Real-World fNIRS Brain Activity Measurements during Ashtanga Vinyasa Yoga
Abstract
1. Introduction
2. Background
2.1. Yoga
2.2. Ashtanga Vinyasa Yoga
2.3. Existing Research on Yoga
2.4. The Prefrontal Cortex
3. Materials and Methods
3.1. Single-Subject Observational Study
3.2. Participant
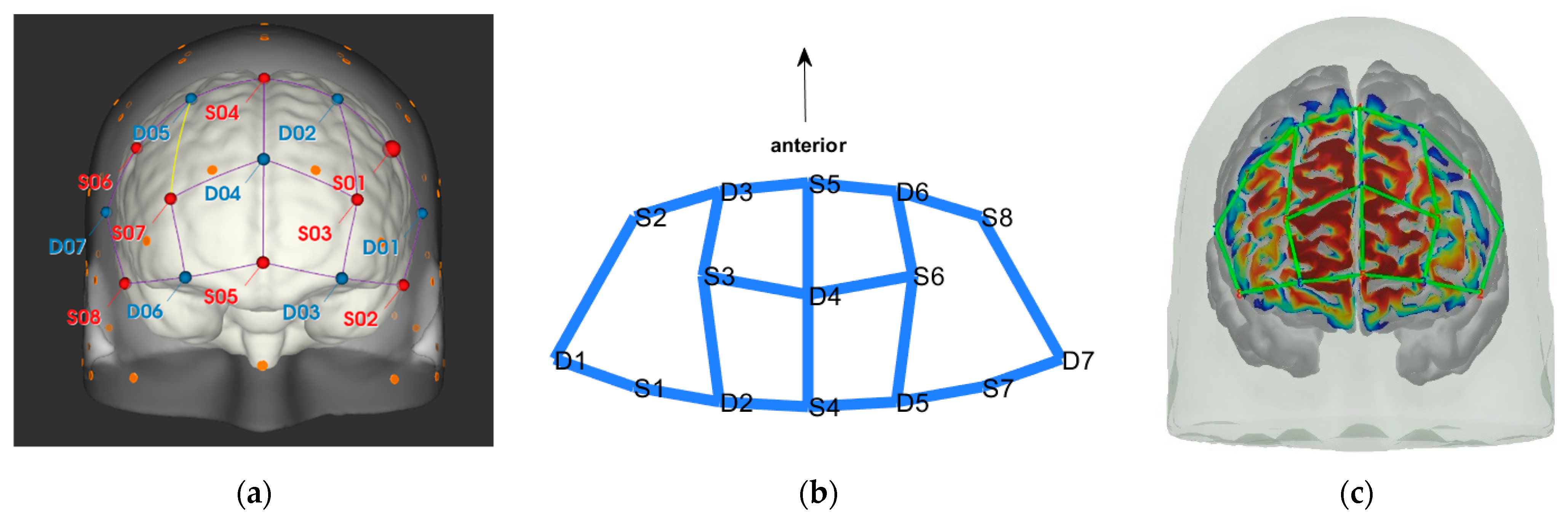
3.3. Data Collection
3.4. Data Analysis
3.4.1. Video Coding
3.4.2. fNIRS Analysis
3.4.3. Selection of Contrasts
4. Results
4.1. Posture Combinations with Expected Greater Statistical Difference
4.2. Postures Combinations with Some Unexpected Differences (Mid-Range)
4.3. Posture Combinations with Unexpected Little Difference
5. Discussion
5.1. Lateralization
5.2. Reflections on Motion Artifacts
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Sanskrit | English | Comment |
|---|---|---|
| Warm-up | ||
| Samasthitih | Mountain Pose | Conducted. Participant listened to chant in video. |
| Surya Namaskara A x5 | Sun Salutation A | Conducted. |
| Surya Namaskara B x5 | Sun Salutation A | Conducted. |
| Standing postures | ||
| Padangusthasana | Big Toe Pose | Conducted. |
| Pada Hastasana | Hand to Foot Pose/hands under feet | Conducted. |
| Utthita Trikonasana | Triangle | Conducted. |
| Parivṛtta Trikonasana | Revolved Triangle | Conducted. |
| Utthita Parsvakonasana | Extended Side Angle | Conducted. |
| Parivṛtta Parsvakonasana | Revolved Side Angle | Conducted. |
| Prasarita Padottanasana, A, B, C and D | Wide Leg Forward Fold A, B, C, and D | Conducted, but adapted to accommodate the sensors. The head was not placed on the ground. |
| Parsvottanasana | Side Intense Stretch | Conducted. |
| Utthita Hasta Padangusthasana | Extended Hand to Big Toe Pose | Conducted. |
| Utthita Parsvasahita | Extended Hand to Big Toe Side and Hold Pose | |
| Ardha Baddha Padmottanasana | Half Bound Lotus Standing Forward Bend | Conducted. |
| Utkatasana | Chair Pose | Conducted. |
| Virabhadrasana I/A | Warrior 1/A | Conducted. |
| Virabhadrasana II/B | Warrior 2/B | Conducted. |
| Seated postures | ||
| Dandasana | Staff Pose | Conducted. |
| Pascimottanasana A, B | West Intense Stretch | Conducted. |
| Purvottanasana | East Intense Stretch | Conducted. |
| Ardha Baddha Padma Pascimottanasana | Half Bound Lotus Forward Fold | Conducted. |
| Triyang Mukha Eka Pada Pascimottanasana | One Leg Folded Back, Forward Fold | Conducted. |
| Janu Sirsasana A, B and C | Head to Knee Pose A, B, and C | Conducted. |
| Maricyasana A, B C and D | Marichi’s Pose. A seated pose with twist variations. | Conducted. |
| Navasana x5 | Boat | Conducted. |
| The first half of the Primary Series is finished | ||
| Baddha Konasana A and B | Bound Angle Pose A (upright) and B (fold) | Conducted sometimes. |
| Finishing sequence | ||
| Urdhva Dhanurasana | Wheel Pose | Not conducted. |
| Pascimottanasana—10 breaths | Seated Forward Fold/Bend | Conducted. |
| Salamba Sarvangasana—10 breaths | Shoulderstand | Not conducted. |
| Halasana | Plow | Not conducted. |
| Karna Pidasana | Ear Pressure Pose | Not conducted. |
| Urdhva Padmasana | Upward Lotus Pose | Not conducted. |
| Pindasana | Embryo | Not conducted. |
| Matsyasana | Fish Pose | Not conducted. |
| Uttana Padasana | Raised Leg Pose | Not conducted. |
| Sirsasana A and B—10 breaths | Headstand A and B | Not conducted. |
| Balasana | Child’s Pose | Conducted. The head was not placed on the ground. |
| Baddha Padmasana (–10 breaths) | Bound Lotus and Bow | Conducted. |
| Padmasana | Lotus position | Conducted |
| Utplutih | Scale Pose/Lotus Uplifting | Conducted |
| Samasthitih | Mountain Pose | Conducted. Participant listened to chant in video. |
| Savasana | Corpse Pose | Not conducted. |
Appendix B
| Sanskrit | English |
|---|---|
| Samasthitih (start of practice) | Mountain_Pose_Start |
| Surya Namaskar A | Sun_Salutation_A |
| Surya Namaskar B | Sun_Salutation_B |
