Problems with Social Cognition and Decision-Making in Huntington’s Disease: Why Is it Important?
Abstract
1. Introduction
2. Social Impairment in HD
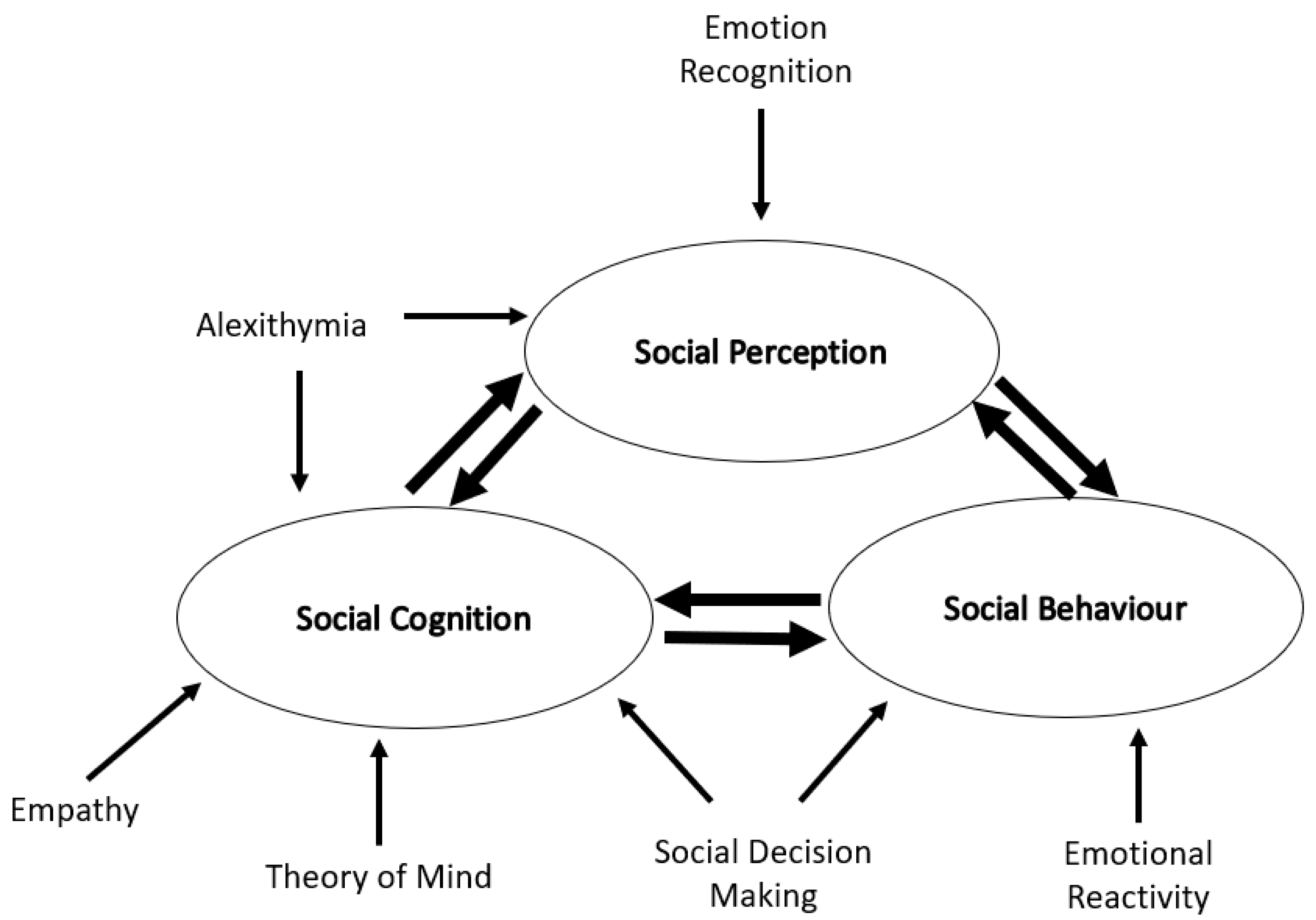
3. How HD Changes Social Cognition
3.1. Emotional Recognition
3.2. Alexithymia
3.3. Theory of Mind
3.4. Empathy
| Processes | Assessment Scales | Neural Correlates | |
|---|---|---|---|
| Emotion The subjective experience of physiological arousal in response to an event. Examples include happiness, sadness, surprise, fear, anger, disgust and contempt | Recognition: The ability to identify emotions in another person. | Ekman faces [71] | Amygdala, insula, globus pallidus, lateral orbitofrontal cortex [72] |
| Awareness (Alexithymia): The ability to identify and describe emotion in oneself. | Toronto Alexithymia Scale [38] Perth Alexithymia Questionnaire [73] | Amygdala, dorsomedial prefrontal cortext, insula, precuneus, dorsal anterior cingulate [39] | |
| Reactivity: The ease at which someone become emotionally aroused and the intensity of emotional experiences | Emotion Reactivity Scale [74] Emotion Intensity Scale [75] | Amygdala, ventromedial prefrontal cortex [76] | |
| Theory of Mind The ability to attribute mental states e.g., beliefs, intents, desires and emotions, to oneself an others Examples include: panicked, playful, jealous, excited | Mentalising: The ability to interpret the mental state of oneself and other. | Reading the Mind in the Eyes [77] Faux Pas [78] Happe-Frith animations [79] | Bilateral temporparietal junction, medial prefrontal cortex, right superior temporal sulcus [80,81] |
| Empathy: The ability to feel what other people are feeling. | Empathy Quotient [82] Multidimensional Emotional Empathy Scale [83] Toronto Empathy Questionnaire [84] | Temporoparietal junction [85], medial prefrontal cortex [86] |
4. How HD Changes Decision Making and Social Decision-Making
5. Potential Treatment Approaches
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Bates, G.P.; Tabrizi, S.J.; Jones, L. Huntington’s Disease, 4th ed.; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Tabrizi, S.J.; Scahill, R.I.; Owen, G.; Durr, A.; Leavitt, B.R.; Roos, R.A.; Borowsky, B.; Landwehrmeyer, B.; Frost, C.; Johnson, H.; et al. Predictors of phenotypic progression and disease onset in premanifest and early-stage Huntington’s disease in the TRACK-HD study: Analysis of 36-month observational data. Lancet Neurol. 2013, 12, 637–649. [Google Scholar] [CrossRef]
- Stout, J.C.; Paulsen, J.S.; Queller, S.; Solomon, A.C.; Whitlock, K.B.; Campbell, J.C.; Carlozzi, N.; Duff, K.; Beglinger, L.J.; Langbehn, D.R.; et al. Neurocognitive signs in prodromal Huntington disease. Neuropsychology 2011, 25, 1–14. [Google Scholar] [CrossRef]
- Beglinger, L.J.; O’Rourke, J.J.; Wang, C.; Langbehn, D.R.; Duff, K.; Paulsen, J.S. Earliest functional declines in Huntington disease. Psychiatry Res. 2010, 178, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.; Zhang, J.; Begeti, F.; Guzman, N.V.; Lezar, A.; Rowe, J.; Barker, R.A.; Hampshire, A. The role of the amygdala during emotional processing in Huntington’s disease: From pre-manifest to late stage disease. Neuropsychologia 2015. [Google Scholar] [CrossRef]
- Dogan, I.; Sass, C.; Mirzazade, S.; Kleiman, A.; Werner, C.J.; Pohl, A.; Schiefer, J.; Binkofski, F.; Schulz, J.B.; Shah, N.J.; et al. Neural correlates of impaired emotion processing in manifest Huntington’s disease. Soc. Cogn. Affect. Neurosci. 2014, 9, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Larsen, I.U.; Vinther-Jensen, T.; Nielsen, J.E.; Gade, A.; Vogel, A. Social Cognition, Executive Functions and Self-Report of Psychological Distress in Huntington’s Disease. PLoS Curr. 2016, 8. [Google Scholar] [CrossRef]
- Henley, S.M.; Novak, M.J.; Frost, C.; King, J.; Tabrizi, S.J.; Warren, J.D. Emotion recognition in Huntington’s disease: A systematic review. Neurosci. Biobehav. Rev. 2012, 36, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.M.; Young, A.W.; Barker, W.A.; Curtis, A.; Gibson, D. Impaired recognition of disgust in Huntington’s disease gene carriers. Brain 1997, 120 Pt 11, 2029–2038. [Google Scholar] [CrossRef]
