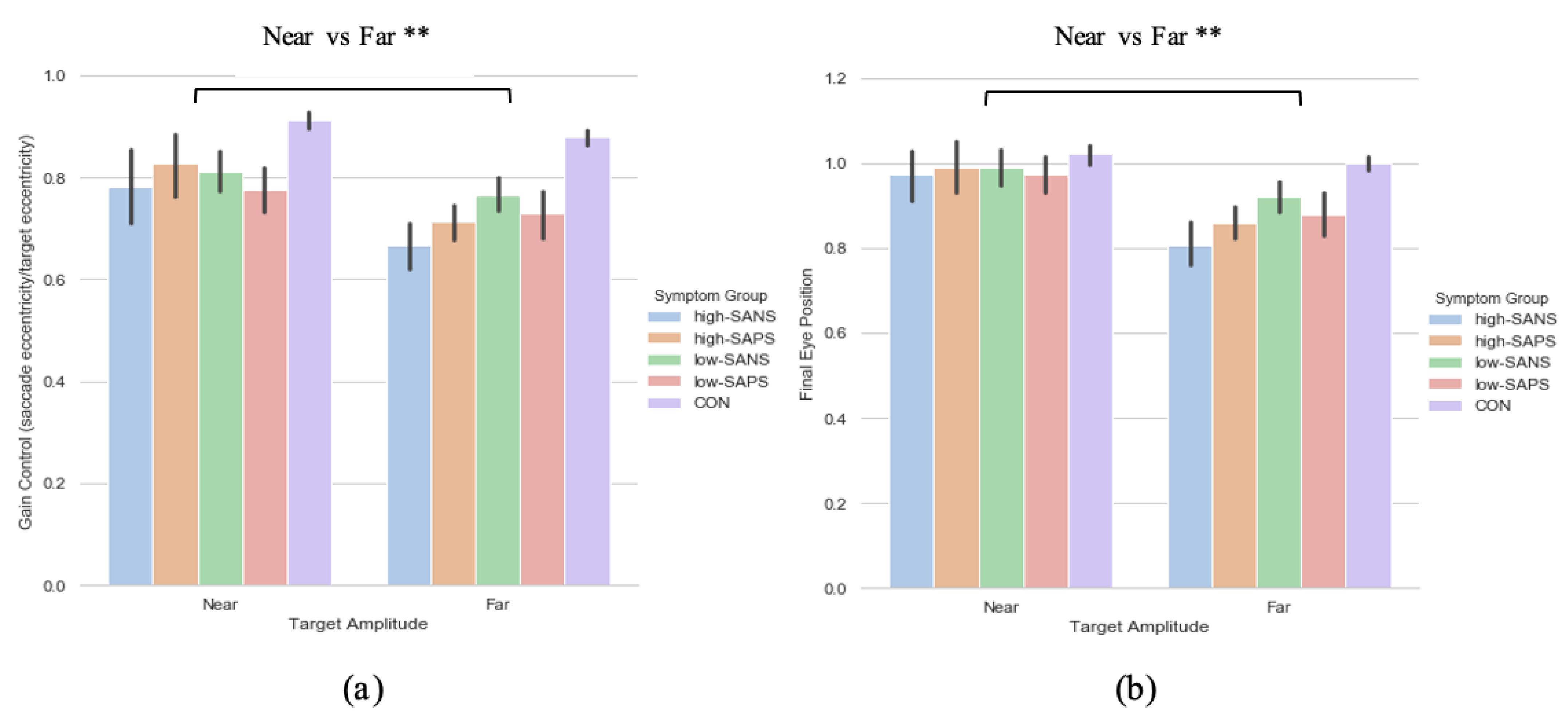
3.2. Symptoms-Based Analysis (1)
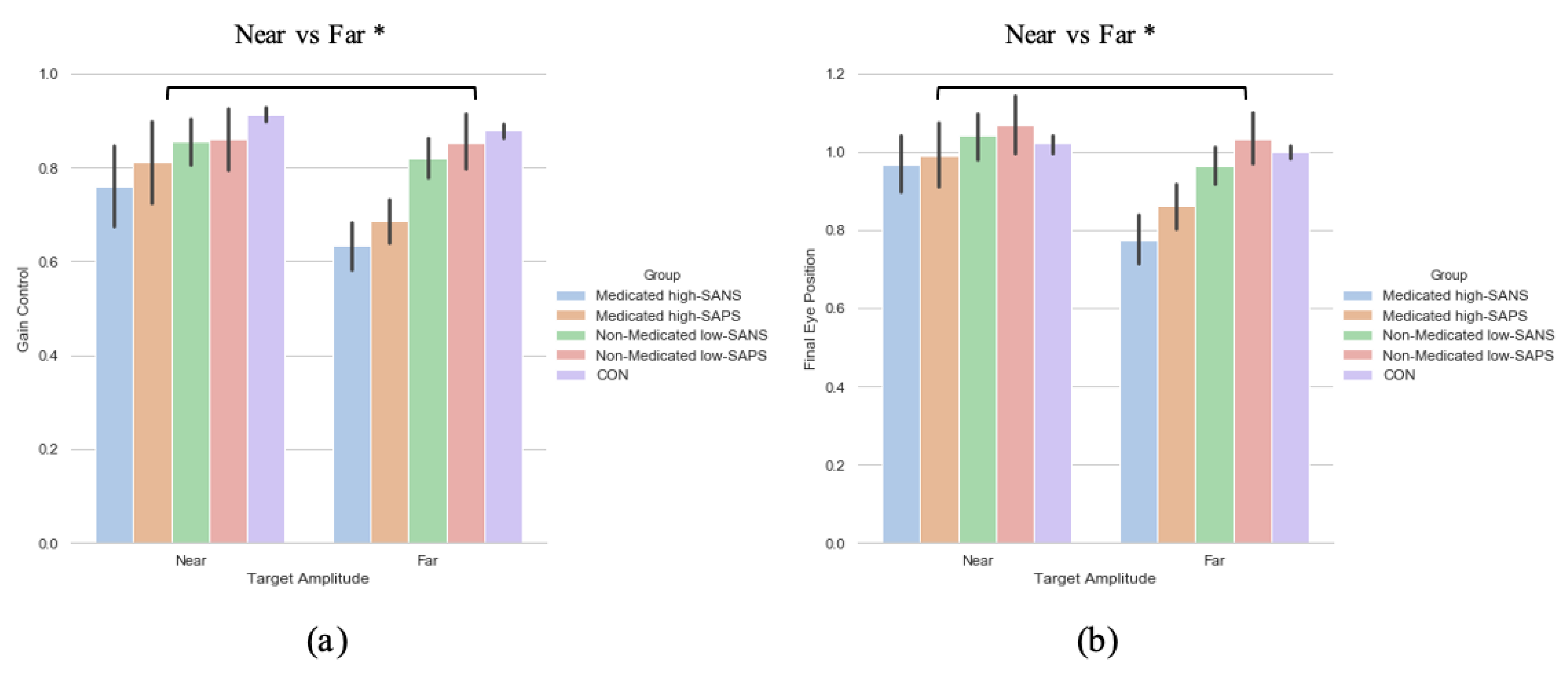
3.2.1. Primary Saccade Gain
3.2.2. Saccade Final Eye Position
3.2.3. Saccade Latency
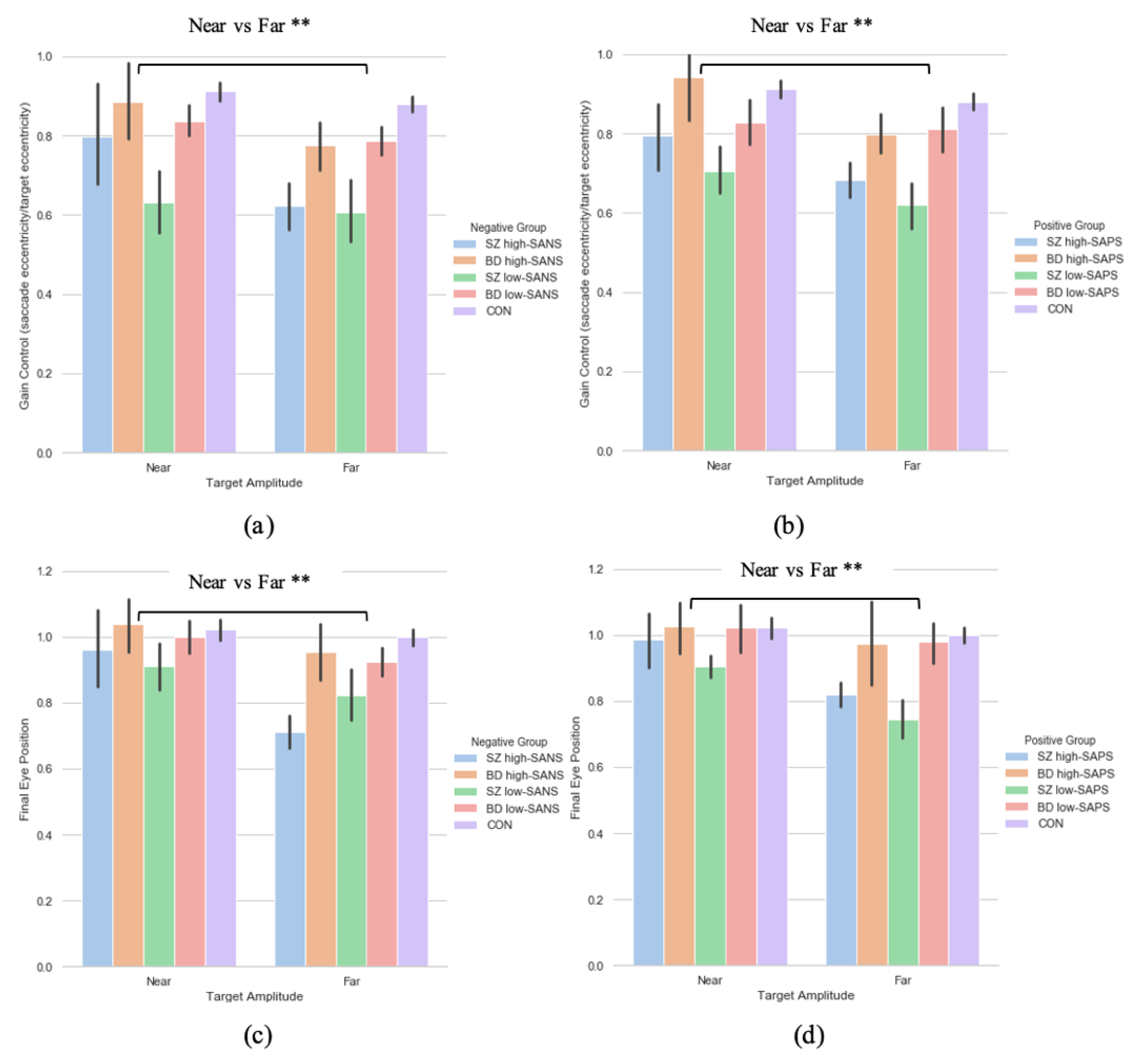
3.3. Symptoms-Based Analysis (2)
3.3.1. Primary Saccade Gain
3.3.2. Saccade Final Eye Position
3.3.3. Saccade Latency
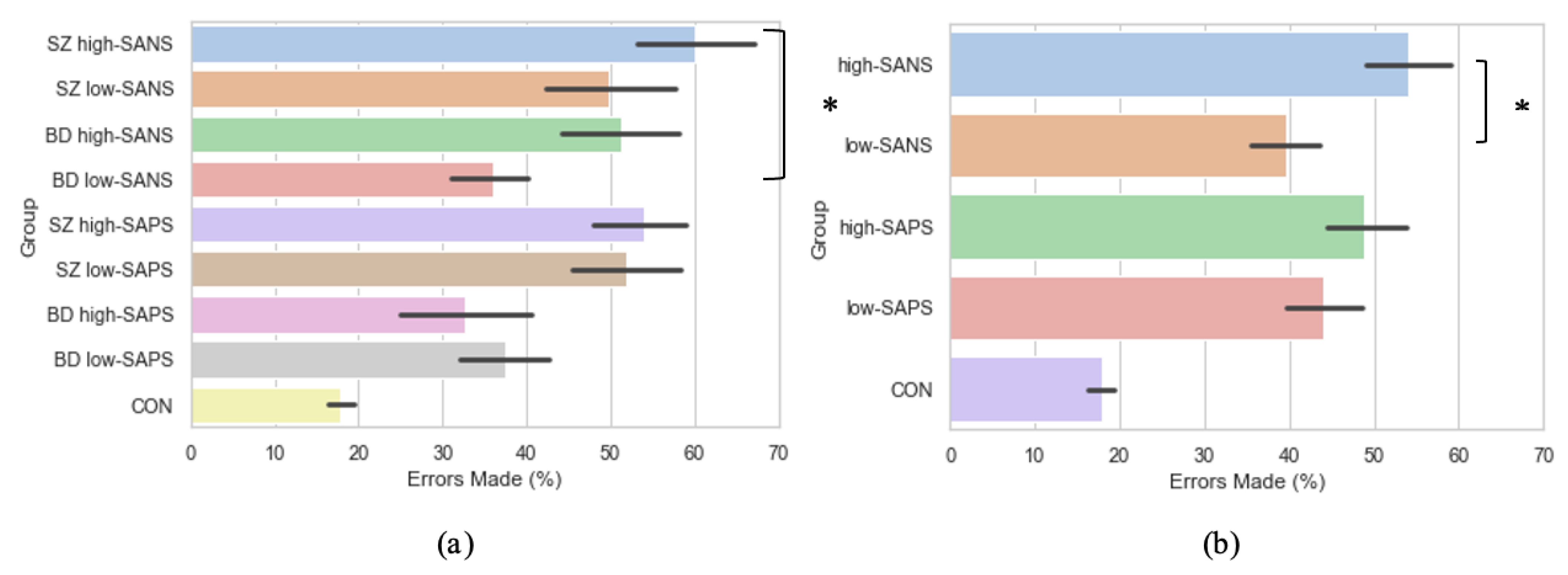
3.4. Neuroleptic Medication on Symptomalogy
3.5. Symptomalogy on Error Rates
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ryan, J.D.; Shen, K. The eyes are a window into memory. Curr. Opin. Behav. Sci. 2020, 32, 1–6. [Google Scholar] [CrossRef]
- Damiano, C.; Walther, D.B. Distinct roles of eye movements during memory encoding and retrieval. Cognition 2019, 184, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.H.; Corneil, B.D.; Munoz, D.P.; Meredith, M.A. Engagement of visual fixation suppresses sensory responsiveness and multisensory integration in the primate superior colliculus. Eur. J. Neurosci. 2003, 18, 2867–2873. [Google Scholar] [CrossRef]
- Munoz, D.P.; Fecteau, J.H. Vying for dominance: Dynamic interactions control visual fixation and saccadic initiation in the superior colliculus. Prog. Brain Res. 2002, 140, 3–19. [Google Scholar] [CrossRef]
- Sawaguchi, T.; Goldman-Rakic, P.S. The role of D1-dopamine receptor in working memory: Local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed-response task. J. Neurophysiol. 1994, 71, 515–528. [Google Scholar] [CrossRef]
- Dias, E.C.; Segraves, M.A. Muscimol-induced inactivation of monkey frontal eye field: Effects on visually and memory-guided saccades. J. Neurophysiol. 1994, 81, 2191–2214. [Google Scholar] [CrossRef]
- Hikosaka, O.; Wurtz, R.H. Visual and oculomotor functions of monkey substantia nigra pars reticulata. IV. Relation of substantia nigra to superior colliculus. J. Neurophysiol. 1983, 49, 1285–1301. [Google Scholar] [CrossRef] [PubMed]