| Adho Mukha Svanasana | Downward_Facing_Dog |
| Padangusthasana | Big_toe_pose |
| Pada Hastasana | Hands_under_feet |
| Utthita Trikonasana (right foot forward) | Trikonasana_Right |
| Utthita Trikonasana (left foot forward) | Trikonasana_Left |
| Parivrtta Trikonasana (right foot forward) | Revolved_triangle_Right |
| Parivrtta Trikonasana (left foot forward) | Revolved_triangle_Left |
| Utthita Parsvakonasana (right foot forward) | Extended_side_angle_Right |
| Utthita Parsvakonasana (left foot forward) | Extended_side_angle_Left |
| Parivṛtta Parsvakonasana (right foot forward) | Revolved_side_angle_Right |
| Parivṛtta Parsvakonasana (left foot forward) | Revolved_side_angle_Left |
| Prasarita Padottanasana | Wide_leg_forward_fold |
| Parsvottanasana (right foot forward) | Side_intense_stretch_Right |
| Parsvottanasana (left foot forward) | Side_intense_stretch_Left |
| Utthita Hasta Padangusthasana (right toe) | Extended_hand_to_Right_big_toe |
| Utthita Parsvasahita (right foot uplifted) | Extended_hand_to_Right_big_toe_hold |
| Utthita Hasta Padangusthasana (left toe) | Extended_hand_to_Left_big_toe |
| Utthita Parsvasahita (left foot uplifted) | Extended_hand_to_Left_big_toe_hold |
| Ardha Baddha Padmottanasana (right foot bound) | Right_foot_in_half_bound_lotus |
| Ardha Baddha Padmottanasana (left foot bound) | Left_foot_in_half_bound_lotus |
| Utkatasana | Chair_Pose |
| Virabhadrasana I (right foot forward) | Warrier_1_Right |
| Virabhadrasana I (left foot forward) | Warrier_1_Left |
| Virabhadrasana II (right foot forward) | Warrier_2_Left |
| Virabhadrasana II (left foot forward) | Warrier_2_Right |
| Dandasana | Staff_pose |
| Pascimottanasana A, B | West_intense_stretch_A_B |
| Purvottanasana | East_intense_stretch |
| Ardha Baddha Padma Pascimottanasana (right foot) | Right_foot_in_half_bound_lotus_forward_fold |
| Ardha Baddha Padma Pascimottanasana (left foot) | Left_foot_in_half_bound_lotus_forward_fold |
| Triyang Mukha Eka Pada Pascimottanasana (right leg) | Right_leg_folded_back_forward_fold |
| Triyang Mukha Eka Pada Pascimottanasana (left leg) | Left_leg_folded_back_forward_fold |
| Janu Sirsasana A (right leg) | Head_to_knee_pose_A_Right_Leg_folded |
| Janu Sirsasana A (left leg) | Head_to_knee_pose_A_Left_Leg_folded |
| Janu Sirsasana B (right heel) | Head_to_knee_pose_B_sit_on_Right_heel |
| Janu Sirsasana B (left heel) | Head_to_knee_pose_B_sit_on_Left_heel |
| Janu Sirsasana C (right toe) | Head_to_knee_pose_C_Right_toe_stretch |
| Janu Sirsasana C (left toe) | Head_to_knee_pose_C_Left_toe_stretch |
| Maricyasana A (right knee bent up) | MA_Right_knee_bent_bind |
| Maricyasana A (left knee bent up) | MA_Left_knee_bent_bind |
| Maricyasana B (left leg Lotus) | MB_Left_leg_lotus_Right_knee_bent_bind |
| Maricyasana B (right leg Lotus) | MB_Right_leg_lotus_Left_knee_bent_bind |
| Maricyasana C (right knee bent up) | MC_Right_knee_bent_twist_bind |
| Maricyasana C (left knee bent up) | MC_Left_knee_bent_twist_bind |
| Maricyasana D (left leg Lotus) | MD_Left_leg_lotus_Right_knee_bent_twist_bind |
| Maricyasana D (right leg Lotus) | MD_Right_leg_lotus_Left_knee_bent_twist_bind |
| Navasana | Boat |
| Baddha Konasana | Bound_angle_upright_and_fold |
| Pascimottanasana | Forward_fold_end |
| Balasana | Childs_pose |
| Baddha Padmasana | Bound_lotus_bow |
| Padmasana | Lotus |
| Utplutih | Lotus_uplifting |
| Samasthitih (end of practice) | Mountain_Pose_Stop |
Appendix C
| Source | Detector | Type | Beta | Se | Tstat | Dfe | Q | Power |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | hbo | 197.46 | 60.05 | 3.29 | 321 | 0.00 | 0.79 |
| 1 | 2 | hbr | 95.15 | 20.27 | 4.69 | 321 | 0.00 | 0.99 |
| 2 | 3 | hbo | 313.03 | 90.63 | 3.45 | 321 | 0.00 | 0.83 |
| 3 | 3 | hbo | 313.97 | 60.74 | 5.17 | 321 | 0.00 | 1.00 |
| 3 | 3 | hbr | 49.19 | 13.82 | 3.56 | 321 | 0.00 | 0.86 |
