Longitudinal Impact of Physical Activity on Brain Pulsatility Index and Cognition in Older Adults with Cardiovascular Risk Factors: A NIRS Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Screening Procedure
2.2. Blood Draw and Blood Pressure Measurement
2.3. Cognitive Assessment
2.4. Assessment of the CVRF and Intensity of Physical Activity
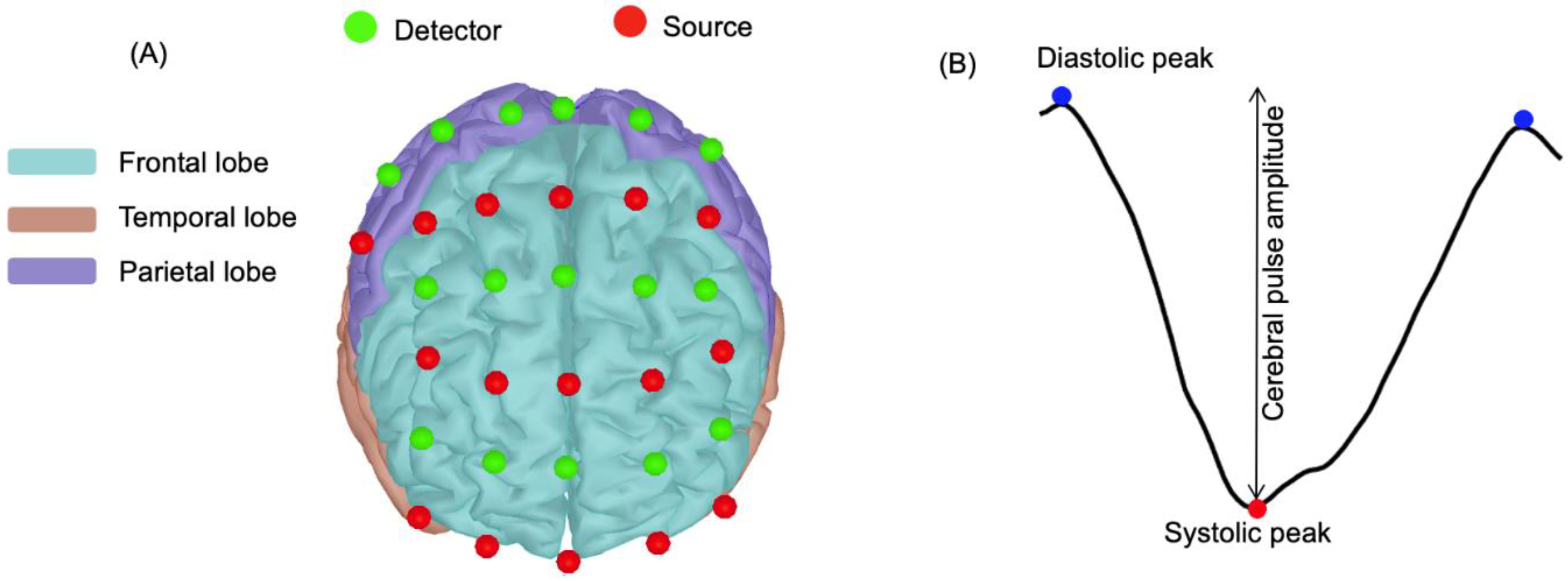
2.5. NIRS Device and Optodes Configuration
2.6. Walking Paradigm for NIRS Recording
2.7. Longitudinal Follow-Up of Physical Activity
2.8. Pulsatility Parameters
2.9. Analysis of the NIRS Data for Extracting Pulsatility Parameters
2.10. Data Exploration and Statistical Analysis
3. Results
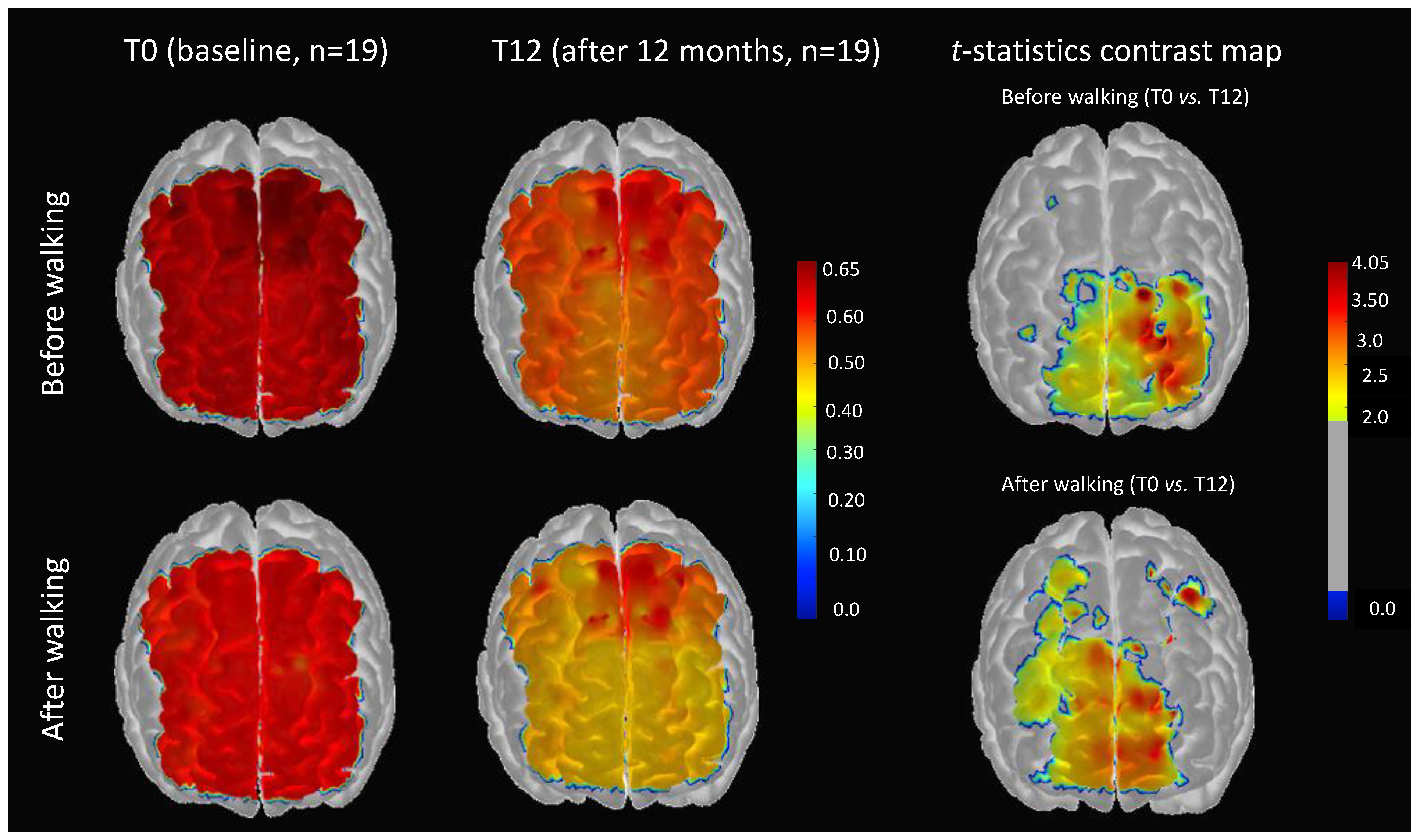
3.1. Impact of 12 Months of Physical Activity on Cerebral Pulse Amplitude
3.2. Impact of 12 Months of Physical Activity on Changes of Cerebral Pulse Amplitude Immediately after Short-Duration Walking
3.3. Relationship Between Reduction in Cerebral Pulse Amplitude and Behavioral Cognitive Performance after 12 Months of Physical Activity
4. Discussion
4.1. Cerebral Pulse Amplitude Is a Proxy Index of Arterial Stiffness
4.2. Impact of 12 Months of Physical Activity on Cerebral Pulsatility Index
4.3. Impact of 12 Months of Physical Activity on Changes in the Cerebral Pulsatility Index Immediately after Short-Duration Walking
4.4. Reducing Cerebral Pulsatility Index Is Associated with Improving Executive Functions
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonald, D.A. Blood flow in arteries. Edward Arnold Publ. 2011. [Google Scholar] [CrossRef]
- Mitchell, G.F. Aortic stiffness, pressure and flow pulsatility, and target organ damage. J. Appl. Physiol. 2018, 125, 1871–1880. [Google Scholar] [CrossRef]
- O’Rourke, M.F.; Hashimoto, J. Mechanical factors in arterial aging: A clinical perspective. J. Am. Coll. Cardiol. 2007, 50, 1–13. [Google Scholar] [CrossRef]
- Pase, M.P.; Grima, N.A.; Stough, C.K.; Scholey, A.; Pipingas, A. Cardiovascular disease risk and cerebral blood flow velocity. Stroke 2012, 43, 2803–2805. [Google Scholar] [CrossRef]
- Wåhlin, A.; Ambarki, K.; Birgander, R.; Malm, J.; Eklund, A. Intracranial pulsatility is associated with regional brain volume in elderly individuals. Neurobiol. Aging 2014, 35, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Ainslie, P.N.; Cotter, J.D.; George, K.P.; Lucas, S.; Murrell, C.; Shave, R.; Atkinson, G. Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. J. Physiol. 2008, 586, 4005–4010. [Google Scholar] [CrossRef] [PubMed]