- Muri, R.M.; Gaymard, B.; Rivaud, S.; Vermersch, A.; Hess, C.W.; Pierrot-Deseilligny, C. Hemispheric asymmetry in cortical control of memory-guided saccades. A transcranial magnetic stimulation study. Neuropsychologia 2000, 38, 1105–1111. [Google Scholar] [CrossRef]
- Nyffeler, T.; Pierrot-Deseilligny, C.; Pflugshaupt, T.; von Wartburg, R.; Hess, C.W.; Muri, R.M. Information processing in long delay memory-guided saccades: Further insights from TMS. Exp. Brain Res. 2004, 154, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Ploner, C.J.; Gaymard, B.M.; Rivaud-Pechoux, S.; Baulac, M.; Clemenceau, S.; Samson, S.; Pierrot-Deseilligny, C. Lesions affecting the parahippocampal cortex yield spatial memory deficits in humans. Cereb. Cortex 2000, 10, 1211–1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ploner, C.J.; Rivaud-Pechoux, S.; Gaymard, B.M.; Agid, Y.; Pierrot-Deseilligny, C. Errors of memory-guided saccades in humans with lesions of the frontal eye field and the dorsolateral prefrontal cortex. J. Neurophysiol. 1999, 82, 1086–1090. [Google Scholar] [CrossRef]
- Ringo, J.L.; Sobotka, S.; Diltz, M.D.; Bunce, C.M. Eye movements modulate activity in hippocampal, parahippocampal, and inferotemporal neurons. J. Neurophysiol. 1994, 71, 1285–1288. [Google Scholar] [CrossRef]
- Sobotka, S.; Nowicka, A.; Ringo, J.L. Activity linked to externally cued saccades in single units recorded from hippocampal, parahippocampal, and inferotemporal areas of macaques. J. Neurophysiol. 1997, 78, 2156–2163. [Google Scholar] [CrossRef]
