Revisiting the Morphology and Classification of the Paracingulate Gyrus with Commentaries on Ambiguous Cases
Abstract
1. Introduction
2. Materials and Methods
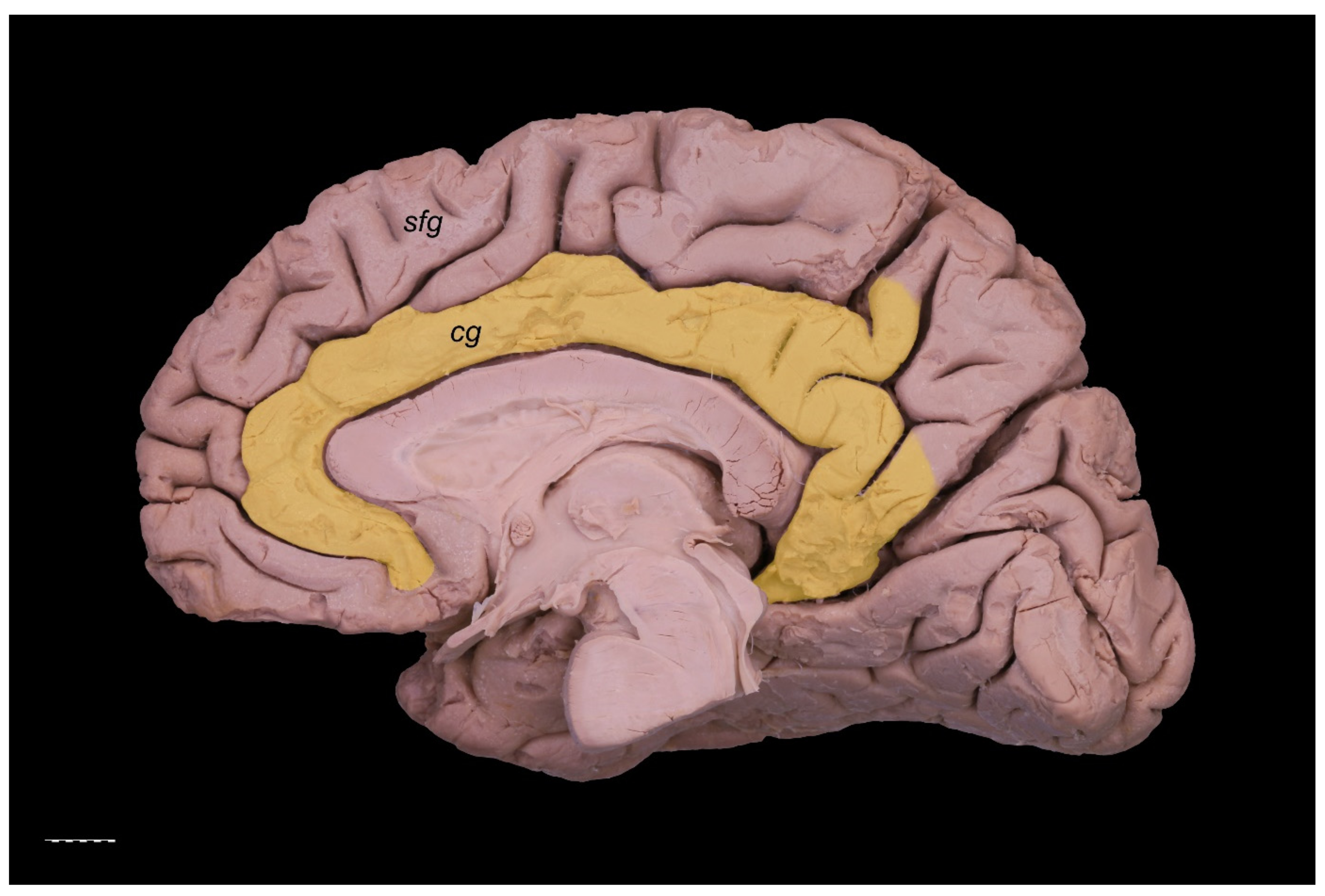
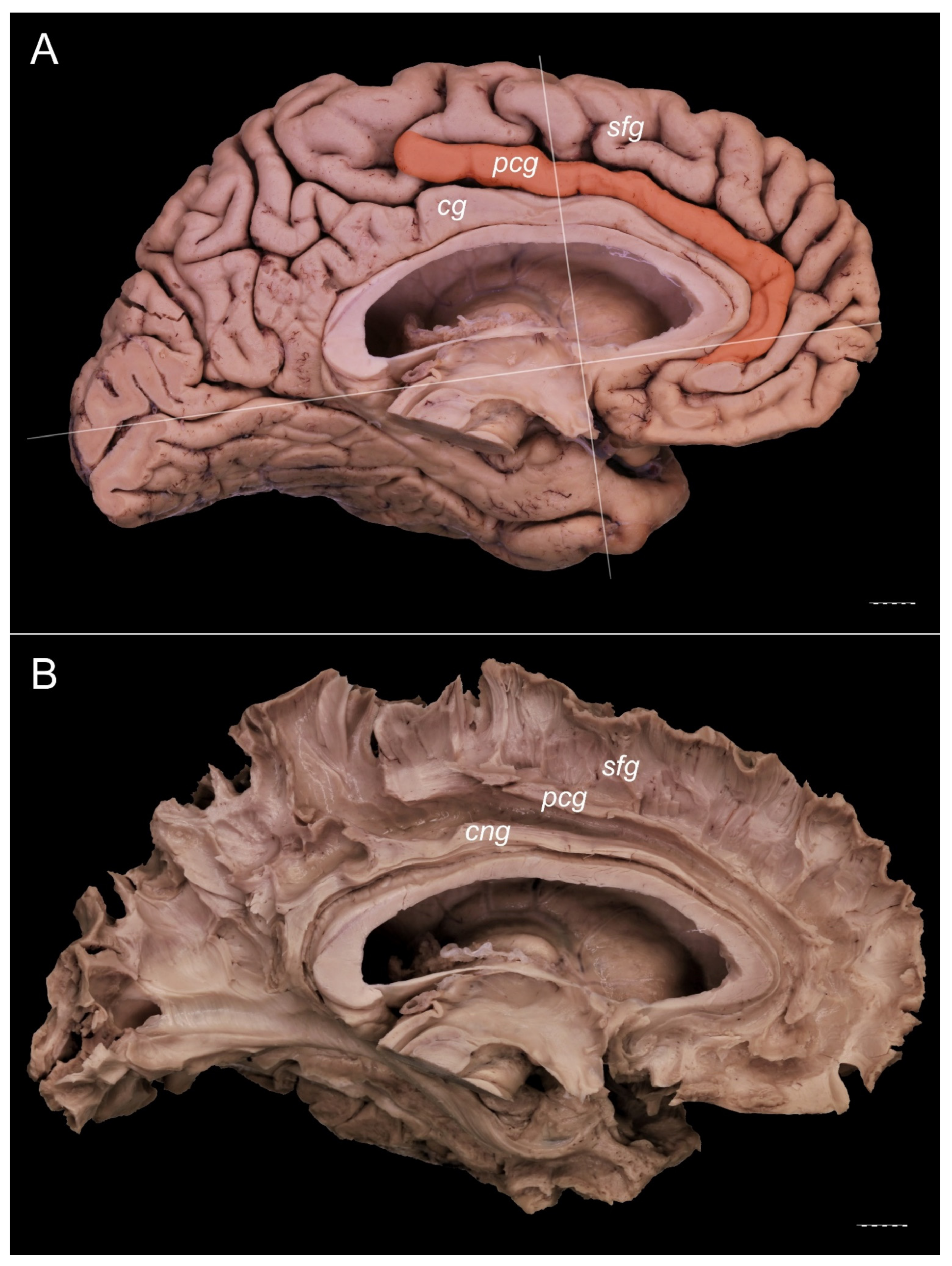
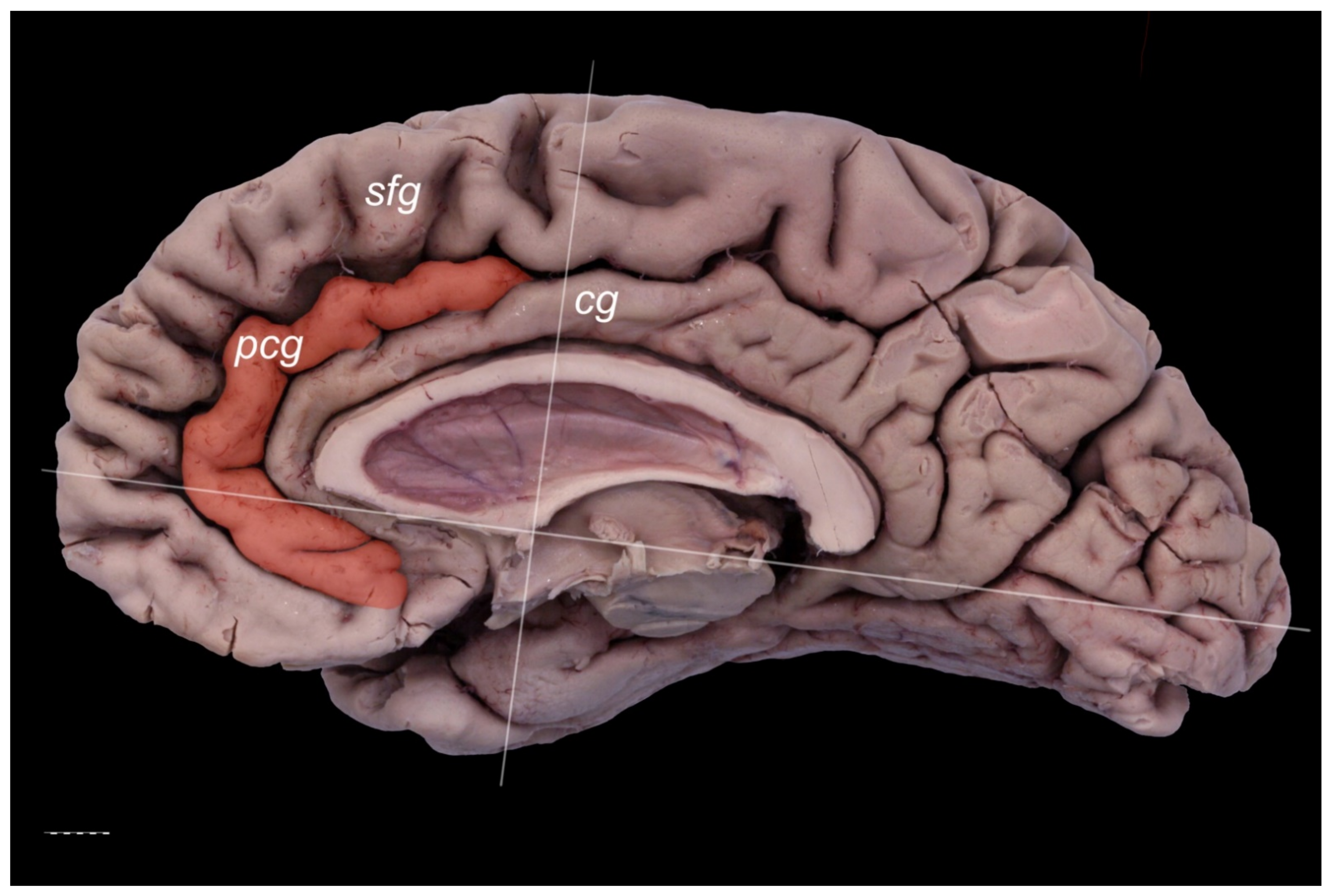
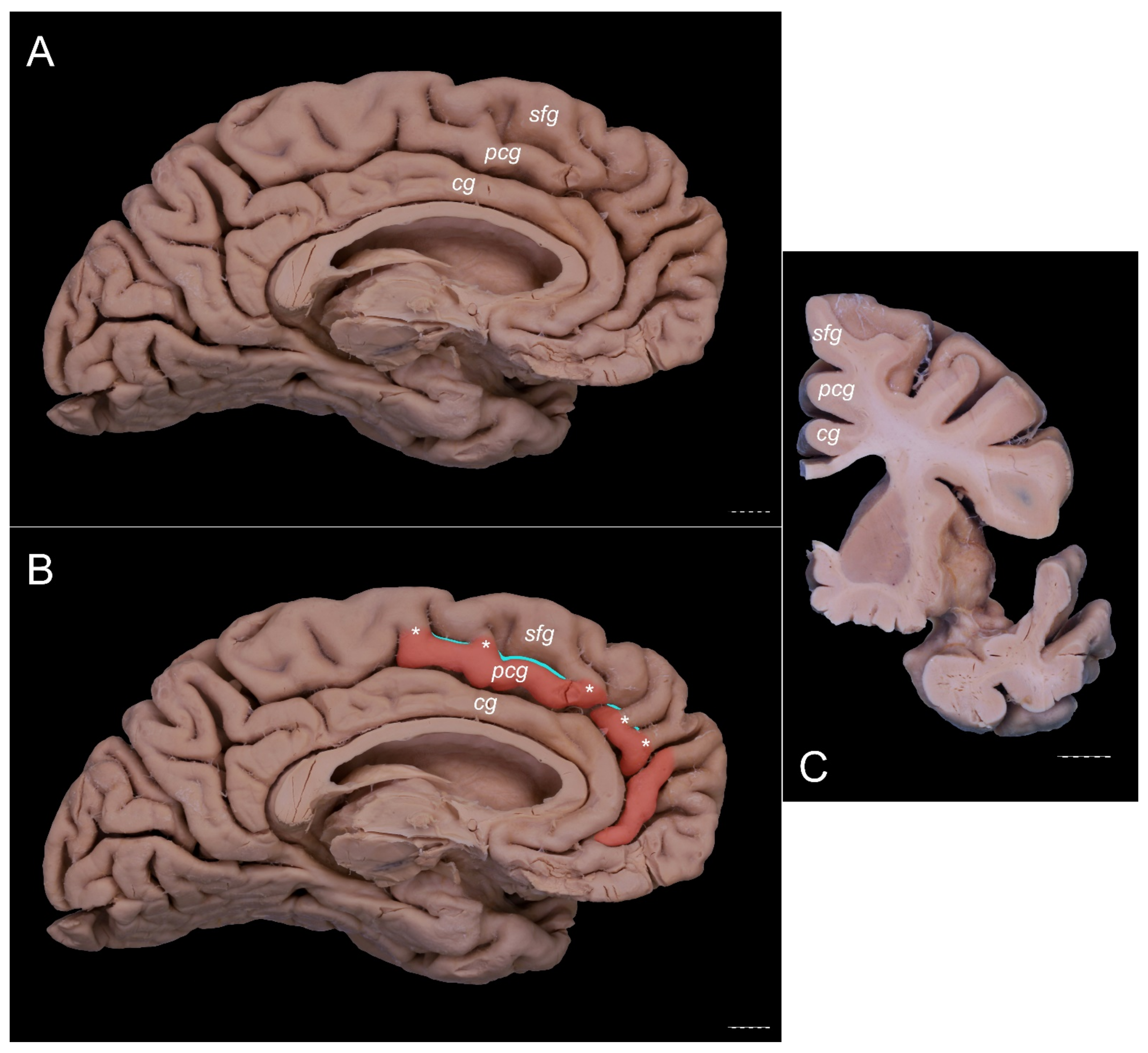
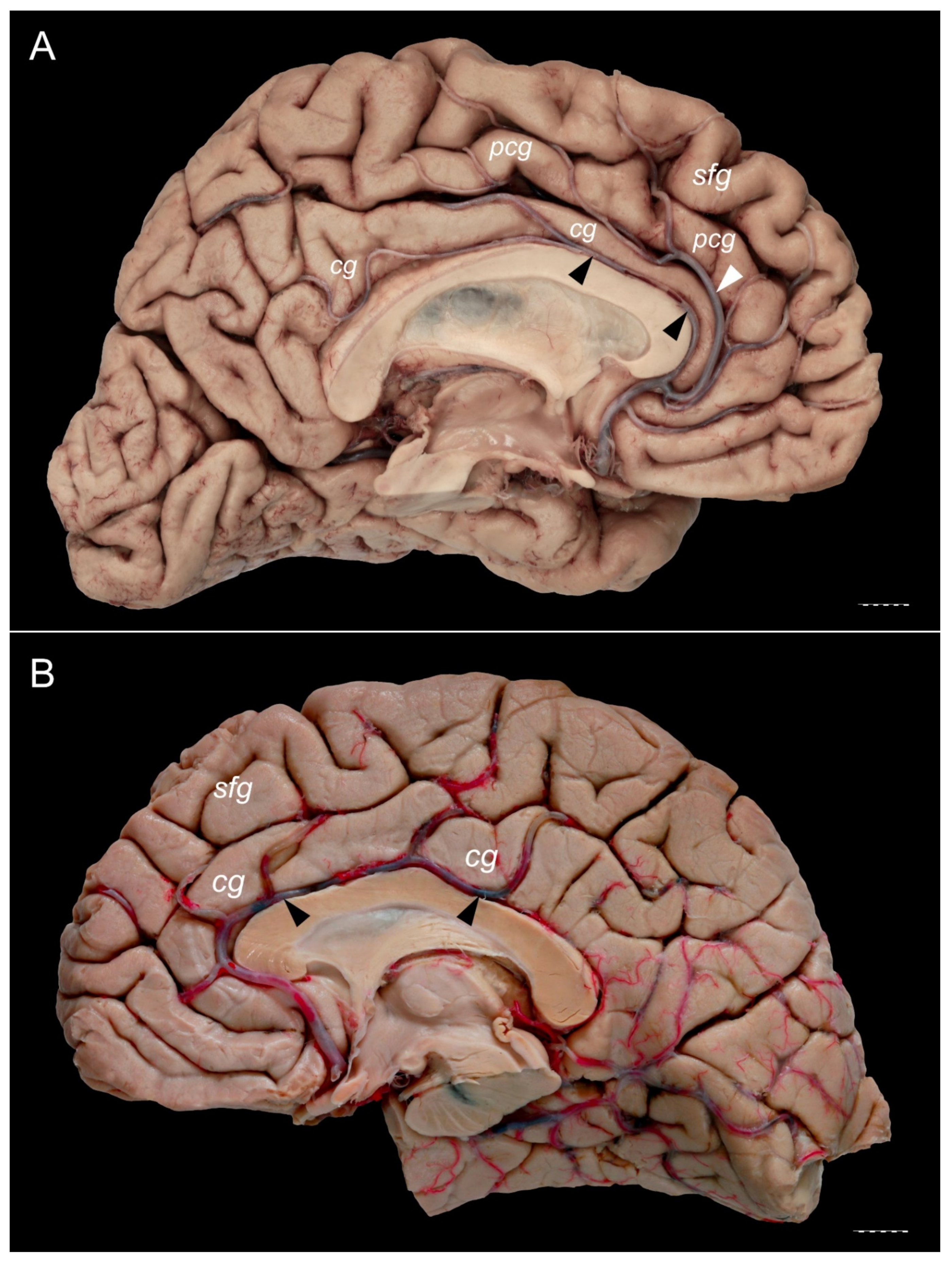
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marshall, J.C.; Fink, G.R. Cerebral localization, then and now. NeuroImage 2003, 20 (Suppl. 1), S2–S7. [Google Scholar] [CrossRef]
- Languis, M.L.; Miller, D.C. Luria’s Theory of Brain Functioning: A Model for Research in Cognitive Psychophysiology. Educ. Psychol. 1992, 27, 493–511. [Google Scholar] [CrossRef]
- Stufflebeam, S.M.; Rosen, B.R. Mapping Cognitive Function. Neuroimaging Clin. N. Am. 2007, 17, 469–484. [Google Scholar] [CrossRef]
- Csonka, M.; Mardmomen, N.; Webster, P.J.; Brefczynski-Lewis, J.A.; Frum, C.; Lewis, J.W. Meta-Analyses Support a Taxonomic Model for Representations of Different Categories of Audio-Visual Interaction Events in the Human Brain. Cereb. Cortex Commun. 2021, 2, tgab002. [Google Scholar] [CrossRef]
- Amiez, C.; Sallet, J.; Novek, J.; Hadj-Bouziane, F.; Giacometti, C.; Andersson, J.; Hopkins, W.D.; Petrides, M. Chimpanzee histology and functional brain imaging show that the paracingulate sulcus is not human-specific. Commun. Biol. 2021, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Żytkowski, A.; Tubbs, R.S.; Iwanaga, J.; Clarke, E.; Polguj, M.; Wysiadecki, G. Anatomical normality and variability: Historical perspective and methodological considerations. Transl. Res. Anat. 2021, 23, 100105. [Google Scholar] [CrossRef]