| 3 | 4 | hbo | 253.23 | 58.59 | 4.32 | 321 | 0.00 | 0.97 |
| 4 | 2 | hbo | 192.11 | 71.50 | 2.69 | 321 | 0.02 | 0.58 |
| 4 | 2 | hbr | 153.95 | 21.13 | 7.29 | 321 | 0.00 | 1.00 |
| 4 | 4 | hbo | 337.58 | 92.19 | 3.66 | 321 | 0.00 | 0.88 |
| 4 | 4 | hbr | 94.87 | 30.08 | 3.15 | 321 | 0.00 | 0.75 |
| 4 | 5 | hbo | 205.60 | 40.26 | 5.11 | 321 | 0.00 | 1.00 |
| 4 | 5 | hbr | 99.92 | 14.15 | 7.06 | 321 | 0.00 | 1.00 |
| 5 | 3 | hbo | 485.21 | 68.56 | 7.08 | 321 | 0.00 | 1.00 |
| 5 | 3 | hbr | 87.51 | 24.00 | 3.65 | 321 | 0.00 | 0.88 |
| 5 | 4 | hbo | 322.83 | 57.88 | 5.58 | 321 | 0.00 | 1.00 |
| 5 | 6 | hbo | 691.47 | 87.80 | 7.88 | 321 | 0.00 | 1.00 |
| 5 | 6 | hbr | 58.16 | 19.36 | 3.00 | 321 | 0.01 | 0.70 |
| 6 | 4 | hbo | 179.67 | 71.04 | 2.53 | 321 | 0.02 | 0.51 |
| 6 | 6 | hbo | 272.10 | 85.00 | 3.20 | 321 | 0.00 | 0.76 |
| 7 | 5 | hbr | 115.30 | 21.91 | 5.26 | 321 | 0.00 | 1.00 |
| 8 | 6 | hbo | 225.62 | 94.04 | 2.40 | 321 | 0.03 | 0.46 |
| Source | Detector | Type | Beta | Se | Tstat | Dfe | Q | Power |
|---|---|---|---|---|---|---|---|---|
| 2 | 3 | hbo | −346.99 | 103.30 | −3.36 | 321 | 0.00 | 0.81 |
| 2 | 3 | hbr | −109.30 | 26.27 | −4.16 | 321 | 0.00 | 0.95 |
| 3 | 3 | hbo | −281.68 | 75.93 | −3.71 | 321 | 0.00 | 0.89 |
| 3 | 3 | hbr | −49.84 | 17.72 | −2.81 | 321 | 0.01 | 0.63 |
| 3 | 4 | hbo | −215.96 | 69.01 | −3.13 | 321 | 0.00 | 0.74 |
| 3 | 4 | hbr | −44.20 | 15.20 | −2.91 | 321 | 0.01 | 0.66 |
| 4 | 4 | hbo | −354.04 | 101.43 | −3.49 | 321 | 0.00 | 0.84 |
| 4 | 4 | hbr | −107.66 | 37.51 | −2.87 | 321 | 0.01 | 0.65 |
| 5 | 3 | hbo | −558.02 | 80.00 | −6.98 | 321 | 0.00 | 1.00 |
| 5 | 3 | hbr | −197.23 | 28.89 | −6.83 | 321 | 0.00 | 1.00 |
| 5 | 4 | hbo | −346.45 | 69.69 | −4.97 | 321 | 0.00 | 0.99 |
| 5 | 4 | hbr | −77.77 | 20.22 | −3.85 | 321 | 0.00 | 0.91 |
| 5 | 6 | hbo | −818.60 | 99.86 | −8.20 | 321 | 0.00 | 1.00 |
| 5 | 6 | hbr | −101.37 | 22.93 | −4.42 | 321 | 0.00 | 0.97 |
| 6 | 4 | hbo | −274.98 | 82.53 | −3.33 | 321 | 0.00 | 0.80 |
| 6 | 4 | hbr | −81.21 | 27.58 | −2.94 | 321 | 0.01 | 0.67 |
| 6 | 6 | hbo | −351.87 | 104.43 | −3.37 | 321 | 0.00 | 0.81 |
| 6 | 6 | hbr | −89.55 | 25.90 | −3.46 | 321 | 0.00 | 0.83 |
| 7 | 5 | hbr | −61.91 | 26.04 | −2.38 | 321 | 0.03 | 0.45 |
| 8 | 6 | hbo | −462.97 | 105.39 | −4.39 | 321 | 0.00 | 0.97 |
| 8 | 6 | hbr | −112.37 | 32.59 | −3.45 | 321 | 0.00 | 0.83 |
| Source | Detector | Type | Beta | Se | Tstat | Dfe | Q | Power |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | hbo | 145.86 | 47.68 | 3.06 | 321 | 0.01 | 0.71 |
| 2 | 3 | hbo | 366.15 | 69.53 | 5.27 | 321 | 0.00 | 1.00 |
| 2 | 3 | hbr | 65.47 | 18.41 | 3.56 | 321 | 0.00 | 0.86 |
| 3 | 3 | hbo | 175.83 | 50.31 | 3.49 | 321 | 0.00 | 0.84 |
| 3 | 4 | hbo | 149.83 | 48.33 | 3.10 | 321 | 0.01 | 0.73 |
| 3 | 4 | hbr | 34.79 | 10.21 | 3.41 | 321 | 0.00 | 0.82 |
| 4 | 4 | hbr | 75.27 | 25.77 | 2.92 | 321 | 0.01 | 0.67 |
| 4 | 5 | hbo | 101.00 | 33.64 | 3.00 | 321 | 0.01 | 0.69 |
| 4 | 5 | hbr | 38.79 | 12.10 | 3.21 | 321 | 0.00 | 0.76 |
| 5 | 3 | hbo | 374.76 | 56.25 | 6.66 | 321 | 0.00 | 1.00 |
| 5 | 3 | hbr | 61.96 | 19.22 | 3.22 | 321 | 0.00 | 0.77 |
| 5 | 4 | hbo | 202.77 | 47.24 | 4.29 | 321 | 0.00 | 0.96 |
| 5 | 4 | hbr | 45.67 | 13.59 | 3.36 | 321 | 0.00 | 0.81 |
| 5 | 6 | hbo | 406.25 | 76.05 | 5.34 | 321 | 0.00 | 1.00 |
| 5 | 6 | hbr | 80.07 | 15.84 | 5.05 | 321 | 0.00 | 0.99 |
| 6 | 6 | hbo | 222.71 | 68.88 | 3.23 | 321 | 0.00 | 0.77 |
| 6 | 6 | hbr | 58.18 | 17.87 | 3.26 | 321 | 0.00 | 0.78 |
| 7 | 7 | hbr | 47.33 | 20.23 | 2.34 | 321 | 0.04 | 0.44 |
| 8 | 6 | hbo | 263.54 | 76.20 | 3.46 | 321 | 0.00 | 0.83 |
| 1 | 2 | hbo | 145.86 | 47.68 | 3.06 | 321 | 0.01 | 0.71 |
| 2 | 3 | hbo | 366.15 | 69.53 | 5.27 | 321 | 0.00 | 1.00 |
| Source | Detector | Type | Beta | Se | Tstat | Dfe | Q | Power |
|---|---|---|---|---|---|---|---|---|
| 5 | 3 | hbo | 453.27 | 111.13 | 4.08 | 321 | 0.00 | 0.94 |
