Structural Degradation in Midcingulate Cortex Is Associated with Pathological Aggression in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Housing Conditions
2.2. RI Test
2.3. Perfusion and Tissue Preparation
2.4. Cell Death and Neuronal Density across MCC Layers
2.5. Microglia and Astroglia across MCC Layers
2.6. cFos Staining for Functional Activation of MCC
3. Results
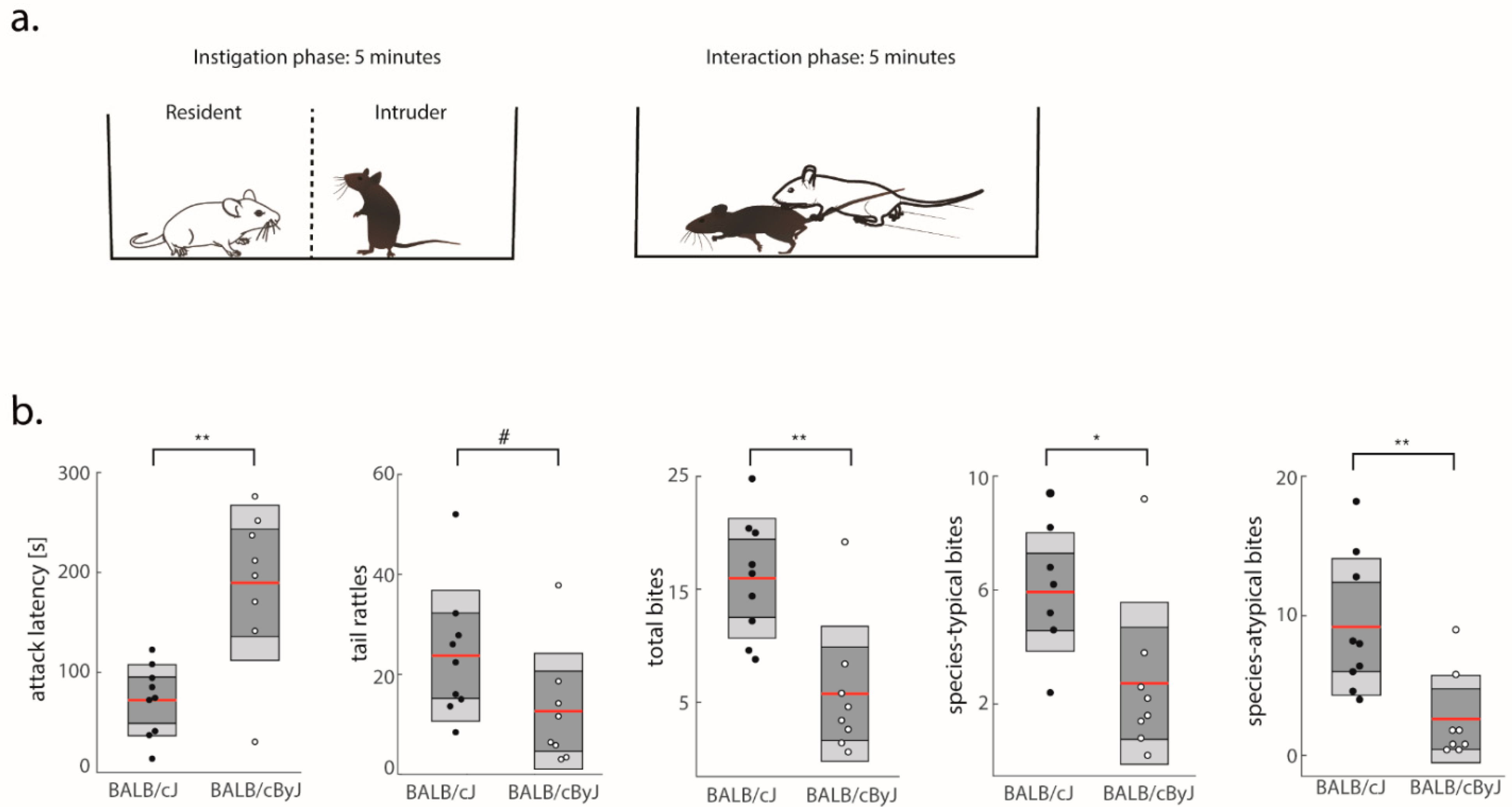
3.1. BALB/cJ Mice Are More Aggressive Than BALB/cByJ Mice
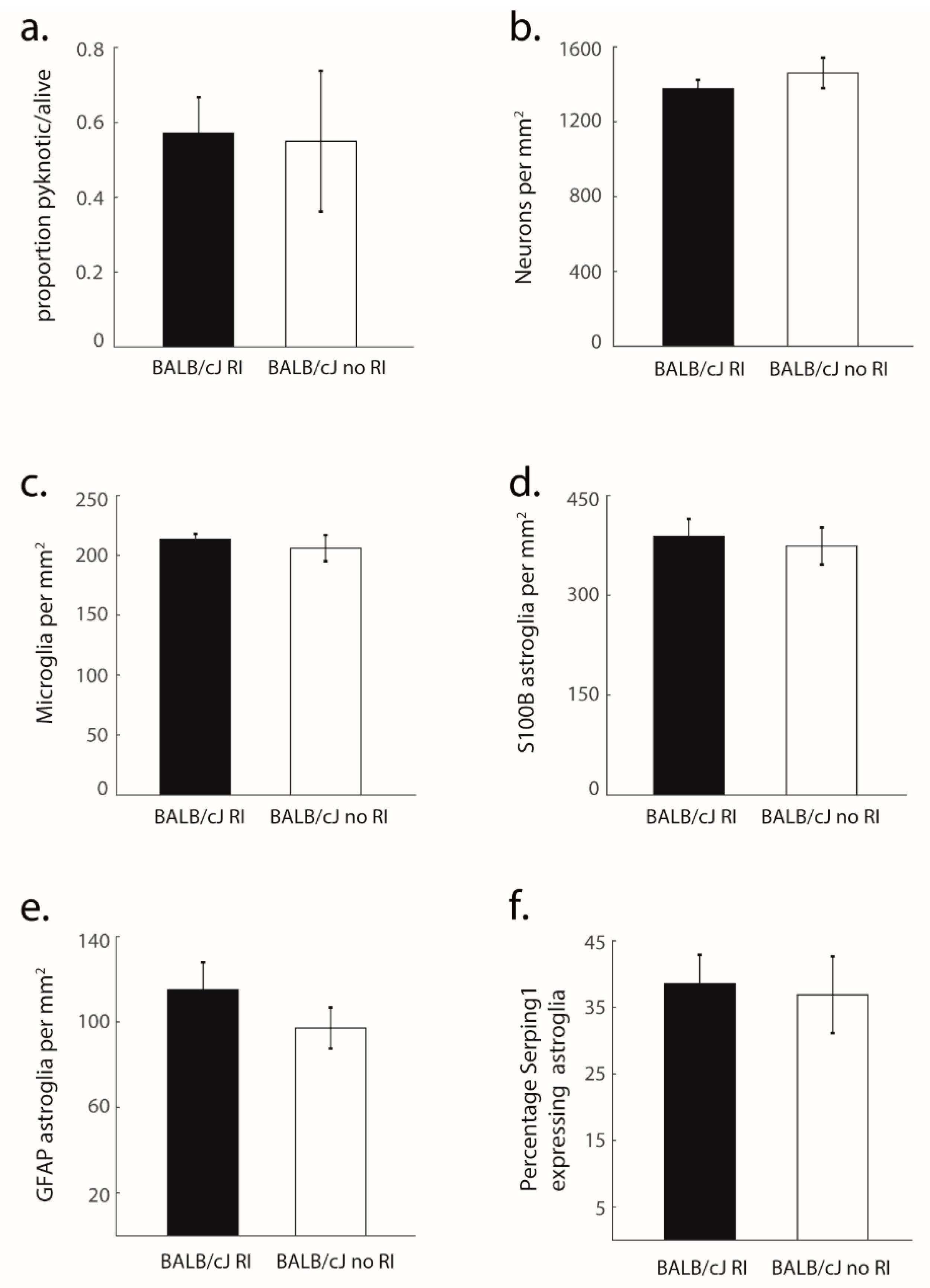
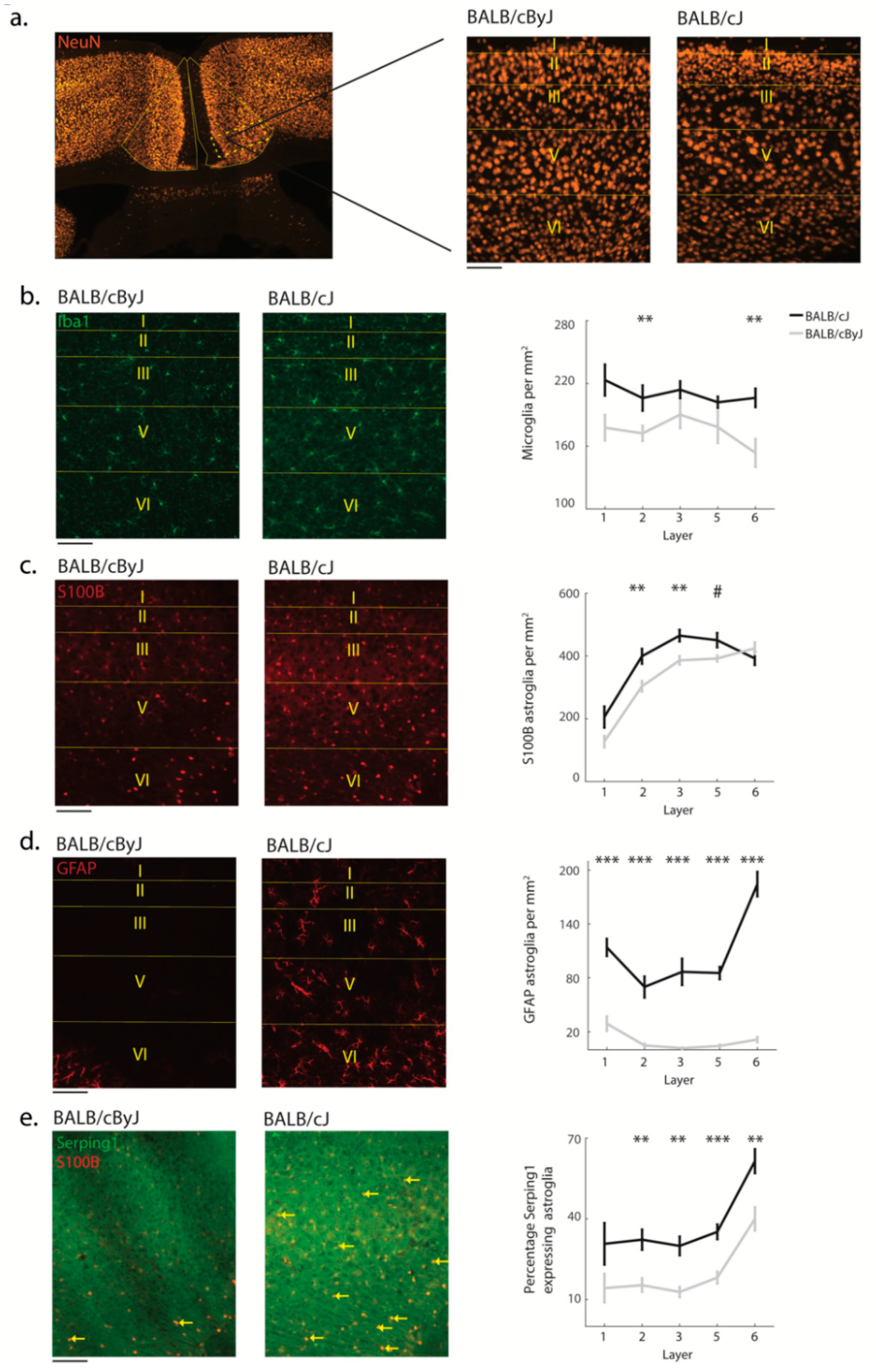
3.2. Structural Degradation in MCC of BALB/cJ Mice
3.3. Glial Processes Are in Line with Neuron Death
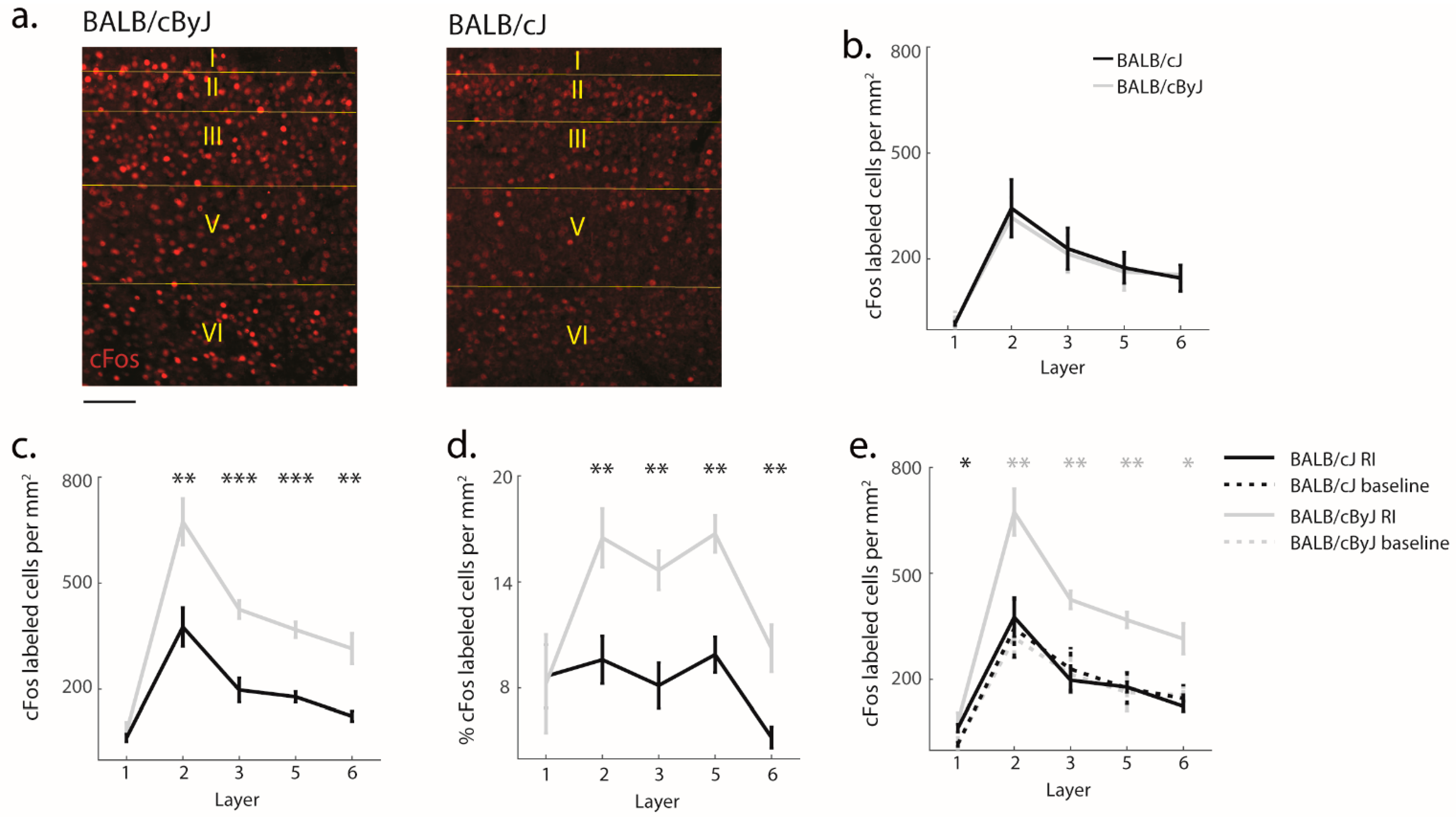
3.4. Functional Implications of Structural Degradation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lischinsky, J.E.; Lin, D. Neural mechanisms of aggression across species. Nat. Neurosci. 2020, 23, 1317–1328. [Google Scholar] [CrossRef]
- Tinbergen, N. On war and peace in animals and man. An ethologist’s approach to the biology of aggression. Science 1968, 160, 1411–1418. [Google Scholar] [CrossRef]
- Anderson, C.A.; Bushman, B.J. Human aggression. Annu. Rev. Psychol. 2002, 53, 27–51. [Google Scholar] [CrossRef] [PubMed]
- Alegria, A.A.; Radua, J.; Rubia, K. Meta-Analysis of fMRI Studies of Disruptive Behavior Disorders. Am. J. Psychiatry 2016, 173, 1119–1130. [Google Scholar] [CrossRef] [Green Version]
- Haller, J.; Kruk, M.R. Normal and abnormal aggression: Human disorders and novel laboratory models. Neurosci. Biobehav. Rev. 2006, 30, 292–303. [Google Scholar] [CrossRef]
- Eastley, R.; Wilcock, G.K. Prevalence and correlates of aggressive behaviours occurring in patients with Alzheimer’s disease. Int. J. Geriatr. Psychiatry 1997, 12, 484–487. [Google Scholar] [CrossRef]