- Wei, X.; Yin, Y.; Rong, M.; Zhang, J.; Wang, L.; Wu, Y.; Cai, Q.; Yu, C.; Wang, J.; Jiang, T. Paracingulate Sulcus Asymmetry in the Human Brain: Effects of Sex, Handedness, and Race. Sci. Rep. 2017, 7, 42033. [Google Scholar] [CrossRef]
- Yücel, M.; Stuart, G.W.; Maruff, P.; Velakoulis, D.; Crowe, S.F.; Savage, G.; Pantelis, C. Hemispheric and Gender-related Differences in the Gross Morphology of the Anterior Cingulate/Paracingulate Cortex in Normal Volunteers: An MRI Morphometric Study. Cereb. Cortex 2001, 11, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Rametti, G.; Junqué, C.; Bartrés-Faz, D.; Zubiaurre-Elorza, L.; Catalán, R.; Penadés, R.; Bargalló, N.; Bernardo, M.; Bernardo, M. Anterior cingulate and paracingulate sulci morphology in patients with schizophrenia. Schizophr. Res. 2010, 121, 66–74. [Google Scholar] [CrossRef]
- Fornito, A. Individual Differences in Anterior Cingulate/Paracingulate Morphology Are Related to Executive Functions in Healthy Males. Cereb. Cortex 2004, 14, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Amiez, C.; Wilson, C.R.; Procyk, E. Variations of cingulate sulcal organization and link with cognitive performance. Sci. Rep. 2018, 8, 13988. [Google Scholar] [CrossRef]
- Paus, T.; Tomaiuolo, F.; Otaky, N.; Macdonald, D.; Petrides, M.; Atlas, J.; Morris, R.; Evans, A.C. Human Cingulate and Paracingulate Sulci: Pattern, Variability, Asymmetry, and Probabilistic Map. Cereb. Cortex 1996, 6, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Talairach, J.; Tournoux, P. Co-Planar Stereotaxic Atlas of the Human Brain; Thieme Medical Publishers: New York, NY, USA, 1988. [Google Scholar]