- Liu, Z.-X.; Shen, K.; Olsen, R.K.; Ryan, J.D. Visual Sampling Predicts Hippocampal Activity. J. Neurosci. 2017, 37, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Voss, J.L.; Cohen, N.J. Hippocampal-cortical contributions to strategic exploration during perceptual discrimination. Hippocampus 2017, 27, 642–652. [Google Scholar] [CrossRef]
- Lucas, H.D.; Duff, M.C.; Cohen, N.J. The Hippocampus Promotes Effective Saccadic Information Gathering in Humans. J. Cogn. Neurosci. 2019, 31, 186–201. [Google Scholar] [CrossRef]
- Shen, K.; Bezgin, G.; Selvam, R.; McIntosh, A.R.; Ryan, J.D. An Anatomical Interface between Memory and Oculomotor Systems. J. Cogn. Neurosci. 2016, 28, 1772–1783. [Google Scholar] [CrossRef] [PubMed]
- Johnston, K.; Everling, S. Neurophysiology and neuroanatomy of reflexive and voluntary saccades in non-human primates. Brain Cogn. 2008, 68, 271–283. [Google Scholar] [CrossRef]
- Hoffman, K.L.; Dragan, M.C.; Leonard, T.K.; Micheli, C.; Montefusco-Siegmund, R.; Valiante, T.A. Saccades during visual exploration align hippocampal 3-8 Hz rhythms in human and non-human primates. Front. Syst. Neurosci. 2013, 7, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jutras, M.J.; Fries, P.; Buffalo, E.A. Oscillatory activity in the monkey hippocampus during visual exploration and memory formation. Proc. Natl. Acad. Sci. USA 2013, 110, 13144–13149. [Google Scholar] [CrossRef] [Green Version]
- Meister, M.L.R.; Buffalo, E.A. Getting directions from the hippocampus: The neural connection between looking and memory. Neurobiol. Learn. Mem. 2016, 134 Pt A, 135–144. [Google Scholar] [CrossRef] [Green Version]
- Kragel, J.E.; Vanhaerents, S.; Templer, J.W.; Schuele, S.; Rosenow, J.M.; Nilakantan, A.S.; Bridge, D.J. Hippocampal theta coordinates memory processing during visual exploration. eLife 2020, 9, e52108. [Google Scholar] [CrossRef]
- Crawford, T.J.; Haeger, B.; Kennard, C.; Reveley, M.A.; Henderson, L. Saccadic abnormalities in psychotic patients. I. Neuroleptic-free psychotic patients. Psychol. Med. 1995, 25, 461–471. [Google Scholar] [CrossRef]