| 5 | 3 | hbr | 116.32 | 36.45 | 3.19 | 321 | 0.00 | 0.76 |
| 5 | 4 | hbo | 256.29 | 91.15 | 2.81 | 321 | 0.01 | 0.63 |
| 5 | 6 | hbo | 568.80 | 145.42 | 3.91 | 321 | 0.00 | 0.92 |
| 5 | 6 | hbr | 160.80 | 31.36 | 5.13 | 321 | 0.00 | 1.00 |
| 5 | 3 | hbo | 453.27 | 111.13 | 4.08 | 321 | 0.00 | 0.94 |
| Source | Detector | Type | Beta | Se | Tstat | Dfe | Q | Power |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | hbo | −715.08 | 219.45 | −3.26 | 321 | 0.02 | 0.78 |
| 1 | 2 | hbo | −295.74 | 92.86 | −3.18 | 321 | 0.02 | 0.76 |
| 2 | 3 | hbo | −414.64 | 136.13 | −3.05 | 321 | 0.03 | 0.71 |
| 3 | 4 | hbo | −292.00 | 85.87 | −3.40 | 321 | 0.02 | 0.82 |
| 4 | 5 | hbr | 65.32 | 22.85 | 2.86 | 321 | 0.04 | 0.64 |
| 1 | 1 | hbo | −715.08 | 219.45 | −3.26 | 321 | 0.02 | 0.78 |
| Source | Detector | Type | Beta | Se | Tstat | Dfe | Q | Power |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | hbr | −86.34 | 26.26 | −3.29 | 321 | 0.01 | 0.79 |
| 3 | 3 | hbr | −53.18 | 18.16 | −2.93 | 321 | 0.03 | 0.67 |
| 4 | 2 | hbr | −141.34 | 27.43 | −5.15 | 321 | 0.00 | 1.00 |
| 4 | 5 | hbr | −88.99 | 19.47 | −4.57 | 321 | 0.00 | 0.98 |
| 7 | 5 | hbr | −99.69 | 27.36 | −3.64 | 321 | 0.00 | 0.87 |
| 1 | 2 | hbr | −86.34 | 26.26 | −3.29 | 321 | 0.01 | 0.79 |
| Source | Detector | Type | Beta | Se | Tstat | Dfe | Q | Power |
|---|---|---|---|---|---|---|---|---|
| 2 | 3 | hbo | 359.06 | 97.59 | 3.68 | 321 | 0.01 | 0.88 |
| Source | Detector | Type | Beta | Se | Tstat | Dfe | Q | Power |
|---|---|---|---|---|---|---|---|---|
| 5 | 3 | hbo | 363.78 | 95.76 | 3.80 | 321 | 0.01 | 0.90 |
| Source | Detector | Type | Beta | Se | Tstat | Dfe | Q | Power |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | hbo | −393.35 | 78.26 | −5.03 | 321 | 0.00 | 0.99 |
References
- Pinti, P.; Tachtsidis, I.; Hamilton, A.; Hirsch, J.; Aichelburg, C.; Gilbert, S.; Burgess, P.W. The Present and Future Use of Functional Near-infrared Spectroscopy (FNIRS) for Cognitive Neuroscience. Ann. N. Y. Acad. Sci. 2020, 1464, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Herold, F.; Wiegel, P.; Scholkmann, F.; Müller, N.G. Applications of Functional Near-Infrared Spectroscopy (FNIRS) Neuroimaging in Exercise–Cognition Science: A Systematic, Methodology-Focused Review. J. Clin. Med. 2018, 7, 466. [Google Scholar] [CrossRef]
- Paszkiel, S.; Szpulak, P. Methods of Acquisition, Archiving and Biomedical Data Analysis of Brain Functioning. In Proceedings of the Biomedical Engineering and Neuroscience; Hunek, W.P., Paszkiel, S., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 158–171. [Google Scholar]
- Zohdi, H.; Scholkmann, F.; Wolf, U. Individual Differences in Hemodynamic Responses Measured on the Head Due to a Long-Term Stimulation Involving Colored Light Exposure and a Cognitive Task: A SPA-FNIRS Study. Brain Sci. 2021, 11, 54. [Google Scholar] [CrossRef]
- Maior, H.A.; Wilson, M.L.; Sharples, S. Workload Alerts—Using Physiological Measures of Mental Workload to Provide Feedback During Tasks. ACM Trans. Comput.-Hum. Interact. 2018, 25, 1–30. [Google Scholar] [CrossRef]
- Pike, M.F.; Maior, H.A.; Porcheron, M.; Sharples, S.C.; Wilson, M.L. Measuring the Effect of Think Aloud Protocols on Workload Using FNIRS. In Proceedings of the SIGCHI Conference on Human Factors in Computing Systems; Association for Computing Machinery: New York, NY, USA, 2014; pp. 3807–3816. [Google Scholar]
- Solovey, E.T.; Girouard, A.; Chauncey, K.; Hirshfield, L.M.; Sassaroli, A.; Zheng, F.; Fantini, S.; Jacob, R.J.K. Using FNIRS Brain Sensing in Realistic HCI Settings: Experiments and Guidelines. In Proceedings of the 22nd Annual ACM Symposium on User Interface Software and Technology, Victoria, BC, Canada, 4–7 October 2009; Association for Computing Machinery: New York, NY, USA, 2009; pp. 157–166. [Google Scholar]