- Sprengelmeyer, R.; Schroeder, U.; Young, A.W.; Epplen, J.T. Disgust in pre-clinical Huntington’s disease: A longitudinal study. Neuropsychologia 2006, 44, 518–533. [Google Scholar] [CrossRef] [PubMed]
- Hennenlotter, A.; Schroeder, U.; Erhard, P.; Haslinger, B.; Stahl, R.; Weindl, A.; von Einsiedel, H.G.; Lange, K.W.; Ceballos-Baumann, A.O. Neural correlates associated with impaired disgust processing in pre-symptomatic Huntington’s disease. Brain 2004, 127, 1446–1453. [Google Scholar] [CrossRef]
- Montagne, B.; Kessels, R.P.; Kammers, M.P.; Kingma, E.; de Haan, E.H.; Roos, R.A.; Middelkoop, H.A. Perception of emotional facial expressions at different intensities in early-symptomatic Huntington’s disease. Eur. Neurol. 2006, 55, 151–154. [Google Scholar] [CrossRef]
- Sprengelmeyer, R.; Young, A.W.; Calder, A.J.; Karnat, A.; Lange, H.; Homberg, V.; Perrett, D.I.; Rowland, D. Loss of disgust. Perception of faces and emotions in Huntington’s disease. Brain 1996, 119 Pt 5, 1647–1665. [Google Scholar] [CrossRef]
- Wang, K.; Hoosain, R.; Yang, R.M.; Meng, Y.; Wang, C.Q. Impairment of recognition of disgust in Chinese with Huntington’s or Wilson’s disease. Neuropsychologia 2003, 41, 527–537. [Google Scholar] [CrossRef]
- Hayes, C.J.; Stevenson, R.J.; Coltheart, M. Disgust and Huntington’s disease. Neuropsychologia 2007, 45, 1135–1151. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, I.J.; Heims, H.; Neville, E.A.; Rickards, H. Huntington’s disease patients show impaired perception of disgust in the gustatory and olfactory modalities. J. Neuropsychiatry Clin. Neurosci. 2005, 17, 119–121. [Google Scholar] [CrossRef] [PubMed]
- Yitzhak, N.; Gurevich, T.; Inbar, N.; Lecker, M.; Atias, D.; Avramovich, H.; Aviezer, H. Recognition of emotion from subtle and non-stereotypical dynamic facial expressions in Huntington’s disease. Cortex 2020, 126, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Kordsachia, C.C.; Labuschagne, I.; Andrews, S.C.; Stout, J.C. Diminished facial EMG responses to disgusting scenes and happy and fearful faces in Huntington’s disease. Cortex 2018, 106, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Milders, M.; Crawford, J.R.; Lamb, A.; Simpson, S.A. Differential deficits in expression recognition in gene-carriers and patients with Huntington’s disease. Neuropsychologia 2003, 41, 1484–1492. [Google Scholar] [CrossRef]
- Labuschagne, I.; Jones, R.; Callaghan, J.; Whitehead, D.; Dumas, E.M.; Say, M.J.; Hart, E.P.; Justo, D.; Coleman, A.; Dar Santos, R.C.; et al. Emotional face recognition deficits and medication effects in pre-manifest through stage-II Huntington’s disease. Psychiatry Res. 2013, 207, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Ille, R.; Holl, A.K.; Kapfhammer, H.P.; Reisinger, K.; Schafer, A.; Schienle, A. Emotion recognition and experience in Huntington’s disease: Is there a differential impairment? Psychiatry Res. 2011, 188, 377–382. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Robotham, L.; Sauter, D.A.; Bachoud-Levi, A.C.; Trinkler, I. The impairment of emotion recognition in Huntington’s disease extends to positive emotions. Cortex 2011, 47, 880–884. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Snowden, J.S.; Austin, N.A.; Sembi, S.; Thompson, J.C.; Craufurd, D.; Neary, D. Emotion recognition in Huntington’s disease and frontotemporal dementia. Neuropsychologia 2008, 46, 2638–2649. [Google Scholar] [CrossRef] [PubMed]
- De Gelder, B.; Van den Stock, J.; Balaguer Rde, D.; Bachoud-Levi, A.C. Huntington’s disease impairs recognition of angry and instrumental body language. Neuropsychologia 2008, 46, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Calder, A.J.; Keane, J.; Young, A.W.; Lawrence, A.D.; Mason, S.; Barker, R.A. The relation between anger and different forms of disgust: Implications for emotion recognition impairments in Huntington’s disease. Neuropsychologia 2010, 48, 2719–2729. [Google Scholar] [CrossRef] [PubMed]
- Cortes, D.S.; Tornberg, C.; Banziger, T.; Elfenbein, H.A.; Fischer, H.; Laukka, P. Effects of aging on emotion recognition from dynamic multimodal expressions and vocalizations. Sci. Rep. 2021, 11, 2647. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.A.; Stout, J.C.; Solomon, A.C.; Langbehn, D.R.; Aylward, E.H.; Cruce, C.B.; Ross, C.A.; Nance, M.; Kayson, E.; Julian-Baros, E.; et al. Beyond disgust: Impaired recognition of negative emotions prior to diagnosis in Huntington’s disease. Brain 2007, 130, 1732–1744. [Google Scholar] [CrossRef]