- Cox, R. AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Comput. Biomed. Res. 1996, 29, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, E.; Klingler, J. Atlas Cerebri Humani; S. Karger: Basel, Switzerland, 1956; pp. 6, 15–20. [Google Scholar]
- De Castro, I.; Christoph, D.D.H.; Dos Santos, D.P.; Landeiro, J.A. Internal structure of the cerebral hemispheres: An introduction of fiber dissection technique. Arq. Neuro Psiquiatria 2005, 63, 252–258. [Google Scholar] [CrossRef][Green Version]
- Wysiadecki, G.; Clarke, E.; Polguj, M.; Haładaj, R.; Żytkowski, A.; Topol, M. Klingler’s method of brain dissection: Review of the technique including its usefulness in practical neuroanatomy teaching, neurosurgery and neuroimaging. Folia Morphol. 2019, 78, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, T.A.; Balasa, A.; Jeżewski, M.P.; Michałowski, Ł.; Marchel, A. White matter dissection with the Klingler technique: A literature review. Brain Struct. Funct. 2021, 226, 13–47. [Google Scholar] [CrossRef]
- Donkelaar, H.J.T.; Tzourio-Mazoyer, N.; Mai, J.K. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Front. Neuroanat. 2018, 12, 93. [Google Scholar] [CrossRef]
- Ono, M.; Kubik, S.; Abernathey, C.D. Atlas of the Cerebral Sulci; Thieme: New York, NY, USA, 1990. [Google Scholar]
- Le Provost, J.-B.; Bartres-Faz, D.; Paillere-Martinot, M.-L.; Artiges, E.; Pappata, S.; Recasens, C.; Perez-Gomez, M.; Bernardo, M.; Baeza, I.; Bayle, F.; et al. Paracingulate sulcus morphology in men with early-onset schizophrenia. Br. J. Psychiatry 2003, 182, 228–232. [Google Scholar] [CrossRef]
- Yagmurlu, K.; Middlebrooks, E.; Tanriover, N.; Rhoton, A.L. Fiber tracts of the dorsal language stream in the human brain. J. Neurosurg. 2016, 124, 1396–1405. [Google Scholar] [CrossRef]