- Treacy Solovey, E.; Afergan, D.; Peck, E.M.; Hincks, S.W.; Jacob, R.J.K. Designing Implicit Interfaces for Physiological Computing: Guidelines and Lessons Learned Using FNIRS. ACM Trans. Comput.-Hum. Interact. 2015, 21, 35:1–35:27. [Google Scholar] [CrossRef]
- Cui, X.; Bray, S.; Bryant, D.M.; Glover, G.H.; Reiss, A.L. A Quantitative Comparison of NIRS and FMRI across Multiple Cognitive Tasks. NeuroImage 2011, 54, 2808–2821. [Google Scholar] [CrossRef] [PubMed]
- Eggebrecht, A.T.; White, B.R.; Ferradal, S.L.; Chen, C.; Zhan, Y.; Snyder, A.Z.; Dehghani, H.; Culver, J.P. A Quantitative Spatial Comparison of High-Density Diffuse Optical Tomography and FMRI Cortical Mapping. NeuroImage 2012, 61, 1120–1128. [Google Scholar] [CrossRef]
- Huppert, T.J.; Hoge, R.D.; Diamond, S.G.; Franceschini, M.A.; Boas, D.A. A Temporal Comparison of BOLD, ASL, and NIRS Hemodynamic Responses to Motor Stimuli in Adult Humans. NeuroImage 2006, 29, 368–382. [Google Scholar] [CrossRef] [PubMed]
- Balardin, J.B.; Zimeo Morais, G.A.; Furucho, R.A.; Trambaiolli, L.; Vanzella, P.; Biazoli, C.J.; Sato, J.R. Imaging Brain Function with Functional Near-Infrared Spectroscopy in Unconstrained Environments. Front. Hum. Neurosci. 2017, 11. [Google Scholar] [CrossRef]
- Piper, S.K.; Krueger, A.; Koch, S.P.; Mehnert, J.; Habermehl, C.; Steinbrink, J.; Obrig, H.; Schmitz, C.H. A Wearable Multi-Channel FNIRS System for Brain Imaging in Freely Moving Subjects. Neuroimage 2014, 85. [Google Scholar] [CrossRef]
- Pinti, P.; Aichelburg, C.; Lind, F.; Power, S.; Swingler, E.; Merla, A.; Hamilton, A.; Gilbert, S.; Burgess, P.; Tachtsidis, I. Using Fiberless, Wearable FNIRS to Monitor Brain Activity in Real-World Cognitive Tasks. JoVE (J. Vis. Exp.) 2015, e53336. [Google Scholar] [CrossRef]
- Baker, J.M.; Rojas-Valverde, D.; Gutiérrez, R.; Winkler, M.; Fuhrimann, S.; Eskenazi, B.; Reiss, A.L.; Mora Ana, M. Portable Functional Neuroimaging as an Environmental Epidemiology Tool: A How-To Guide for the Use of FNIRS in Field Studies. Environ. Health Perspect. 2017, 125, 094502. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, K.; Oka, N.; Yamamoto, K.; Takahashi, H.; Kato, T. Functional Brain Imaging Using Near-Infrared Spectroscopy during Actual Driving on an Expressway. Front. Hum. Neurosci. 2013, 7. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.-P.; Tan, F.-L.; Zhang, Z.; Jiang, Y.-H.; Zhao, Y.; Zhu, C.-Z. Feasibility of Functional Near-Infrared Spectroscopy (FNIRS) to Investigate the Mirror Neuron System: An Experimental Study in a Real-Life Situation. Front. Hum. Neurosci. 2018, 12. [Google Scholar] [CrossRef]
- Nihashi, T.; Ishigaki, T.; Satake, H.; Ito, S.; Kaii, O.; Mori, Y.; Shimamoto, K.; Fukushima, H.; Suzuki, K.; Umakoshi, H.; et al. Monitoring of Fatigue in Radiologists during Prolonged Image Interpretation Using FNIRS. Jpn. J. Radiol. 2019, 37, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, D.; Leehr, E.J.; Rubel, J.; Maier, M.J.; Pagliaro, V.; Deutsch, K.; Hudak, J.; Metzger, F.G.; Fallgatter, A.J.; Ehlis, A.-C. Cortical Oxygenation during Exposure Therapy – in Situ FNIRS Measurements in Arachnophobia. NeuroImage Clin. 2020, 26, 102219. [Google Scholar] [CrossRef]
- Slutter, M.W.J.; Thammasan, N.; Poel, M. Exploring the Brain Activity Related to Missing Penalty Kicks: An FNIRS Study. Front. Comput. Sci. 2021, 3. [Google Scholar] [CrossRef]
- Okamoto, M.; Dan, H.; Shimizu, K.; Takeo, K.; Amita, T.; Oda, I.; Konishi, I.; Sakamoto, K.; Isobe, S.; Suzuki, T.; et al. Multimodal Assessment of Cortical Activation during Apple Peeling by NIRS and FMRI. NeuroImage 2004, 21, 1275–1288. [Google Scholar] [CrossRef]
- Ayaz, H.; Onaral, B.; Izzetoglu, K.; Shewokis, P.A.; McKendrick, R.; Parasuraman, R. Continuous Monitoring of Brain Dynamics with Functional near Infrared Spectroscopy as a Tool for Neuroergonomic Research: Empirical Examples and a Technological Development. Front. Hum. Neurosci. 2013, 7. [Google Scholar] [CrossRef]