- McDowell, J.E.; Clementz, B.A. Behavioral and brain imaging studies of saccadic performance in schizophrenia. Biol. Psychol. 2001, 57, 5–22. [Google Scholar] [CrossRef]
- Everling, S.; Krappmann, P.; Preuss, S.; Brand, A.; Flohr, H. Hypometric primary saccades of schizophrenics in a delayed-response task. Exp. Brain Res. 1996, 111, 289–295. [Google Scholar] [CrossRef]
- Landgraf, S.; Amado, I.; Bourdel, M.C.; Leonardi, S.; Krebs, M.O. Memory-guided saccade abnormalities in schizophrenic patients and their healthy, full biological siblings. Psychol. Med. 2008, 38, 861–870. [Google Scholar] [CrossRef]
- McDowell, J.E.; Clementz, B.A. Ocular-motor delayed-response task performance among schizophrenia patients. Neuropsychobiology 1996, 34, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Müller, N.; Riedel, M.; Eggert, T.; Straube, A. Internally and externally guided voluntary saccades in unmedicated and medicated schizophrenic patients. Part II. Saccadic latency, gain, and fixation suppression errors. Eur. Arch. Psychiatry Clin. Neurosci. 1999, 249, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Holzman, P.S. Schizophrenics show spatial working memory deficits. Arch. Gen. Psychiatry 1992, 49, 975–982. [Google Scholar] [CrossRef]
- Park, S.; Holzman, P.S. Association of working memory deficit and eye tracking dysfunction in schizophrenia. Schizophr. Res. 1993, 11, 55–61. [Google Scholar] [CrossRef]
- Ross, D.E.; Ochs, A.L.; Hill, M.R.; Goldberg, S.C.; Pandurangi, A.K.; Winfrey, C.J. Erratic eye tracking in schizophrenic patients as revealed by high-resolution techniques. Biol. Psychiatry 1998, 24, 675–688. [Google Scholar] [CrossRef]