- Seals, D.R.; Walker, A.E.; Pierce, G.L.; Lesniewski, L.A. Habitual exercise and vascular ageing. J. Physiol. 2009, 587, 5541–5549. [Google Scholar] [CrossRef] [PubMed]
- Bherer, L.; Erickson, K.I.; Liu-Ambrose, T. A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. J. Aging Res. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Bliss, E.S.; Wong, R.H.X.; Howe, P.R.C.; Mills, D.E. Benefits of exercise training on cerebrovascular and cognitive function in ageing. J. Cereb. Blood Flow Metab. 2021, 41, 447–470. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Tanabe, T.; Otsuki, T.; Sugawara, J.; Ajisaka, R.; Matsuda, M. Acute exercise increases systemic arterial compliance after 6-month exercise training in older women. Hypertens. Res. 2008, 31, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H.; Vincent, T.; Peng, K.; Nigam, A.; Gayda, M.; Fraser, S.; Joanette, Y.; Lesage, F.; Bherer, L. Coronary artery disease and its impact on the pulsatile brain: A functional NIRS study. Hum. Brain Mapp. 2021. [Google Scholar] [CrossRef] [PubMed]
- Perissiou, M.; Bailey, T.G.; Windsor, M.; Nam, M.C.Y.; Greaves, K.; Leicht, A.S.; Askew, C.D. Effects of exercise intensity and cardiorespiratory fitness on the acute response of arterial stiffness to exercise in older adults. Eur. J. Appl. Physiol. 2018, 118, 1673–1688. [Google Scholar] [CrossRef]
- Wielemborek-Musial, K.; Szmigielska, K.; Leszczynska, J.; Jegier, A. Blood Pressure Response to Submaximal Exercise Test in Adults. BioMed. Res. Int. 2016, 2016. [Google Scholar] [CrossRef]
- Chuang, S.Y.; Cheng, H.M.; Bai, C.H.; Yeh, W.T.; Chen, J.R.; Pan, W.H. Blood Pressure, Carotid Flow Pulsatility, and the Risk of Stroke: A Community-Based Study. Stroke 2016, 47, 2262–2268. [Google Scholar] [CrossRef] [PubMed]
- Ozari, H.O.; Oktenli, C.; Celik, S.; Tangi, F.; Ipcioglu, O.; Terekeci, H.M.; Nalbant, S. Are increased carotid artery pulsatility and resistance indexes early signs of vascular abnormalities in young obese males? J. Clin. Ultrasound 2012, 40, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A.; Ahmadi, M.; Shemshaki, H. The value of transcranial Doppler derived pulsatility index for diagnosing cerebral small-vessel disease. Adv. Biomed. Res. 2015, 4, 54. [Google Scholar] [CrossRef]
- Al-Jehani, H. The use of transcranial doppler pulsatility index to guide intracranial pressure monitoring in intoxicated traumatic brain injury patients. Saudi J. Med. Med. Sci. 2014, 2, 162. [Google Scholar] [CrossRef]