- Aoki, Y.; Inokuchi, R.; Nakao, T.; Yamasue, H. Neural bases of antisocial behavior: A voxel-based meta-analysis. Soc. Cogn. Affect. Neurosci. 2014, 9, 1223–1231. [Google Scholar] [CrossRef]
- Dermody, N.; Wong, S.; Ahmed, R.; Piguet, O.; Hodges, J.R.; Irish, M. Uncovering the Neural Bases of Cognitive and Affective Empathy Deficits in Alzheimer’s Disease and the Behavioral-Variant of Frontotemporal Dementia. J. Alzheimer’s Dis. 2016, 53, 801–816. [Google Scholar] [CrossRef] [PubMed]
- Miskovich, T.A.; Anderson, N.E.; Harenski, C.L.; Harenski, K.A.; Baskin-Sommers, A.R.; Larson, C.L.; Kiehl, K.A. Abnormal cortical gyrification in criminal psychopathy. Neuroimage Clin. 2018, 19, 876–882. [Google Scholar] [CrossRef]
- Sarkheil, P.; Ibrahim, C.N.; Schneider, F.; Mathiak, K.; Klasen, M. Aberrant functional connectivity profiles of brain regions associated with salience and reward processing in female patients with borderline personality disorder. Brain Imaging Behav. 2020, 14, 485–495. [Google Scholar] [CrossRef]
- Wong, T.Y.; Sid, A.; Wensing, T.; Eickhoff, S.B.; Habel, U.; Gur, R.C.; Nickl-Jockschat, T. Neural networks of aggression: ALE meta-analyses on trait and elicited aggression. Brain Struct. Funct. 2019, 224, 133–148. [Google Scholar] [CrossRef]
- Rosell, D.R.; Siever, L.J. The neurobiology of aggression and violence. CNS Spectr. 2015, 20, 254–279. [Google Scholar] [CrossRef]
- Siever, L.J. Neurobiology of aggression and violence. Am. J. Psychiatry 2008, 165, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Miczek, K.A.; de Almeida, R.M.; Kravitz, E.A.; Rissman, E.F.; de Boer, S.F.; Raine, A. Neurobiology of escalated aggression and violence. J. Neurosci. 2007, 27, 11803–11806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boes, A.D.; Tranel, D.; Anderson, S.W.; Nopoulos, P. Right anterior cingulate: A neuroanatomical correlate of aggression and defiance in boys. Behav. Neurosci. 2008, 122, 677–684. [Google Scholar] [CrossRef] [Green Version]