- Causse, M.; Chua, Z.; Peysakhovich, V.; Del Campo, N.; Matton, N. Mental Workload and Neural Efficiency Quantified in the Prefrontal Cortex Using FNIRS. Sci. Rep. 2017, 7, 5222. [Google Scholar] [CrossRef]
- Ahn, S.; Nguyen, T.; Jang, H.; Kim, J.G.; Jun, S.C. Exploring Neuro-Physiological Correlates of Drivers’ Mental Fatigue Caused by Sleep Deprivation Using Simultaneous EEG, ECG, and FNIRS Data. Front. Hum. Neurosci. 2016, 10. [Google Scholar] [CrossRef]
- Sibi, S.; Balters, S.; Mok, B.K.; Steinert, M.; Ju, W. Assessing Driver Cortical Activity under Varying Levels of Automation with Functional near Infrared Spectroscopy. In Proceedings of the 2017 IEEE Intelligent Vehicles Symposium (IV), Los Angeles, CA, USA, 11–14 June 2017; IEEE: Los Angeles, CA, USA, 2017; pp. 1509–1516. [Google Scholar]
- Perpetuini, D.; Chiarelli, A.M.; Cardone, D.; Filippini, C.; Bucco, R.; Zito, M.; Merla, A. Complexity of Frontal Cortex FNIRS Can Support Alzheimer Disease Diagnosis in Memory and Visuo-Spatial Tests. Entropy 2019, 21, 26. [Google Scholar] [CrossRef]
- Lin, C.-C.; Barker, J.W.; Sparto, P.J.; Furman, J.M.; Huppert, T.J. Functional Near-Infrared Spectroscopy (FNIRS) Brain Imaging of Multi-Sensory Integration during Computerized Dynamic Posturography in Middle-Aged and Older Adults. Exp. Brain Res. 2017, 235, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- de Campos, A.C.; Sukal-Moulton, T.; Huppert, T.; Alter, K.; Damiano, D.L. Brain Activation Patterns Underlying Upper Limb Bilateral Motor Coordination in Unilateral Cerebral Palsy: An FNIRS Study. Dev. Med. Child Neurol. 2020, 62, 625–632. [Google Scholar] [CrossRef]
- Büssing, A.; Michalsen, A.; Khalsa, S.B.S.; Telles, S.; Sherman, K.J. Effects of Yoga on Mental and Physical Health: A Short Summary of Reviews. Evid.-Based Complement. Altern. Med. 2012, 2012, 165410. [Google Scholar] [CrossRef]
- Gard, T.; Noggle, J.J.; Park, C.L.; Vago, D.R.; Wilson, A. Potential Self-Regulatory Mechanisms of Yoga for Psychological Health. Front. Hum. Neurosci. 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Pascoe, M.C.; Bauer, I.E. A Systematic Review of Randomised Control Trials on the Effects of Yoga on Stress Measures and Mood. J. Psychiatr. Res. 2015, 68, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Telles, S.; Joshi, M.; Dash, M.; Raghuraj, P.; Naveen, K.V.; Nagendra, H.R. An Evaluation of the Ability to Voluntarily Reduce the Heart Rate after a Month of Yoga Practice. Integr. Psych. Behav. 2004, 39, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Cowen, V.S.; Adams, T.B. Heart Rate in Yoga Asana Practice: A Comparison of Styles. J. Bodyw. Mov. Ther. 2007, 11, 91–95. [Google Scholar] [CrossRef]
- Jarry, J.L.; Chang, F.M.; La Civita, L. Ashtanga Yoga for Psychological Well-Being: Initial Effectiveness Study. Mindfulness 2017, 8, 1269–1279. [Google Scholar] [CrossRef]
- Mikkonen, J.; Pedersen, P.; McCarthy, P.W. A Survey of Musculoskeletal Injury among Ashtanga Vinyasa Yoga Practitioners. Int. J. Yoga Therap. 2008, 18, 59–64. [Google Scholar] [CrossRef]
- Sorosky, S.; Stilp, S.; Akuthota, V. Yoga and Pilates in the Management of Low Back Pain. Curr. Rev. Musculoskelet. Med. 2008, 1, 39–47. [Google Scholar] [CrossRef]
- Smith, B.R. Body, Mind and Spirit? Towards an Analysis of the Practice of Yoga. Body Soc. 2007, 13, 25–46. [Google Scholar] [CrossRef]
- Smith, B.R. Adjusting the Quotidian: Ashtanga Yoga as Everyday Practice; Citeseer: Princeton, NJ, USA, 2004. [Google Scholar]
- Cowen, V.S.; Adams, T.B. Physical and Perceptual Benefits of Yoga Asana Practice: Results of a Pilot Study. J. Bodyw. Mov. Ther. 2005, 9, 211–219. [Google Scholar] [CrossRef]
- Merriam-Webster Omphaloskepsis. Available online: https://www.merriam-webster.com/dictionary/omphaloskepsis (accessed on 10 November 2020).