- Novak, M.J.; Warren, J.D.; Henley, S.M.; Draganski, B.; Frackowiak, R.S.; Tabrizi, S.J. Altered brain mechanisms of emotion processing in pre-manifest Huntington’s disease. Brain 2012, 135, 1165–1179. [Google Scholar] [CrossRef]
- Kipps, C.M.; Duggins, A.J.; McCusker, E.A.; Calder, A.J. Disgust and happiness recognition correlate with anteroventral insula and amygdala volume respectively in preclinical Huntington’s disease. J. Cogn. Neurosci. 2007, 19, 1206–1217. [Google Scholar] [CrossRef]
- Zarotti, N.; Simpson, J.; Fletcher, I.; Squitieri, F.; Migliore, S. Exploring emotion regulation and emotion recognition in people with presymptomatic Huntington’s disease: The role of emotional awareness. Neuropsychologia 2018, 112, 1–9. [Google Scholar] [CrossRef]
- Zarotti, N.; Fletcher, I.; Simpson, J. New Perspectives on Emotional Processing in People with Symptomatic Huntington’s Disease: Impaired Emotion Regulation and Recognition of Emotional Body Languagedagger. Arch. Clin. Neuropsychol. 2019, 34, 610–624. [Google Scholar] [CrossRef]
- Sprengelmeyer, R.; Young, A.W.; Baldas, E.M.; Ratheiser, I.; Sutherland, C.A.M.; Muller, H.P.; Gron, G.; Sussmuth, S.D.; Landwehrmeyer, G.B.; Orth, M. The neuropsychology of first impressions: Evidence from Huntington’s disease. Cortex 2016, 85, 100–115. [Google Scholar] [CrossRef]
- Campellone, T.R.; Kring, A.M. Who do you trust? The impact of facial emotion and behaviour on decision making. Cogn. Emot. 2013, 27, 603–620. [Google Scholar] [CrossRef] [PubMed]
- Kornreich, C.; Philippot, P.; Foisy, M.L.; Blairy, S.; Raynaud, E.; Dan, B.; Hess, U.; Noel, X.; Pelc, I.; Verbanck, P. Impaired emotional facial expression recognition is associated with interpersonal problems in alcoholism. Alcohol. Alcohol. 2002, 37, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Clark, U.S.; Neargarder, S.; Cronin-Golomb, A. Specific impairments in the recognition of emotional facial expressions in Parkinson’s disease. Neuropsychologia 2008, 46, 2300–2309. [Google Scholar] [CrossRef]
- Narme, P.; Mouras, H.; Roussel, M.; Duru, C.; Krystkowiak, P.; Godefroy, O. Emotional and cognitive social processes are impaired in Parkinson’s disease and are related to behavioral disorders. Neuropsychology 2013, 27, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Luminet, O.; Bagby, R.M.; Taylor, G.J. Alexithymia: Advances in Research, Theory, and Clinical Practice; Cambridge University Press: Cambridge, UK, 2018. [Google Scholar]
- Bagby, R.M.; Taylor, G.J.; Parker, J.D. The Twenty-item Toronto Alexithymia Scale--II. Convergent, discriminant, and concurrent validity. J. Psychosom. Res. 1994, 38, 33–40. [Google Scholar] [CrossRef]
- Van der Velde, J.; Servaas, M.N.; Goerlich, K.S.; Bruggeman, R.; Horton, P.; Costafreda, S.G.; Aleman, A. Neural correlates of alexithymia: A meta-analysis of emotion processing studies. Neurosci. Biobehav. Rev. 2013, 37, 1774–1785. [Google Scholar] [CrossRef]
- Goerlich-Dobre, K.S.; Witteman, J.; Schiller, N.O.; van Heuven, V.J.; Aleman, A.; Martens, S. Blunted feelings: Alexithymia is associated with a diminished neural response to speech prosody. Soc. Cogn. Affect. Neurosci. 2014, 9, 1108–1117. [Google Scholar] [CrossRef]
- Wingbermuhle, E.; Theunissen, H.; Verhoeven, W.M.; Kessels, R.P.; Egger, J.I. The neurocognition of alexithymia: Evidence from neuropsychological and neuroimaging studies. Acta Neuropsychiatr. 2012, 24, 67–80. [Google Scholar] [CrossRef]
- Craig, A.D. How do you feel--now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009, 10, 59–70. [Google Scholar] [CrossRef]
- Trinkler, I.; Devignevielle, S.; Achaibou, A.; Ligneul, R.V.; Brugieres, P.; Cleret de Langavant, L.; De Gelder, B.; Scahill, R.; Schwartz, S.; Bachoud-Levi, A.C. Embodied emotion impairment in Huntington’s Disease. Cortex 2017, 92, 44–56. [Google Scholar] [CrossRef]
- Trinkler, I.; Cleret de Langavant, L.; Bachoud-Levi, A.C. Joint recognition-expression impairment of facial emotions in Huntington’s disease despite intact understanding of feelings. Cortex 2013, 49, 549–558. [Google Scholar] [CrossRef]