- van Heukelum, S.; Drost, L.; Mogavero, F.; Jager, A.; Havenith, M.N.; Glennon, J.C. Aggression in BALB/cJ mice is differentially predicted by the volumes of anterior and midcingulate cortex. Brain Struct. Funct. 2018, 224, 1009–1019. [Google Scholar] [CrossRef] [Green Version]
- van Heukelum, S.; Mogavero, F.; van de Wal, M.A.; Geers, F.E.; França, A.S.; Buitelaar, J.K.; Havenith, M.N. Gradient of Parvalbumin- and Somatostatin-Expressing Interneurons Across Cingulate Cortex Is Differentially Linked to Aggression and Sociability in BALB/cJ Mice. Front. Psychiatry 2019, 10, 809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterzer, P.; Stadler, C.; Krebs, A.; Kleinschmidt, A.; Poustka, F. Abnormal neural responses to emotional visual stimuli in adolescents with conduct disorder. Biol. Psychiatry 2005, 57, 7–15. [Google Scholar] [CrossRef]
- Kumari, V.; Uddin, S.; Premkumar, P.; Young, S.; Gudjonsson, G.H.; Raghuvanshi, S.; Das, M. Lower anterior cingulate volume in seriously violent men with antisocial personality disorder or schizophrenia and a history of childhood abuse. Aust. N. Z. J. Psychiatry 2014, 48, 153–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Heukelum, S.; Mars, R.; Guthrie, M.; Buitelaar, J.K.; Beckmann, C.F.; Tiesinga, P.H.; Vogt, B.A.; Glennon, J.; Havenith, M.N. Where is Cingulate Cortex? A Cross-Species View. Trends Neurosci. 2020, 43, 285–299. [Google Scholar] [CrossRef]
- Vogt, B.A. Cingulate Neurobiology and Disease; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Vogt, B.A. Midcingulate cortex: Structure, connections, homologies, functions and diseases. J. Chem. Neuroanat. 2016, 74, 28–46. [Google Scholar] [CrossRef]