- Caldani, S.; Bucci, M.P.; Lamy, J.C.; Seassau, M.; Bendjemaa, N.; Gadel, R.; Gaillard, R.; Krebs, M.-O.; Amado, I. Saccadic eye movements as markers of schizophrenia spectrum: Exploration in at-risk mental states. Schizophr. Res. 2017, 181, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Caldani, S.; Amado, I.; Bendjemaa, N.; Vialatte, F.; Mam-Lam-Fook, C.; Gaillard, R.; Krebs, M.-O.; Bucci, M.P. Oculomotricity and neurological soft signs: Can we refine the endophenotype? A study in subjects belonging to the spectrum of schizophrenia. Psychiatry Res. 2017, 256, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Radant, A.D.; Claypoole, K.; Wingerson, D.K.; Cowley, D.S.; Roy-Byrne, P.P. Relationships between neuropsychological and oculomotor measures in schizophrenia patients and normal controls. Biol. Psychiatry 1997, 42, 797–805. [Google Scholar] [CrossRef]
- Calkins, M.E.; Iacono, W.G.; Ones, D.S. Eye movement dysfunction in first-degree relatives of patients with schizophrenia: A meta-analytic evaluation of candidate endophenotypes. Brain Cogn. 2008, 68, 436–461. [Google Scholar] [CrossRef] [Green Version]
- Thomas, E.; Rossell, S.; Tan, E.; Carruthers, S.; Sumner, P.; Bozaoglu, K.; Gurvich, C. T61. Antisaccade and memory guided saccade performance across the schizophrenia continuum. Schizophr. Bull. 2018, 44 (Suppl. 1), S137–S138. [Google Scholar] [CrossRef]
- Thomas, E.H.; Rossell, S.L.; Tan, E.J.; Neill, E.; Van Rheenen, T.E.; Carruthers, S.P.; Sumner, P.J.; Louise, S.; Bozaoglu, K.; Gurvich, C. Do schizotypy dimensions reflect the symptoms of schizophrenia? Aust. N. Z. J. Psychiatry 2019, 53, 236–247. [Google Scholar] [CrossRef] [Green Version]
- Thomas, E.H.; Steffens, M.; Harms, C.; Rossell, S.L.; Gurvich, C.; Ettinger, U. Schizotypy, neuroticism, and saccadic eye movements: New data and meta-analysis. Psychophysiology 2021, 58, e13706. [Google Scholar] [CrossRef]