- Komaitis, S.; Skandalakis, G.P.; Kalyvas, A.V.; Drosos, E.; Lani, E.; Emelifeonwu, J.; Liakos, F.; Piagkou, M.; Kalamatianos, T.; Stranjalis, G.; et al. Dorsal component of the superior longitudinal fasciculus revisited: Novel insights from a focused fiber dissection study. J. Neurosurg. 2020, 132, 1265–1278. [Google Scholar] [CrossRef]
- Paus, T. Primate anterior cingulate cortex: Where motor control, drive and cognition interface. Nat. Rev. Neurosci. 2001, 2, 417–424. [Google Scholar] [CrossRef]
- Niendam, T.A.; Laird, A.R.; Ray, K.L.; Dean, Y.M.; Glahn, D.C.; Carter, C.S. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn. Affect. Behav. Neurosci. 2012, 12, 241–268. [Google Scholar] [CrossRef]
- Gennari, S.P.; Millman, R.E.; Hymers, M.; Mattys, S.L. Anterior paracingulate and cingulate cortex mediates the effects of cognitive load on speech sound discrimination. NeuroImage 2018, 178, 735–743. [Google Scholar] [CrossRef]
- Shackman, A.J.; Salomons, T.V.; Slagter, H.; Fox, A.S.; Winter, J.; Davidson, R.J. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat. Rev. Neurosci. 2011, 12, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Vogt, B.A.; Nimchinsky, E.A.; Vogt, L.J.; Hof, P.R. Human cingulate cortex: Surface features, flat maps, and cytoarchitecture. J. Comp. Neurol. 1995, 359, 490–506. [Google Scholar] [CrossRef] [PubMed]
- Meier, T.B.; Drevets, W.C.; Wurfel, B.E.; Ford, B.N.; Morris, H.M.; Victor, T.A.; Bodurka, J.; Teague, K.; Dantzer, R.; Savitz, J. Relationship between neurotoxic kynurenine metabolites and reductions in right medial prefrontal cortical thickness in major depressive disorder. Brain Behav. Immun. 2016, 53, 39–48. [Google Scholar] [CrossRef]