- Eddy, C.M.; Rickards, H.E. Interaction without intent: The shape of the social world in Huntington’s disease. Soc. Cogn. Affect. Neurosci. 2015, 10, 1228–1235. [Google Scholar] [CrossRef]
- Wood, R.L.; Williams, C.; Lewis, R. Role of alexithymia in suicide ideation after traumatic brain injury. J. Int. Neuropsychol. Soc. 2010, 16, 1108–1114. [Google Scholar] [CrossRef]
- Neumann, D.; Zupan, B.; Malec, J.F.; Hammond, F. Relationships between alexithymia, affect recognition, and empathy after traumatic brain injury. J. Head Trauma Rehabil. 2014, 29, E18–E27. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; Wood, R.L. The impact of alexithymia on relationship quality and satisfaction following traumatic brain injury. J. Head Trauma Rehabil. 2013, 28, E21–E30. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.L.; Doughty, C. Alexithymia and avoidance coping following traumatic brain injury. J. Head Trauma Rehabil. 2013, 28, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Baird, J.A.; Astington, J.W. The role of mental state understanding in the development of moral cognition and moral action. New Dir. Child. Adolesc. Dev. 2004, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Carrington, S.J.; Bailey, A.J. Are there theory of mind regions in the brain? A review of the neuroimaging literature. Hum. Brain Mapp. 2009, 30, 2313–2335. [Google Scholar] [CrossRef]
- Adjeroud, N.; Besnard, J.; El Massioui, N.; Verny, C.; Prudean, A.; Scherer, C.; Gohier, B.; Bonneau, D.; Allain, P. Theory of mind and empathy in preclinical and clinical Huntington’s disease. Soc. Cogn. Affect. Neurosci. 2016, 11, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Philpott, A.L.; Andrews, S.C.; Staios, M.; Churchyard, A.; Fisher, F. Emotion Evaluation and Social Inference Impairments in Huntington’s Disease. J. Huntingtons. Dis. 2016, 5, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Larsen, I.U.; Vinther-Jensen, T.; Gade, A.; Nielsen, J.E.; Vogel, A. Do I misconstrue? Sarcasm detection, emotion recognition, and theory of mind in Huntington disease. Neuropsychology 2016, 30, 181–189. [Google Scholar] [CrossRef]
- Eddy, C.M.; Sira Mahalingappa, S.; Rickards, H.E. Is Huntington’s disease associated with deficits in theory of mind? Acta Neurol. Scand. 2012, 126, 376–383. [Google Scholar] [CrossRef]
- Brune, M.; Blank, K.; Witthaus, H.; Saft, C. “Theory of mind” is impaired in Huntington’s disease. Mov. Disord. 2011, 26, 671–678. [Google Scholar] [CrossRef]
- Snowden, J.S.; Gibbons, Z.C.; Blackshaw, A.; Doubleday, E.; Thompson, J.; Craufurd, D.; Foster, J.; Happe, F.; Neary, D. Social cognition in frontotemporal dementia and Huntington’s disease. Neuropsychologia 2003, 41, 688–701. [Google Scholar] [CrossRef]
- Brune, M.; von Hein, S.M.; Claassen, C.; Hoffmann, R.; Saft, C. Altered third-party punishment in Huntington’s disease: A study using neuroeconomic games. Brain Behav. 2021, 11, e01908. [Google Scholar] [CrossRef]
- Eddy, C.M.; Rickards, H.E. Theory of mind can be impaired prior to motor onset in Huntington’s disease. Neuropsychology 2015, 29, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Allain, P.; Havet-Thomassin, V.; Verny, C.; Gohier, B.; Lancelot, C.; Besnard, J.; Fasotti, L.; Le Gall, D. Evidence for deficits on different components of theory of mind in Huntington’s disease. Neuropsychology 2011, 25, 741–751. [Google Scholar] [CrossRef]
- Olivetti Belardinelli, M.; Hunefeldt, T.; Meloni, R.; Squitieri, F.; Maffi, S.; Migliore, S. Abnormal visual scanning and impaired mental state recognition in pre-manifest Huntington disease. Exp. Brain Res. 2021, 239, 141–150. [Google Scholar] [CrossRef]
- Lagravinese, G.; Avanzino, L.; Raffo De Ferrari, A.; Marchese, R.; Serrati, C.; Mandich, P.; Abbruzzese, G.; Pelosin, E. Theory of Mind Is Impaired in Mild to Moderate Huntington’s Disease Independently from Global Cognitive Functioning. Front. Psychol. 2017, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Krendl, A.C.; Kennedy, D.P.; Hugenberg, K.; Perry, B.L. Social cognitive abilities predict unique aspects of older adults’ personal social networks. J. Gerontol. B Psychol. Sci. Soc. Sci. 2021. [Google Scholar] [CrossRef]