- Carter, C.; Robertson, L.; Nordahl, T.; Chaderjian, M.; Kraft, L.; O’Shora-Celaya, L. Spatial working memory deficits and their relationship to negative symptoms in unmedicated schizophrenia patients. Biol. Psychiatry 1996, 40, 930–932. [Google Scholar] [CrossRef]
- Curtin, A.; Sun, J.; Zhao, Q.; Onaral, B.; Wang, J.; Tong, S.; Ayaz, H. Visuospatial task-related prefrontal activity is correlated with negative symptoms in schizophrenia. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Wolkin, A.; Sanfilipo, M.; Wolf, A.P.; Angrist, B.; Brodie, J.D.; Rotrosen, J. Negative symptoms and hypofrontality in chronic schizophrenia. Arch. Gen. Psychiatry 1992, 49, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, B.D.; O’Brien, B.A.; Evans, W.J.; McDermott, B.E.; Sautter, F.J.; Winstead, D.K. Abnormal saccadic eye movements associated with positive family history schizophrenics. Biol. Psychiatry 1995, 38, 487–491. [Google Scholar] [CrossRef]
- Norouzi, H.; Tavakoli, N.; Daliri, M.R. Alpha oscillation during the performance of a new variant of working memory-guided saccade task: Evidence from behavioral and electroencephalographic analyses. Int. J. Psychophysiol. 2021, 166, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Crawford, T.J.; Haeger, B.; Kennard, C.; Reveley, M.A.; Henderson, L. Saccadic abnormalities in psychotic patients. II. The role of neuroleptic treatment. Psychol. Med. 1995, 25, 473–483. [Google Scholar] [CrossRef]
- Carvalho, N.; Laurent, E.; Noiret, N.; Chopard, G.; Haffen, E.; Bennabi, D.; Vandel, P. Eye movement in unipolar and bipolar depression: A systematic review of the literature. Front. Psychol. 2015, 6, 1809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correll, C.U.; Schooler, N.R. Negative Symptoms in Schizophrenia: A Review and Clinical Guide for Recognition, Assessment, and Treatment. Neuropsychiatr. Dis. Treat. 