- Scuteri, A.; Wang, H. Pulse wave velocity as a marker of cognitive impairment in the elderly. J. Alzheimer’s Dis. 2014, 42, S401–S410. [Google Scholar] [CrossRef]
- Qawqzeh, Y.K. The Analysis of PPG Time Indices to Predict Aging Atherosclerosis. Learn. Anal. Intell. Syst. 2020. [Google Scholar] [CrossRef]
- Lawes, C. Blood pressure indices and cardiovascular disease in the Asia Pacific region: A pooled analysis. Hypertension 2003, 42, 69–75. [Google Scholar] [CrossRef]
- Chiarelli, A.M.; Fletcher, M.A.; Tan, C.H.; Low, K.A.; Maclin, E.L.; Zimmerman, B.; Fabiani, M. Individual differences in regional cortical volumes across the life span are associated with regional optical measures of arterial elasticity. NeuroImage 2017, 162, 199–213. [Google Scholar] [CrossRef]
- Mohammadi, H.; Peng, K.; Kassab, A.; Nigam, A.; Bherer, L.; Lesage, F.; Joanette, Y. Cortical Thinning is Associated with Brain Pulsatility in Older Adults: An MRI and NIRS Study. Neurobiol. Aging 2021. [Google Scholar] [CrossRef]
- Fabiani, M.; Low, K.A.; Tan, C.H.; Zimmerman, B.; Fletcher, M.A.; Schneider-Garces, N.; Gratton, G. Taking the pulse of aging: Mapping pulse pressure and elasticity in cerebral arteries with optical methods. Psychophysiology 2014, 51, 1072–1088. [Google Scholar] [CrossRef]
- Tan, C.H.; Low, K.A.; Kong, T.; Fletcher, M.A.; Zimmerman, B.; MacLin, E.L.; Fabiani, M. Mapping cerebral pulse pressure and arterial compliance over the adult lifespan with optical imaging. PLoS ONE 2017, 12, e0171305. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Low, K.A.; Schneider-Garces, N.; Zimmerman, B.; Fletcher, M.A.; Maclin, E.L.; Fabiani, M. Optical measures of changes in cerebral vascular tone during voluntary breath holding and a Sternberg memory task. Biol. Psychol. 2016, 118, 184–194. [Google Scholar] [CrossRef]
- Boidin, M.; Lapierre, G.; Paquette Tanir, L.; Nigam, A.; Juneau, M.; Guilbeault, V.; Gayda, M. Effect of aquatic interval training with Mediterranean diet counseling in obese patients: Results of a preliminary study. Ann. Phys. Rehabil. Med. 2015, 58, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Gayda, M.; Brun, C.; Juneau, M.; Levesque, S.; Nigam, A. Long-term cardiac rehabilitation and exercise training programs improve metabolic parameters in metabolic syndrome patients with and without coronary heart disease. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Fine, E.M.; Delis, D.C. Delis–Kaplan Executive Functioning System. In Encyclopedia of Clinical Neuropsychology; APA PsycTests: Washington, DC, USA, 2011; pp. 796–801. [Google Scholar] [CrossRef]