- Cachia, A.; Borst, G.; Tissier, C.; Fisher, C.; Plaze, M.; Gay, O.; Rivière, D.; Gogtay, N.; Giedd, J.; Mangin, J.-F.; et al. Longitudinal stability of the folding pattern of the anterior cingulate cortex during development. Dev. Cogn. Neurosci. 2016, 19, 122–127. [Google Scholar] [CrossRef]
- Fornito, A.; Wood, S.J.; Whittle, S.; Fuller, J.; Adamson, C.; Saling, M.M.; Velakoulis, D.; Pantelis, C.; Yücel, M. Variability of the paracingulate sulcus and morphometry of the medial frontal cortex: Associations with cortical thickness, surface area, volume, and sulcal depth. Hum. Brain Mapp. 2008, 29, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Fornito, A.; Yücel, M.; Wood, S.J.; Proffitt, T.; McGorry, P.D.; Velakoulis, D.; Pantelis, C. Morphology of the paracingulate sulcus and executive cognition in schizophrenia. Schizophr. Res. 2006, 88, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Garrison, J.R.; Fernyhough, C.; McCarthy-Jones, S.; Haggard, M.; The Australian Schizophrenia Research Bank; Simons, J. Paracingulate sulcus morphology is associated with hallucinations in the human brain. Nat. Commun. 2015, 6, 8956. [Google Scholar] [CrossRef]
- Park, H.Y.; Hwang, J.Y.; Jung, W.H.; Shin, N.Y.; Shim, G.; Jang, J.H.; Kwon, J.S. Altered asymmetry of the anterior cingulate cortex in subjects at genetic high risk for psychosis. Schizophr. Res. 2013, 150, 512–518. [Google Scholar] [CrossRef]
- Koo, M.-S.; Levitt, J.J.; Salisbury, D.F.; Nakamura, M.; Shenton, M.E.; McCarley, R.W. A Cross-Sectional and Longitudinal Magnetic Resonance Imaging Study of Cingulate Gyrus Gray Matter Volume Abnormalities in First-Episode Schizophrenia and First-Episode Affective Psychosis. Arch. Gen. Psychiatry 2008, 65, 746–760. [Google Scholar] [CrossRef] [PubMed]