2020, 16, 519–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreasen, N.C. The Scale for the Assessment of Negative Symptoms (SANS): Conceptual and theoretical foundations. Br. J. Psychiatry 1984, 155, 49–52. [Google Scholar] [CrossRef]
- Andreasen, N.C. Schedule for the Assessment of Positive Symptoms (SAPS); University of Iowa Press: Iowa City, IA, USA, 1984. [Google Scholar]
- Ettinger, U.; Kumari, V.; Chitnis, X.A.; Corr, P.J.; Crawford, T.J.; Fannon, D.G.; O’Ceallaigh, S.; Sumich, A.L.; Doku, V.C.; Sharma, T. Volumetric neural correlates of antisaccade eye movements in first-episode psychosis. Am. J. Psychiatry 2004, 161, 1918–1921. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, U.; Picchioni, M.; Hall, M.H.; Schulze, K.; Toulopoulou, T.; Landau, S.; Crawford, T.J.; Murray, R.M. Antisaccade performance in monozygotic twins discordant for schizophrenia: The Maudsley twin study. Am. J. Psychiatry 2006, 163, 543–545. [Google Scholar] [CrossRef]
- Tien, A.Y.; Ross, D.E.; Pearlson, G.; Strauss, M.E. Eye movements and psychopathology in schizophrenia and bipolar disorder. J. Nerv. Ment. Dis. 1996, 184, 331–338. [Google Scholar] [CrossRef]
- Winograd-Gurvich, C.; Fitzgerald, P.B.; Georgiou-Karistianis, N.; Millist, L.; White, O. Inhibitory control and spatial working memory: A saccadic eye movement study of negative symptoms in schizophrenia. Psychiatry Res. 2008, 157, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.S.; Crawford, T.J. Positive and Negative Symptoms are associated with distinct effects on Predictive Saccades. Schizophr. Res. Under Review, available upon request.