- Wilson, P.W.F.; D’Agostino, R.B.; Levy, D.; Belanger, A.M.; Silbershatz, H.; Kannel, W.B. Prediction of coronary heart disease using risk factor categories. Circulation 1998, 97, 1837–1847. [Google Scholar] [CrossRef]
- Logan, S.L.; Gottlieb, B.H.; Maitl, S.B.; Meegan, D.; Spriet, L.L. The physical activity scale for the elderly (PASE) questionnaire; Does it predict physical health? Int. J. Environ. Res. Public Health 2013, 10, 3967–3986. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.A.V. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Lareau, E.; Guillaume, S.; Frederic, L.; Dang, N.; Mohamad, S. Near infrared spectrometer combined with multichannel eeg for functional brain imaging. In Proceedings of the 2011 5th International Symposium on Medical Information and Communication Technology, Montreux, Switzerland, 27–30 March 2011; pp. 122–126. [Google Scholar]
- Kassab, A.; Le Lan, J.; Tremblay, J.; Vannasing, P.; Dehbozorgi, M.; Pouliot, P.; Nguyen, D.K. Multichannel wearable fNIRS-EEG system for long-term clinical monitoring. Hum. Brain Mapp. 2018, 39, 7–23. [Google Scholar] [CrossRef]
- Klem, G.H.; Lüders, H.O.; Jasper, H.H.; Elger, C. The ten-twenty electrode system of the International Federation. The International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999, 52, 3–6. [Google Scholar]
- Tadel, F.; Baillet, S.; Mosher, J.C.; Pantazis, D.; Leahy, R.M. Brainstorm: A user-friendly application for MEG/EEG analysis. Comput. Intell. Neurosci. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Tortora, G.J. A Brief Atlas of the Skeleton, Surface Anatomy and Selected Medical Images; John Wiley: Jakarta, Indonesia, 2006. [Google Scholar]
- Greenwood, P.M. Functional Plasticity in Cognitive Aging: Review and Hypothesis. Neuropsychology 2007, 21, 657–673. [Google Scholar] [CrossRef] [PubMed]
- Agbangla, N.F.; Audiffren, M.; Albinet, C.T. Use of near-infrared spectroscopy in the investigation of brain activation during cognitive aging: A systematic review of an emerging area of research. Ageing Res. Rev. 2017, 38, 52–66. [Google Scholar] [CrossRef]
- Desjardins, M. Vascular correlates of aging in the brain: Evidence from imaging data. IRBM 2015, 36, 158–165. [Google Scholar] [CrossRef]