- Rollins, C.P.E.; Garrison, J.R.; Arribas, M.; Seyedsalehi, A.; Li, Z.; Chan, R.C.K.; Yang, J.; Wang, D.; Liò, P.; Yan, C.; et al. Evidence in cortical folding patterns for prenatal predispositions to hallucinations in schizophrenia. Transl. Psychiatry 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cachia, A.; Amad, A.; Brunelin, J.; Krebs, M.-O.; Plaze, M.; Thomas, P.; Jardri, R. Deviations in cortex sulcation associated with visual hallucinations in schizophrenia. Mol. Psychiatry 2014, 20, 1101–1107. [Google Scholar] [CrossRef]
- Shim, G.; Jung, W.H.; Choi, J.-S.; Jung, M.H.; Jang, J.H.; Park, J.-Y.; Choi, C.-H.; Kang, H.; Kwon, J.S. Reduced cortical folding of the anterior cingulate cortex in obsessive–compulsive disorder. J. Psychiatry Neurosci. 2009, 34, 443–449. [Google Scholar] [PubMed]
- Kay, B.P.; Holland, S.K.; Privitera, M.D.; Szaflarski, J.P. Differences in paracingulate connectivity associated with epileptiform discharges and uncontrolled seizures in genetic generalized epilepsy. Epilepsia 2014, 55, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Szaflarski, J.P.; Kay, B.; Gotman, J.; Privitera, M.D.; Holland, S.K. The relationship between the localization of the generalized spike and wave discharge generators and the response to valproate. Epilepsia 2013, 54, 471–480. [Google Scholar] [CrossRef]
- James, J.C.; Richter, D.; Tomaske, L.; Schneider, R.; Lukas, C.; Kaemmerer, F.; Gold, R.; Krogias, C. Usefulness of Computed Tomographic Perfusion Imaging for Appropriate Diagnosis of Acute Cerebral Vessel Occlusion in Case of Anatomic Variations of the Circle of Willis. Neurointervention 2021. [Google Scholar] [CrossRef] [PubMed]