- Adams, A.G.; Henry, J.D.; Molenberghs, P.; Robinson, G.A.; Nott, Z.; von Hippel, W. The relationship between social cognitive difficulties in the acute stages of stroke and later functional outcomes. Soc. Neurosci. 2020, 15, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.L.K.; Wright, C.; Atherton, C. Deception Detection and Truth Detection Are Dependent on Different Cognitive and Emotional Traits: An Investigation of Emotional Intelligence, Theory of Mind, and Attention. Pers. Soc. Psychol. Bull. 2019, 45, 794–807. [Google Scholar] [CrossRef]
- Decety, J.; Lamm, C. Human empathy through the lens of social neuroscience. ScientificWorldJournal 2006, 6, 1146–1163. [Google Scholar] [CrossRef]
- Singer, T. The neuronal basis and ontogeny of empathy and mind reading: Review of literature and implications for future research. Neurosci. Biobehav. Rev. 2006, 30, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Maurage, P.; Lahaye, M.; Grynberg, D.; Jeanjean, A.; Guettat, L.; Verellen-Dumoulin, C.; Halkin, S.; Heeren, A.; Billieux, J.; Constant, E. Dissociating emotional and cognitive empathy in pre-clinical and clinical Huntington’s disease. Psychiatry Res. 2016, 237, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Baez, S.; Herrera, E.; Gershanik, O.; Garcia, A.M.; Bocanegra, Y.; Kargieman, L.; Manes, F.; Ibanez, A. Impairments in negative emotion recognition and empathy for pain in Huntington’s disease families. Neuropsychologia 2015, 68, 158–167. [Google Scholar] [CrossRef]
- Beadle, J.N.; de la Vega, C.E. Impact of Aging on Empathy: Review of Psychological and Neural Mechanisms. Front. Psychiatry 2019, 10, 331. [Google Scholar] [CrossRef]
- Ekman, P.; Friesen, W.V. Constants across cultures in the face and emotion. J. Pers. Soc. Psychol. 1971, 17, 124–129. [Google Scholar] [CrossRef]
- Murphy, F.C.; Nimmo-Smith, I.; Lawrence, A.D. Functional neuroanatomy of emotions: A meta-analysis. Cogn. Affect. Behav. Neurosci. 2003, 3, 207–233. [Google Scholar] [CrossRef]
- Preece, D.; Becerra, R.; Campitelli, G. Assessing Emotional Reactivity: Psychometric Properties of the Perth Emotional Reactivity Scale and the Development of a Short Form. J. Pers. Assess. 2019, 101, 589–597. [Google Scholar] [CrossRef]
- Nock, M.K.; Wedig, M.M.; Holmberg, E.B.; Hooley, J.M. The emotion reactivity scale: Development, evaluation, and relation to self-injurious thoughts and behaviors. Behav. Ther. 2008, 39, 107–116. [Google Scholar] [CrossRef]
- Larsen, R.J.; Diener, E.; Cropanzano, R.S. Cognitive operations associated with individual differences in affect intensity. J. Pers. Soc. Psychol. 1987, 53, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Padgaonkar, N.T.; Phuong Uy, J.; DePasque, S.; Galvan, A.; Peris, T.S. Neural correlates of emotional reactivity and regulation in youth with and without anxiety. Depress. Anxiety 2021. [Google Scholar] [CrossRef] [PubMed]
- Baron-Cohen, S.; Wheelwright, S.; Hill, J.; Raste, Y.; Plumb, I. The “Reading the Mind in the Eyes” Test revised version: A study with normal adults, and adults with Asperger syndrome or high-functioning autism. J. Child. Psychol. Psychiatry 2001, 42, 241–251. [Google Scholar] [CrossRef]
- Stone, V.E.; Baron-Cohen, S.; Knight, R.T. Frontal lobe contributions to theory of mind. J. Cogn. Neurosci. 1998, 10, 640–656. [Google Scholar] [CrossRef] [PubMed]
- White, S.J.; Coniston, D.; Rogers, R.; Frith, U. Developing the Frith-Happe animations: A quick and objective test of Theory of Mind for adults with autism. Autism. Res. 2011, 4, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Saxe, R.; Kanwisher, N. People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind”. Neuroimage 2003, 19, 1835–1842. [Google Scholar] [CrossRef]
- Gallagher, H.L.; Happe, F.; Brunswick, N.; Fletcher, P.C.; Frith, U.; Frith, C.D. Reading the mind in cartoons and stories: An fMRI study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia 2000, 38, 11–21. [Google Scholar] [CrossRef]
- Baron-Cohen, S.; Wheelwright, S. The empathy quotient: An investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. J. Autism. Dev. Disord. 2004, 34, 163–175. [Google Scholar] [CrossRef]
- Caruso, D.R.; Mayer, J.D. A Measure of Emotional Empathy for Adolescents and Adults. Unpublished Manucript. 1998. Available online: https://scholars.unh.edu/cgi/viewcontent.cgi?article=1021&context=personality_lab (accessed on 1 April 2021).