- Salzman, T.; Tobón Vallejo, D.; Polskaia, N.; Michaud, L.; St-Amant, G.; Lajoie, Y.; Fraser, S. Hemodynamic and behavioral changes in older adults during cognitively demanding dual tasks. Brain Behav. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Themelis, G.; D’Arceuil, H.; Diamond, S.G.; Thaker, S.; Huppert, T.J.; Boas, D.A.; Franceschini, M.A. Near-infrared spectroscopy measurement of the pulsatile component of cerebral blood flow and volume from arterial oscillations. J. Biomed. Opt. 2007, 12, 014033. [Google Scholar] [CrossRef]
- Huppert, T.J.; Diamond, S.G.; Franceschini, M.A.; Boas, D.A. HomER: A review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl. Opt. 2009, 48, D280–D289. [Google Scholar] [CrossRef]
- Pollonini, L.; Olds, C.; Abaya, H.; Bortfeld, H.; Beauchamp, M.S.; Oghalai, J.S. Auditory cortex activation to natural speech and simulated cochlear implant speech measured with functional near-infrared spectroscopy. Hear. Res. 2014, 309, 84–93. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Aasted, C.M.; Yücel, M.A.; Cooper, R.J.; Dubb, J.; Tsuzuki, D.; Becerra, L.; Boas, D.A. Anatomical guidance for functional near-infrared spectroscopy: AtlasViewer tutorial. Neurophotonics 2015, 2, 020801. [Google Scholar] [CrossRef] [PubMed]
- Waldstein, S.R.; Rice, S.C.; Thayer, J.F.; Najjar, S.S.; Scuteri, A.; Zonderman, A.B. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension 2008, 51, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Cockcroft, J.; Van Bortel, L.; Boutouyrie, P.; Giannattasio, C.; Hayoz, D.; Struijker-Boudier, H. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J. 2006, 27, 2588–2605. [Google Scholar] [CrossRef] [PubMed]
- Westerhof, N.; Lankhaar, J.W.; Westerhof, B.E. The arterial windkessel. Med. Biol. Eng. Comput. 2009, 47, 131–141. [Google Scholar] [CrossRef]
- Lopes, S.; Afreixo, V.; Teixeira, M.; Garcia, C.; Leitão, C.; Gouveia, M.; Ribeiro, F. Exercise training reduces arterial stiffness in adults with hypertension: A systematic review and meta-analysis. J. Hypertens. 2021, 39, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.; Boreham, C.; Stehouwer, C. The Benefits of Exercise for Arterial Stiffness. Am. J. Hypertens. 2006, 19, 1037–1038. [Google Scholar] [CrossRef]
- Tanaka, H.; Safar, M.E. Influence of lifestyle modification on arterial stiffness and wave reflections. Am. J. Hypertens. 2005, 18, 137–144. [Google Scholar] [CrossRef]