- Kakou, M.; Destrieux, C.; Velut, S. Microanatomy of the pericallosal arterial complex. J. Neurosurg. 2000, 93, 667–675. [Google Scholar] [CrossRef]
- Kawashima, M.; Matsushima, T.; Sasaki, T. Surgical strategy for distal anterior cerebral artery aneurysms: Microsurgical anatomy. J. Neurosurg. 2003, 99, 517–525. [Google Scholar] [CrossRef]
- Ugur, H.C.; Kahilogullari, G.; Esmer, A.F.; Comert, A.; Odabasi, A.B.; Tekdemir, I.; Elhan, A.; Kanpolat, Y. A neurosurgical view of anatomical variations of the distal anterior cerebral artery: An anatomical study. J. Neurosurg. 2006, 104, 278–284. [Google Scholar] [CrossRef]
- Kahilogullari, G.; Comert, A.; Arslan, M.; Esmer, A.; Tuccar, E.; Elhan, A.; Tubbs, R.; Ugur, H. Callosal branches of the anterior cerebral artery: An anatomical report. Clin. Anat. 2008, 21, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Rădoi, P.M.; Rusu, M.C.; Dincă, D.; Toader, C. Combined rare anatomic variants: Persistent primitive olfactory artery and azygos pericallosal artery. Surg. Radiol. Anat. 2021. [Google Scholar] [CrossRef]
- Kumral, E.; Erdoğan, C.E.; Bayam, F.E.; Arslan, H. Cingulate infarction: A neuropsychological and neuroimaging study. J. Neurol. Sci. 2019, 402, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Labib, M.A.; Gandhi, S.; Cavallo, C.; Nisson, P.L.; Mooney, M.A.; Catapano, J.S.; Lang, M.J.; Chen, T.; Lawton, M.T. Anterior Cerebral Artery Bypass for Complex Aneurysms: Advances in Intracranial-Intracranial Bypass Techniques. World Neurosurg. 2020, 141, e42–e54. [Google Scholar] [CrossRef]