- Streeter, C.C.; Gerbarg, P.L.; Saper, R.B.; Ciraulo, D.A.; Brown, R.P. Effects of Yoga on the Autonomic Nervous System, Gamma-Aminobutyric-Acid, and Allostasis in Epilepsy, Depression, and Post-Traumatic Stress Disorder. Med. Hypotheses 2012, 78, 571–579. [Google Scholar] [CrossRef]
- Cho, H.K.; Moon, W.; Kim, J. Effects of Yoga on Stress and Inflammatory Factors in Patients with Chronic Low Back Pain: A Non-Randomized Controlled Study. Eur. J. Integr. Med. 2015, 7, 118–123. [Google Scholar] [CrossRef]
- Fiori, F.; David, N.; Aglioti, S.M. Processing of Proprioceptive and Vestibular Body Signals and Self-Transcendence in Ashtanga Yoga Practitioners. Front. Hum. Neurosci. 2014, 8. [Google Scholar] [CrossRef]
- Benavides, S.; Caballero, J. Ashtanga Yoga for Children and Adolescents for Weight Management and Psychological Well Being: An Uncontrolled Open Pilot Study. Complement. Ther. Clin. Pract. 2009, 15, 110–114. [Google Scholar] [CrossRef]
- van Aalst, J.; Ceccarini, J.; Schramm, G.; Van Weehaeghe, D.; Rezaei, A.; Demyttenaere, K.; Sunaert, S.; Van Laere, K. Long-Term Ashtanga Yoga Practice Decreases Medial Temporal and Brainstem Glucose Metabolism in Relation to Years of Experience. EJNMMI Res. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Talwadkar, S.; Jagannathan, A.; Raghuram, N. Effect of Trataka on Cognitive Functions in the Elderly. Int. J. Yoga 2014, 7, 96. [Google Scholar] [CrossRef] [PubMed]
- Vhavle, S.P.; Rao, R.M.; Manjunath, N.K. Comparison of Yoga versus Physical Exercise on Executive Function, Attention, and Working Memory in Adolescent Schoolchildren: A Randomized Controlled Trial. Int. J. Yoga 2019, 12, 172. [Google Scholar] [CrossRef]
- Bhargav, H.; Bn, G.; Raghuram, N.; Hr, N. Frontal Hemodynamic Responses to High Frequency Yoga Breathing in Schizophrenia: A Functional Near-Infrared Spectroscopy Study. Front. Psychiatry 2014, 5. [Google Scholar] [CrossRef]
- Dev, P.; Lancet, R.S.; Saurav, S.; Seshadri, N.P.G.; Kumar Singh, B.; Jha, M. Effect of Yoga on Hemodynamic Changes at Prefrontal Cortex during Sustained Attention Task. In Proceedings of the 2019 5th International Conference on Advanced Computing Communication Systems (ICACCS), Coimbatore, India, 15–16 March 2019; pp. 728–731. [Google Scholar]
- Kora, P.; Meenakshi, K.; Swaraja, K.; Rajani, A.; Raju, M.S. EEG Based Interpretation of Human Brain Activity during Yoga and Meditation Using Machine Learning: A Systematic Review. Complement. Ther. Clin. Pract. 2021, 43, 101329. [Google Scholar] [CrossRef]
- Fishburn, F.A.; Norr, M.E.; Medvedev, A.V.; Vaidya, C.J. Sensitivity of FNIRS to Cognitive State and Load. Front. Hum. Neurosci. 2014, 8. [Google Scholar] [CrossRef]
- Miller, E.K.; Freedman, D.J.; Wallis, J.D. The Prefrontal Cortex: Categories, Concepts and Cognition. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2002, 357, 1123–1136. [Google Scholar] [CrossRef]
- Vanderhasselt, M.-A.; De Raedt, R.; Baeken, C. Dorsolateral Prefrontal Cortex and Stroop Performance: Tackling the Lateralization. Psychon. Bull. Rev. 2009, 16, 609–612. [Google Scholar] [CrossRef]
- Funahashi, S. Working Memory in the Prefrontal Cortex. Brain Sci. 2017, 7, 49. [Google Scholar] [CrossRef]
- MacDonald, A.W.; Cohen, J.D.; Stenger, V.A.; Carter, C.S. Dissociating the Role of the Dorsolateral Prefrontal and Anterior Cingulate Cortex in Cognitive Control. Science 2000, 288, 1835–1838. [Google Scholar] [CrossRef]
- Kaller, C.P.; Rahm, B.; Spreer, J.; Weiller, C.; Unterrainer, J.M. Dissociable Contributions of Left and Right Dorsolateral Prefrontal Cortex in Planning. Cereb. Cortex 2011, 21, 307–317. [Google Scholar] [CrossRef]
- Vassena, E.; Gerrits, R.; Demanet, J.; Verguts, T.; Siugzdaite, R. Anticipation of a Mentally Effortful Task Recruits Dorsolateral Prefrontal Cortex: An FNIRS Validation Study. Neuropsychologia 2019, 123, 106–115. [Google Scholar] [CrossRef]
- Mansouri, F.A.; Koechlin, E.; Rosa, M.G.P.; Buckley, M.J. Managing Competing Goals—A Key Role for the Frontopolar Cortex. Nat. Rev. Neurosci. 2017, 18, 645–657. [Google Scholar] [CrossRef]
- Boschin, E.A.; Piekema, C.; Buckley, M.J. Essential Functions of Primate Frontopolar Cortex in Cognition. Proc. Natl. Acad. Sci. USA 2015, 112, E1020–E1027. [Google Scholar] [CrossRef]
- Ashtanga Yoga Full Primary Series with Ty Landrum. Available online: https://www.youtube.com/watch?v=K-s4IIxVBc8&t=2607s (accessed on 10 November 2020).
- Oostenveld, R.; Praamstra, P. The Five Percent Electrode System for High-Resolution EEG and ERP Measurements. Clin. Neurophysiol. 2001, 112, 713–719. [Google Scholar] [CrossRef]
- Aasted, C.M.; Yücel, M.A.; Cooper, R.J.; Dubb, J.; Tsuzuki, D.; Becerra, L.; Petkov, M.P.; Borsook, D.; Dan, I.; Boas, D.A. Anatomical Guidance for Functional Near-Infrared Spectroscopy: AtlasViewer Tutorial. Neurophotonics 2015, 2. [Google Scholar] [CrossRef]
- Fonov, V.; Evans, A.C.; Botteron, K.; Almli, C.R.; McKinstry, R.C.; Collins, D.L. Unbiased Average Age-Appropriate Atlases for Pediatric Studies. NeuroImage 2011, 54, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Fonov, V.; Evans, A.; McKinstry, R.; Almli, C.; Collins, D. Unbiased Nonlinear Average Age-Appropriate Brain Templates from Birth to Adulthood. NeuroImage 2009, 47, S102. [Google Scholar] [CrossRef]
- Santosa, H.; Zhai, X.; Fishburn, F.; Huppert, T. The NIRS Brain AnalyzIR Toolbox. Algorithms 2018, 11, 73. [Google Scholar] [CrossRef]
- NIRStar|FNIRS Systems|NIRS Devices|NIRx. Available online: https://nirx.net/nirstar-1 (accessed on 17 December 2019).