- Spreng, R.N.; McKinnon, M.C.; Mar, R.A.; Levine, B. The Toronto Empathy Questionnaire: Scale development and initial validation of a factor-analytic solution to multiple empathy measures. J. Pers. Assess. 2009, 91, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Knight, L.K.; Stoica, T.; Fogleman, N.D.; Depue, B.E. Convergent Neural Correlates of Empathy and Anxiety During Socioemotional Processing. Front. Hum. Neurosci. 2019, 13, 94. [Google Scholar] [CrossRef] [PubMed]
- Rameson, L.T.; Morelli, S.A.; Lieberman, M.D. The neural correlates of empathy: Experience, automaticity, and prosocial behavior. J. Cogn. Neurosci. 2012, 24, 235–245. [Google Scholar] [CrossRef]
- Sanfey, A.G.; Loewenstein, G.; McClure, S.M.; Cohen, J.D. Neuroeconomics: Cross-currents in research on decision-making. Trends Cogn. Sci. 2006, 10, 108–116. [Google Scholar] [CrossRef]
- Klein, G. Naturalistic decision making. Hum. Factors 2008, 50, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.K.; Harris, L.T. How social cognition can inform social decision making. Front. Neurosci. 2013, 7, 259. [Google Scholar] [CrossRef]
- De Groot, K.; Thurik, R. Disentangling Risk and Uncertainty: When Risk-Taking Measures Are Not About Risk. Front. Psychol. 2018, 9, 2194. [Google Scholar] [CrossRef] [PubMed]
- Adjeroud, N.; Besnard, J.; Verny, C.; Prundean, A.; Scherer, C.; Gohier, B.; Bonneau, D.; Massioui, N.E.; Allain, P. Dissociation between decision-making under risk and decision-making under ambiguity in premanifest and manifest Huntington’s disease. Neuropsychologia 2017, 103, 87–95. [Google Scholar] [CrossRef]
- Campbell, M.C.; Stout, J.C.; Finn, P.R. Reduced autonomic responsiveness to gambling task losses in Huntington’s disease. J. Int. Neuropsychol. Soc. 2004, 10, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Holl, A.K.; Wilkinson, L.; Tabrizi, S.J.; Painold, A.; Jahanshahi, M. Selective executive dysfunction but intact risky decision-making in early Huntington’s disease. Mov. Disord. 2013, 28, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Stout, J.C.; Rodawalt, W.C.; Siemers, E.R. Risky decision making in Huntington’s disease. J. Int. Neuropsychol. Soc. 2001, 7, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Galvez, V.; Fernandez-Ruiz, J.; Bayliss, L.; Ochoa-Morales, A.; Hernandez-Castillo, C.R.; Diaz, R.; Campos-Romo, A. Early Huntington’s Disease: Impulse Control Deficits but Correct Judgment Regarding Risky Situations. J. Huntingtons. Dis. 2017, 6, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Watkins, L.H.; Rogers, R.D.; Lawrence, A.D.; Sahakian, B.J.; Rosser, A.E.; Robbins, T.W. Impaired planning but intact decision making in early Huntington’s disease: Implications for specific fronto-striatal pathology. Neuropsychologia 2000, 38, 1112–1125. [Google Scholar] [CrossRef]
- Minati, L.; Grisoli, M.; Franceschetti, S.; Epifani, F.; Granvillano, A.; Medford, N.; Harrison, N.A.; Piacentini, S.; Critchley, H.D. Neural signatures of economic parameters during decision-making: A functional MRI (FMRI), electroencephalography (EEG) and autonomic monitoring study. Brain Topogr. 2012, 25, 73–96. [Google Scholar] [CrossRef]
- D’Aurizio, G.; Migliore, S.; Curcio, G.; Squitieri, F. Safer Attitude to Risky Decision-Making in Premanifest Huntington’s Disease Subjects. Front. Psychol. 2019, 10, 846. [Google Scholar] [CrossRef] [PubMed]
- Gleichgerrcht, E.; Ibanez, A.; Roca, M.; Torralva, T.; Manes, F. Decision-making cognition in neurodegenerative diseases. Nat. Rev. Neurol. 2010, 6, 611–623. [Google Scholar] [CrossRef]
- Darby, R.R.; Dickerson, B.C. Dementia, Decision Making, and Capacity. Harv. Rev. Psychiatry 2017, 25, 270–278. [Google Scholar] [CrossRef]
- Bora, E.; Velakoulis, D.; Walterfang, M. Social cognition in Huntington’s disease: A meta-analysis. Behav. Brain Res. 2016, 297, 131–140. [Google Scholar] [CrossRef]
- Enzi, B.; Edel, M.A.; Lissek, S.; Peters, S.; Hoffmann, R.; Nicolas, V.; Tegenthoff, M.; Juckel, G.; Saft, C. Altered ventral striatal activation during reward and punishment processing in premanifest Huntington’s disease: A functional magnetic resonance study. Exp. Neurol. 2012, 235, 256–264. [Google Scholar] [CrossRef]
- McCusker, E.; Loy, C.T. The many facets of unawareness in huntington disease. Tremor Other Hyperkinet. Mov. 2014, 4, 257. [Google Scholar] [CrossRef]
- Robson, S.E.; Repetto, L.; Gountouna, V.E.; Nicodemus, K.K. A review of neuroeconomic gameplay in psychiatric disorders. Mol. Psychiatry 2020, 25, 67–81. [Google Scholar] [CrossRef]