- Taaffe, D.R.; Galvão, D.A.; Sharman, J.E.; Coombes, J.S. Reduced central blood pressure in older adults following progressive resistance training. J. Hum. Hypertens. 2007, 21, 96–98. [Google Scholar] [CrossRef]
- Femminella, G.D.; de Lucia, C.; Iacotucci, P.; Formisano, R.; Petraglia, L.; Allocca, E.; Ferrara, N. Neuro-hormonal effects of physical activity in the elderly. Front. Physiol. 2013. [Google Scholar] [CrossRef]
- Shirwany, N.A.; Zou, M.H. Arterial stiffness: A brief review. Acta Pharmacol. Sin. 2010, 31, 1267–1276. [Google Scholar] [CrossRef]
- Higashi, Y.; Sasaki, S.; Kurisu, S.; Yoshimizu, A.; Sasaki, N.; Matsuura, H.; Oshima, T. Regular aerobic exercise augments endothelium-dependent vascular relaxation in normotensive as well as hypertensive subjects: Role of endothelium-derived nitric oxide. Circulation 1999, 100, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Goto, C.; Nishioka, K.; Umemura, T.; Jitsuiki, D.; Sakagutchi, A.; Kawamura, M.; Higashi, Y. Acute Moderate-Intensity Exercise Induces Vasodilation Through an Increase in Nitric Oxide Bioavailiability in Humans. Am. J. Hypertens. 2007, 20, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Akazawa, N.; Tanahashi, K.; Kosaki, K.; Ra, S.G.; Matsubara, T.; Choi, Y.; Maeda, S. Aerobic exercise training enhances cerebrovascular pulsatility response to acute aerobic exercise in older adults. Physiol. Rep. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Salas-Gomez, D.; Fernandez-Gorgojo, M.; Pozueta, A.; Diaz-Ceballos, I.; Lamarain, M.; Perez, C.; Sanchez-Juan, P. Physical Activity Is Associated With Better Executive Function in University Students. Front. Hum. Neurosci. 2020, 14. [Google Scholar] [CrossRef] [PubMed]
- Fallik, D. Aerobic Exercise Improves Executive Function in Adults Aged 20–67. Neurol. Today 2019, 19, 24–26. [Google Scholar] [CrossRef]
- Diamond, A. Effects of Physical Exercise on Executive Functions: Going beyond Simply Moving to Moving with Thought. Ann. Sports Med. Res. 2015, 2, 1011. [Google Scholar] [PubMed]
- Chang, Y.K.; Chen, F.T.; Kuan, G.; Wei, G.X.; Chu, C.H.; Yan, J.; Hung, T.M. Effects of Acute Exercise Duration on the Inhibition Aspect of Executive Function in Late Middle-Aged Adults. Front. Aging Neurosci. 2019, 11, 227. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.; Lin, Y.C. Stopping ability in younger and older adults: Behavioral and event-related potential. Cogn. Affect. Behav. Neurosci. 2017, 17, 348–363. [Google Scholar] [CrossRef]