- Fillinger, C.; Yalcin, I.; Barrot, M.; Veinante, P. Afferents to anterior cingulate areas 24a and 24b and midcingulate areas 24a′ and 24b′ in the mouse. Brain Struct. Funct. 2016, 222, 1509–1532. [Google Scholar] [CrossRef]
- Fillinger, C.; Yalcin, I.; Barrot, M.; Veinante, P. Efferents of anterior cingulate areas 24a and 24b and midcingulate areas 24a′ and 24b′ in the mouse. Brain Struct. Funct. 2017, 223, 1747–1778. [Google Scholar] [CrossRef]
- van Heukelum, S.; Tulva, K.; Geers, F.E.; van Dulm, S.; Ruisch, I.H.; Mill, J.; Viana, J.F.; Beckmann, C.F.; Buitelaar, J.K.; Poelmans, G.; et al. A central role for anterior cingulate cortex in the control of pathological aggression. Curr. Biol. 2021, 31, 2321–2333.e5. [Google Scholar] [CrossRef] [PubMed]
- Biro, L.; Sipos, E.; Bruzsik, B.; Farkas, I.; Zelena, D.; Balázsfi, D.; Toth, M.; Haller, J. Task Division within the Prefrontal Cortex: Distinct Neuron Populations Selectively Control Different Aspects of Aggressive Behavior via the Hypothalamus. J. Neurosci. 2018, 38, 4065–4075. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Nagayasu, K.; Nishitani, N.; Kaneko, S.; Koide, T. Control of Intermale Aggression by Medial Prefrontal Cortex Activation in the Mouse. PLoS ONE 2014, 9, e94657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velez, L.; Sokoloff, G.; Miczek, K.A.; Palmer, A.A.; Dulawa, S.C. Differences in Aggressive Behavior and DNA Copy Number Variants between BALB/cJ and BALB/cByJ Substrains. Behav. Genet. 2009, 40, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Benjamini, Y.H.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Franklin, K.B.J.; Paxinos, G. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates; Academic Press: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Wilhelmsson, U.; Bushong, E.A.; Price, D.L.; Smarr, B.L.; Phung, V.; Terada, M.; Ellisman, M.H.; Pekny, M. Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc. Natl. Acad. Sci. USA 2006, 103, 17513–17518. [Google Scholar] [CrossRef] [Green Version]