- Kishida, K.T.; King-Casas, B.; Montague, P.R. Neuroeconomic approaches to mental disorders. Neuron 2010, 67, 543–554. [Google Scholar] [CrossRef]
- Beadle, J.N.; Paradiso, S.; Kovach, C.; Polgreen, L.; Denburg, N.L.; Tranel, D. Effects of age-related differences in empathy on social economic decision-making. Int. Psychogeriatr. 2012, 24, 822–833. [Google Scholar] [CrossRef]
- Chiong, W.; Hsu, M.; Wudka, D.; Miller, B.L.; Rosen, H.J. Financial errors in dementia: Testing a neuroeconomic conceptual framework. Neurocase 2014, 20, 389–396. [Google Scholar] [CrossRef]
- O’Callaghan, C.; Bertoux, M.; Irish, M.; Shine, J.M.; Wong, S.; Spiliopoulos, L.; Hodges, J.R.; Hornberger, M. Fair play: Social norm compliance failures in behavioural variant frontotemporal dementia. Brain 2016, 139, 204–216. [Google Scholar] [CrossRef]
- Shao, J.; Zhang, Q.; Ren, Y.; Li, X.; Lin, T. Why are older adults victims of fraud? Current knowledge and prospects regarding older adults’ vulnerability to fraud. J. Elder Abuse Negl. 2019, 31, 225–243. [Google Scholar] [CrossRef]
- Zhu, L.; Walsh, D.; Hsu, M. Neuroeconomic measures of social decision-making across the lifespan. Front. Neurosci. 2012, 6, 128. [Google Scholar] [CrossRef] [PubMed]
- Ponce, C.C.; Ordonez, T.N.; Lima-Silva, T.B.; Dos Santos, G.D.; Viola, L.F.; Nunes, P.V.; Forlenza, O.V.; Cachioni, M. Effects of a psychoeducational intervention in family caregivers of people with Alzheimer’s disease. Dement. Neuropsychol. 2011, 5, 226–237. [Google Scholar] [CrossRef]
- Kempnich, C.L.; Wong, D.; Georgiou-Karistianis, N.; Stout, J.C. Feasibility and Efficacy of Brief Computerized Training to Improve Emotion Recognition in Premanifest and Early-Symptomatic Huntington’s Disease. J. Int. Neuropsychol. Soc. 2017, 23, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Beresnevaite, M. Exploring the benefits of group psychotherapy in reducing alexithymia in coronary heart disease patients: A preliminary study. Psychother. Psychosom. 2000, 69, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Bechi, M.; Bosia, M.; Spangaro, M.; Buonocore, M.; Cocchi, F.; Pigoni, A.; Piantanida, M.; Guglielmino, C.; Bianchi, L.; Smeraldi, E.; et al. Combined social cognitive and neurocognitive rehabilitation strategies in schizophrenia: Neuropsychological and psychopathological influences on Theory of Mind improvement. Psychol. Med. 2015, 45, 3147–3157. [Google Scholar] [CrossRef]
- Mazza, M.; Lucci, G.; Pacitti, F.; Pino, M.C.; Mariano, M.; Casacchia, M.; Roncone, R. Could schizophrenic subjects improve their social cognition abilities only with observation and imitation of social situations? Neuropsychol. Rehabil. 2010, 20, 675–703. [Google Scholar] [CrossRef] [PubMed]
- Weisz, E.; Ong, D.C.; Carlson, R.W.; Zaki, J. Building empathy through motivation-based interventions. Emotion 2020. [Google Scholar] [CrossRef]
- Gabery, S.; Murphy, K.; Schultz, K.; Loy, C.T.; McCusker, E.; Kirik, D.; Halliday, G.; Petersen, A. Changes in key hypothalamic neuropeptide populations in Huntington disease revealed by neuropathological analyses. Acta Neuropathol. 2010, 120, 777–788. [Google Scholar] [CrossRef]
- Gabery, S.; Halliday, G.; Kirik, D.; Englund, E.; Petersen, A. Selective loss of oxytocin and vasopressin in the hypothalamus in early Huntington disease: A case study. Neuropathol. Appl. Neurobiol. 2015, 41, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Cheong, R.Y.; Tonetto, S.; von Horsten, S.; Petersen, A. Imbalance of the oxytocin-vasopressin system contributes to the neuropsychiatric phenotype in the BACHD mouse model of Huntington disease. Psychoneuroendocrinology 2020, 119, 104773. [Google Scholar] [CrossRef]
- Unti, E.; Mazzucchi, S.; Frosini, D.; Pagni, C.; Tognoni, G.; Palego, L.; Betti, L.; Miraglia, F.; Giannaccini, G.; Ceravolo, R. Social Cognition and Oxytocin in Huntington’s Disease: New Insights. Brain Sci. 2018, 8, 161. [Google Scholar] [CrossRef] [PubMed]
- Labuschagne, I.; Poudel, G.; Kordsachia, C.; Wu, Q.; Thomson, H.; Georgiou-Karistianis, N.; Stout, J.C. Oxytocin selectively modulates brain processing of disgust in Huntington’s disease gene carriers. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 81, 11–16. [Google Scholar] [CrossRef]
- Heinrichs, M.; Baumgartner, T.; Kirschbaum, C.; Ehlert, U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol. Psychiatry 2003, 54, 1389–1398. [Google Scholar] [CrossRef]
- Heinrichs, M.; Meinlschmidt, G.; Neumann, I.; Wagner, S.; Kirschbaum, C.; Ehlert, U.; Hellhammer, D.H. Effects of suckling on hypothalamic-pituitary-adrenal axis responses to psychosocial stress in postpartum lactating women. J. Clin. Endocrinol. Metab. 2001, 86, 4798–4804. [Google Scholar] [CrossRef]