- Acerbi, F.; Vetrano, I.G.; Falco, J.; Gioppo, A.; Ciuffi, A.; Ziliani, V.; Schiariti, M.; Broggi, M.; Faragò, G.; Ferroli, P. In Situ Side-to-Side Pericallosal-Pericallosal Artery and Callosomarginal-Callosomarginal Artery Bypasses for Complex Distal Anterior Cerebral Artery Aneurysms: A Technical Note. Oper. Neurosurg. 2020, 19, E487–E495. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, J.; Singh, V.; Ohtsuka, A.; Hwang, Y.; Kim, H.J.; Moryś, J.; Ravi, K.S.; Ribatti, D.; Trainor, P.A.; Sañudo, J.R.; et al. Acknowledging the use of human cadaveric tissues in research papers: Recommendations from anatomical journal editors. Clin. Anat. 2021, 34, 2–4. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wysiadecki, G.; Mazurek, A.; Walocha, J.; Majos, A.; Tubbs, R.S.; Iwanaga, J.; Żytkowski, A.; Radek, M. Revisiting the Morphology and Classification of the Paracingulate Gyrus with Commentaries on Ambiguous Cases. Brain Sci. 2021, 11, 872. https://doi.org/10.3390/brainsci11070872
Wysiadecki G, Mazurek A, Walocha J, Majos A, Tubbs RS, Iwanaga J, Żytkowski A, Radek M. Revisiting the Morphology and Classification of the Paracingulate Gyrus with Commentaries on Ambiguous Cases. Brain Sciences. 2021; 11(7):872. https://doi.org/10.3390/brainsci11070872
Chicago/Turabian StyleWysiadecki, Grzegorz, Agata Mazurek, Jerzy Walocha, Agata Majos, R. Shane Tubbs, Joe Iwanaga, Andrzej Żytkowski, and Maciej Radek. 2021. "Revisiting the Morphology and Classification of the Paracingulate Gyrus with Commentaries on Ambiguous Cases" Brain Sciences 11, no. 7: 872. https://doi.org/10.3390/brainsci11070872
APA StyleWysiadecki, G., Mazurek, A., Walocha, J., Majos, A., Tubbs, R. S., Iwanaga, J., Żytkowski, A., & Radek, M. (2021). Revisiting the Morphology and Classification of the Paracingulate Gyrus with Commentaries on Ambiguous Cases. Brain Sciences, 11(7), 872. https://doi.org/10.3390/brainsci11070872