- Blanchard, R.J.; O’Donnell, V.; Blanchard, D.C. Attack and defensive behaviors in the albino mouse. Aggress. Behav. 1979, 5, 341–352. [Google Scholar] [CrossRef]
- Newman, E.L.; Terunuma, M.; Wang, T.L.; Hewage, N.; Bicakci, M.B.; Moss, S.J.; DeBold, J.F.; Miczek, K.A. A Role for Prefrontal Cortical NMDA Receptors in Murine Alcohol-Heightened Aggression. Neuropsychopharmacology 2018, 43, 1224–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duran, R.C.D.; Wang, C.-Y.; Zheng, H.; Deneen, B.; Wu, J.Q. Brain Region-Specific Gene Signatures Revealed by Distinct Astrocyte Subpopulations Unveil Links to Glioma and Neurodegenerative Diseases. eNeuro 2019, 6. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Giles, J.M.; Whitaker, J.W.; Moy, S.S.; Fletcher, C.A. Effect of Environmental Enrichment on Aggression in BALB/cJ and BALB/cByJ Mice Monitored by Using an Automated System. J. Am. Assoc. Lab. Anim. Sci. 2018, 57, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Jager, A.; Amiri, H.; Bielczyk, N.; van Heukelum, S.; Heerschap, A.; Aschrafi, A.; Poelmans, G.; Buitelaar, J.K.; Kozicz, T.; Glennon, J. Cortical control of aggression: GABA signalling in the anterior cingulate cortex. Eur. Neuropsychopharmacol. 2020, 30, 5–16. [Google Scholar] [CrossRef]
- Beans, C. News Feature: What happens when lab animals go wild. Proc. Natl. Acad. Sci. USA 2018, 115, 3196–3199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moy, S.S.; Nadler, J.J.; Young, N.B.; Perez, A.; Holloway, L.P.; Barbaro, R.; Barbaro, J.R.; Wilson, L.M.; Threadgill, D.; Lauder, J.M. Mouse