- Kosfeld, M.; Heinrichs, M.; Zak, P.J.; Fischbacher, U.; Fehr, E. Oxytocin increases trust in humans. Nature 2005, 435, 673–676. [Google Scholar] [CrossRef]
- Domes, G.; Heinrichs, M.; Michel, A.; Berger, C.; Herpertz, S.C. Oxytocin improves “mind-reading” in humans. Biol. Psychiatry 2007, 61, 731–733. [Google Scholar] [CrossRef]
- Hurlemann, R.; Patin, A.; Onur, O.A.; Cohen, M.X.; Baumgartner, T.; Metzler, S.; Dziobek, I.; Gallinat, J.; Wagner, M.; Maier, W.; et al. Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J. Neurosci. 2010, 30, 4999–5007. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, R.F.; Leary, M.R. The need to belong: Desire for interpersonal attachments as a fundamental human motivation. Psychol. Bull. 1995, 117, 497–529. [Google Scholar] [CrossRef] [PubMed]
- Hawkley, L.C.; Cacioppo, J.T. Loneliness matters: A theoretical and empirical review of consequences and mechanisms. Ann. Behav. Med. 2010, 40, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Beutel, M.E.; Klein, E.M.; Brahler, E.; Reiner, I.; Junger, C.; Michal, M.; Wiltink, J.; Wild, P.S.; Munzel, T.; Lackner, K.J.; et al. Loneliness in the general population: Prevalence, determinants and relations to mental health. BMC Psychiatry 2017, 17, 97. [Google Scholar] [CrossRef] [PubMed]
- Stickley, A.; Koyanagi, A. Loneliness, common mental disorders and suicidal behavior: Findings from a general population survey. J. Affect. Disord. 2016, 197, 81–87. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Bennett, D.A.; Schneider, J.A.; Tang, Y.; Arnold, S.E.; Wilson, R.S. The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: A longitudinal cohort study. Lancet Neurol. 2006, 5, 406–412. [Google Scholar] [CrossRef]
- Hoe, M.; Nakagami, E.; Green, M.F.; Brekke, J.S. The causal relationships between neurocognition, social cognition and functional outcome over time in schizophrenia: A latent difference score approach. Psychol. Med. 2012, 42, 2287–2299. [Google Scholar] [CrossRef]
- Poole, J.H.; Tobias, F.C.; Vinogradov, S. The functional relevance of affect recognition errors in schizophrenia. J. Int. Neuropsychol. Soc. 2000, 6, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Green, M.F.; Olivier, B.; Crawley, J.N.; Penn, D.L.; Silverstein, S. Social cognition in schizophrenia: Recommendations from the measurement and treatment research to improve cognition in schizophrenia new approaches conference. Schizophr. Bull. 2005, 31, 882–887. [Google Scholar] [CrossRef] [PubMed]
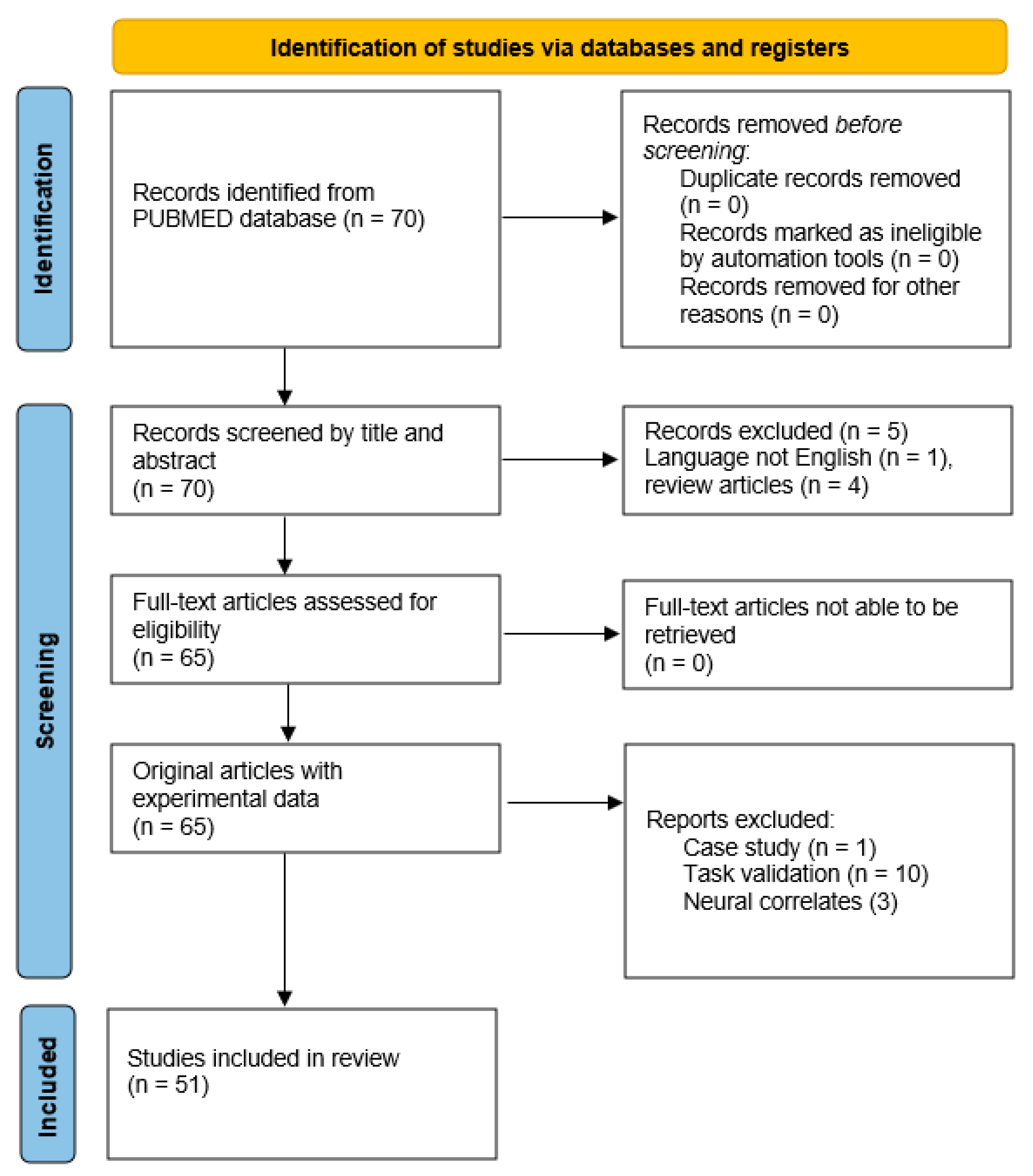
| Genetic testing | Whether or not to have predictive testing When to have predictive testing Whether to share the results of the test with family, friends and the wider world |
| Reproduction | Whether or not to have children at all Whether to have children who are also at risk of HD Whether to use preimplantation testing or prenatal testing or natural conception |
| Sharing HD status | When to tell friend or new partners about gene status Do employers need to know? When do the DVLA need to know? Does the GP need to know? When to tell children that HD is in the family |
| Forward planning | When or if to set up an advanced directive Is it necessary to set up a proxy decision-maker in advance? If so, who will it be? |
| Care choices | Do you want to have a PEG fitted to assist feeding, if so when should it be removed? When is independent living no longer practical or possible? Should care be provided at home or in a nursing care facility. |
| Experimental research | Whether to take part in experimental studies |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mason, S.L.; Schaepers, M.; Barker, R.A. Problems with Social Cognition and Decision-Making in Huntington’s Disease: Why Is it Important? Brain Sci. 2021, 11, 838. https://doi.org/10.3390/brainsci11070838
Mason SL, Schaepers M, Barker RA. Problems with Social Cognition and Decision-Making in Huntington’s Disease: Why Is it Important? Brain Sciences. 2021; 11(7):838. https://doi.org/10.3390/brainsci11070838
Chicago/Turabian StyleMason, Sarah L., Miriam Schaepers, and Roger A. Barker. 2021. "Problems with Social Cognition and Decision-Making in Huntington’s Disease: Why Is it Important?" Brain Sciences 11, no. 7: 838. https://doi.org/10.3390/brainsci11070838
APA StyleMason, S. L., Schaepers, M., & Barker, R. A. (2021). Problems with Social Cognition and Decision-Making in Huntington’s Disease: Why Is it Important? Brain Sciences, 11(7), 838. https://doi.org/10.3390/brainsci11070838