- LabStreamingLayer. Available online: https://labstreaminglayer.readthedocs.io/index.html (accessed on 28 April 2021).
- The IMotions Platform; iMotions: Copenhagen, Denmark, 2020.
- Friard, O.; Gamba, M. BORIS: A Free, Versatile Open-Source Event-Logging Software for Video/Audio Coding and Live Observations. Methods Ecol. Evol. 2016, 7, 1325–1330. [Google Scholar] [CrossRef]
- Jacques, S.L. Optical Properties of Biological Tissues: A Review. Phys. Med. Biol. 2013, 58, R37–R61. [Google Scholar] [CrossRef]
- Barker, J.W.; Aarabi, A.; Huppert, T.J. Autoregressive Model Based Algorithm for Correcting Motion and Serially Correlated Errors in FNIRS. Biomed. Opt. Express 2013, 4, 1366. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Huppert, T.J. Commentary on the Statistical Properties of Noise and Its Implication on General Linear Models in Functional Near-Infrared Spectroscopy. Neurophotonics 2016, 3, 010401. [Google Scholar] [CrossRef]
- Fishburn, F.A.; Ludlum, R.S.; Vaidya, C.J.; Medvedev, A.V. Temporal Derivative Distribution Repair (TDDR): A Motion Correction Method for FNIRS. NeuroImage 2019, 184, 171–179. [Google Scholar] [CrossRef] [PubMed]
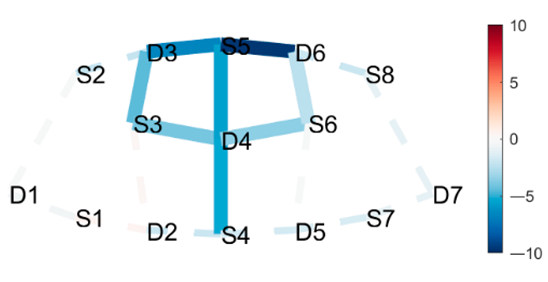
| Posture 1 | Contrast Statistics Visualized on Probe Montage | Posture 2 |
|---|---|---|
| Boat | HbO | Mountain Pose Start |
 |  |  |
| Mountain Pose Start | HbO | MA Right Knee Bent Bind |
 |  |  |
| Boat | HbO | Lotus |
 | 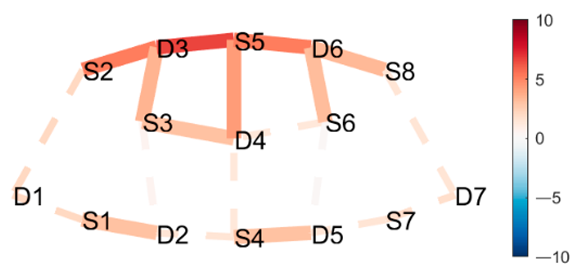 |  |
| Posture 1 | Contrast Statistics Visualized on Probe Montage | Posture 2 |
|---|---|---|
| Head to Knee Pose B, sit on right heel. | HbO | Extended Hand to Right Big Toe Hold |
 |  |  |
| Lotus Uplifting | HbO | Warrior 2 Left |
 |  |  |
| Mountain Pose Start | HbR | Extended Hand to Right Big Toe Hold |
 | 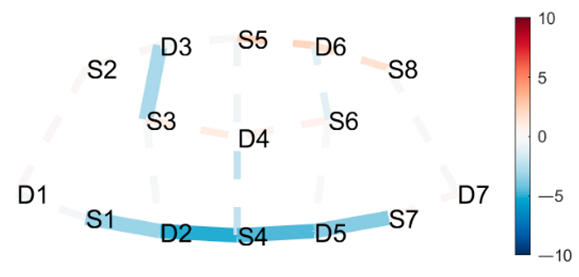 |  |
| Posture 1 | Contrast Statistics Visualized on Probe Montage | Posture 2 |
|---|---|---|
| Lotus Uplifting | HbO | Lotus |
 |  |  |
| West Side Intense Stretch | HbO | Warrior 2 Right |
 | 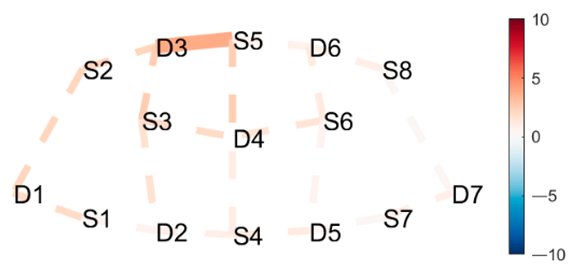 |  |
| Triangle Left | HbO | Triangle Right |
 | 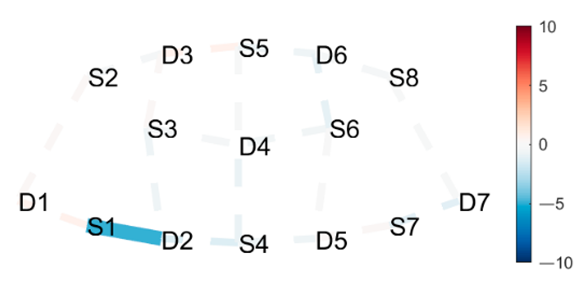 |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dybvik, H.; Steinert, M. Real-World fNIRS Brain Activity Measurements during Ashtanga Vinyasa Yoga. Brain Sci. 2021, 11, 742. https://doi.org/10.3390/brainsci11060742
Dybvik H, Steinert M. Real-World fNIRS Brain Activity Measurements during Ashtanga Vinyasa Yoga. Brain Sciences. 2021; 11(6):742. https://doi.org/10.3390/brainsci11060742
Chicago/Turabian StyleDybvik, Henrikke, and Martin Steinert. 2021. "Real-World fNIRS Brain Activity Measurements during Ashtanga Vinyasa Yoga" Brain Sciences 11, no. 6: 742. https://doi.org/10.3390/brainsci11060742
APA StyleDybvik, H., & Steinert, M. (2021). Real-World fNIRS Brain Activity Measurements during Ashtanga Vinyasa Yoga. Brain Sciences, 11(6), 742. https://doi.org/10.3390/brainsci11060742