behavioral tasks relevant to autism: Phenotypes of 10 inbred strains. Behav. Brain Res. 2007, 176, 4–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lepicard, E.M.; Joubert, C.; Hagneau, I.; Perez-Diaz, F.; Chapouthier, G. Differences in anxiety-related behavior and response to diazepam in BALB/cByJ and C57BL/6J strains of mice. Pharmacol. Biochem. Behav. 2000, 67, 739–748. [Google Scholar] [CrossRef]
- Bailoo, J.D.; Murphy, E.; Varholick, J.; Novak, J.; Palme, R.; Würbel, H. Evaluation of the effects of space allowance on measures of animal welfare in laboratory mice. Sci. Rep. 2018, 8, 713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, D.; Boyle, M.P.; Dollar, P.; Lee, H.; Lein, E.S.; Perona, P.; Anderson, D.J. Functional identification of an aggression locus in the mouse hypothalamus. Nat. Cell Biol. 2011, 470, 221–226. [Google Scholar] [CrossRef] [Green Version]
- Kolling, N.; Wittmann, M.K.; Behrens, T.E.J.; Boorman, E.D.; Mars, R.; Rushworth, M.F.S. Value, search, persistence and model updating in anterior cingulate cortex. Nat. Neurosci. 2016, 19, 1280–1285. [Google Scholar] [CrossRef]
- Heilbronner, S.R.; Hayden, B.Y. Dorsal Anterior Cingulate Cortex: A Bottom-Up View. Annu. Rev. Neurosci. 2016, 39, 149–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bush, G.; Vogt, B.A.; Holmes, J.; Dale, A.M.; Greve, D.; Jenike, M.A.; Rosen, B.R. Dorsal anterior cingulate cortex: A role in reward-based decision making. Proc. Natl. Acad. Sci. USA 2002, 99, 523–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rushworth, M.F.; Behrens, T.E. Choice, uncertainty and value in prefrontal and cingulate cortex. Nat. Neurosci. 2008, 11, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Shackman, A.J.; Salomons, T.V.; Slagter, H.; Fox, A.S.; Winter, J.; Davidson, R.J. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat. Rev. Neurosci. 2011, 12, 154–167. [Google Scholar] [CrossRef]