- Obyedkov, I.; Skuhareuskaya, M.; Skugarevsky, O.; Obyedkov, V.; Buslauski, P.; Skuhareuskaya, T.; Waszkiewicz, N. Saccadic eye movements in different dimensions of schizophrenia and in clinical high-risk state for psychosis. BMC Psychiatry 2019, 19, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, E.S. Memory-Guided Saccades in Psychosis: Effects of Medication and Stimulus Location. Available online: osf.io/ucgv3 (accessed on 16 June 2021).
| Schizophrenia Patients | Bipolar Disorder Patients | Control Participants | |||
|---|---|---|---|---|---|
| Medicated | Non-Medicated | Medicated | Non-Medicated | ||
| Chlorprozamine equivalent units | 1637 (572) | - | 1186 (474) | - | - |
| Age (year) | 39.9 (12.3) | 39.4 (13.2) | 43.6 (12.1) | 42.1 (12.3) | 38.5 (10.8) |
| Disease duration (year) | 13.7 (10.7) | 12.9 (14.8) | 20.5 (11.6) | 14.7 (13.6) | - |
| Age of onset (year) | 26.3 (8.9) | 25.4 (10.6) | 23.1 (8.9) | 27.4 (12.2) | - |
| Negative symptoms (SANS) | 27.5 (17.1) | 21.5 (19.7) | 13.7 (17.6) | 4.6 (6.8) | 1.0 (1.9) |
| Positive symptoms (SAPS) | 16.6 (21.7) | 17.7 (17.8) | 5.1 (9.2) | 3.4 (6.6) | 0.3 (0.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, E.S.; Crawford, T.J. Memory-Guided Saccades in Psychosis: Effects of Medication and Stimulus Location. Brain Sci. 2021, 11, 1071. https://doi.org/10.3390/brainsci11081071
Smith ES, Crawford TJ. Memory-Guided Saccades in Psychosis: Effects of Medication and Stimulus Location. Brain Sciences. 2021; 11(8):1071. https://doi.org/10.3390/brainsci11081071
Chicago/Turabian StyleSmith, Eleanor S., and Trevor J. Crawford. 2021. "Memory-Guided Saccades in Psychosis: Effects of Medication and Stimulus Location" Brain Sciences 11, no. 8: 1071. https://doi.org/10.3390/brainsci11081071
APA StyleSmith, E. S., & Crawford, T. J. (2021). Memory-Guided Saccades in Psychosis: Effects of Medication and Stimulus Location. Brain Sciences, 11(8), 1071. https://doi.org/10.3390/brainsci11081071