- Vogt, B.A.; Berger, G.R.; Derbyshire, S.W.G. Structural and functional dichotomy of human midcingulate cortex. Eur. J. Neurosci. 2003, 18, 3134–3144. [Google Scholar] [CrossRef] [Green Version]
- Laubach, M.; Amarante, L.; Swanson, T.K.; White, S. What, If Anything, Is Rodent Prefrontal Cortex? eNeuro 2018, 5. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heukelum, S.v.; Geers, F.E.; Tulva, K.; van Dulm, S.; Beckmann, C.F.; Buitelaar, J.K.; Glennon, J.C.; Vogt, B.A.; Havenith, M.N.; França, A.S.C. Structural Degradation in Midcingulate Cortex Is Associated with Pathological Aggression in Mice. Brain Sci. 2021, 11, 868. https://doi.org/10.3390/brainsci11070868
Heukelum Sv, Geers FE, Tulva K, van Dulm S, Beckmann CF, Buitelaar JK, Glennon JC, Vogt BA, Havenith MN, França ASC. Structural Degradation in Midcingulate Cortex Is Associated with Pathological Aggression in Mice. Brain Sciences. 2021; 11(7):868. https://doi.org/10.3390/brainsci11070868
Chicago/Turabian StyleHeukelum, Sabrina van, Femke E. Geers, Kerli Tulva, Sanne van Dulm, Christian F. Beckmann, Jan K. Buitelaar, Jeffrey C. Glennon, Brent A. Vogt, Martha N. Havenith, and Arthur S. C. França. 2021. "Structural Degradation in Midcingulate Cortex Is Associated with Pathological Aggression in Mice" Brain Sciences 11, no. 7: 868. https://doi.org/10.3390/brainsci11070868
APA StyleHeukelum, S. v., Geers, F. E., Tulva, K., van Dulm, S., Beckmann, C. F., Buitelaar, J. K., Glennon, J. C., Vogt, B. A., Havenith, M. N., & França, A. S. C. (2021). Structural Degradation in Midcingulate Cortex Is Associated with Pathological Aggression in Mice. Brain Sciences, 11(7), 868. https://doi.org/10.3390/brainsci11070868