- Mitchell, G.F. Effects of central arterial aging on the structure and function of the peripheral vasculature: Implications for end-organ damage. J. Appl. Physiol. 2008, 105, 1652–1660. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.F. Cerebral small vessel disease: Role of aortic stiffness and pulsatile hemodynamics. J. Hypertens. 2015, 33, 2025–2028. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | CVRF_T0 (n = 19) | CVRF_T12 (n = 19) |
|---|---|---|
| Female/male (n) | 13/6 | 13/6 |
| Age (years) | 68.05 (4.99) | 69.05 (4.99) |
| Resting SBP (mmHg) | 124.57 (13.55) | 120.5 (12.09) |
| Resting DBP (mmHg) | 75.9 (5.68) | 75.88 (6.97) |
| Pulse pressure (mmHg) | 54.35 (11.33) | 50.61 (8.93) |
| BMI (kg/m2) | 26.99 (3.93) | 26.81 (3.59) |
| Smoking, n (%) | 4 (21.05) | 4 (21.05) |
| Walking distance (m) | 33.04 (3.90) | 33.23 (5.92) |
| Walking speed (m·s−1) | 1.10 (0.13) | 1.10 (0.19) |
| Physical activity | ||
| PASE score | 127.20 (68.51) | 124.90 (44.38) |
| Frequency of sessions per week | 1.94 (0.76) | 2.01 (0.87) |
| Duration of sessions (hour/week) | 3.45 (2.33) | 2.57 (1.52) |
| Intensity of physical activity * | 4.31 (2.11) | 4.07 (1.65) |
| Blood sample parameters | ||
| Total-cholesterol (mmol·L−1) | 4.47 (1.14) | 4.91 (1.29) |
| LDL-cholesterol (mmol·L−1) | 2.92 (1.92) | 2.81 (0.65) |
| HDL-cholesterol (mmol·L−1) | 1.35 (0.54) | 1.64 (0.29) |
| Medication therapy | ||
| Aspirin, n (%) | 1 (0.05) | 1 (0.05) |
| Beta-blockers, n (%) | 2 (10.53) | 1 (0.05) |
| Statin, n (%) | 4 (21.05) | 4 (21.05) |
| ACE-inhibitor, n (%) | 2 (11.54) | 3 (15.79) |
| ARA, n (%) | 3 (15.79) | 3 (15.79) |
| Stroop Condition | Response Time (T0) | Response Time (T12) | t-Test ** |
|---|---|---|---|
| Naming | 31.52 (7.41) | 29.105 (9.32) | p > 0.1 |
| Reading | 22.05 (3.68) | 21.26 (6.41) | p > 0.1 |
| Inhibition | 58.21 (20.57) | 54.05 (18.06) | p = 0.008 * |
| Switching | 63.05 (5.68) | 56.63 (18.35) | p = 0.025 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammadi, H.; Gagnon, C.; Vincent, T.; Kassab, A.; Fraser, S.; Nigam, A.; Lesage, F.; Bherer, L. Longitudinal Impact of Physical Activity on Brain Pulsatility Index and Cognition in Older Adults with Cardiovascular Risk Factors: A NIRS Study. Brain Sci. 2021, 11, 730. https://doi.org/10.3390/brainsci11060730
Mohammadi H, Gagnon C, Vincent T, Kassab A, Fraser S, Nigam A, Lesage F, Bherer L. Longitudinal Impact of Physical Activity on Brain Pulsatility Index and Cognition in Older Adults with Cardiovascular Risk Factors: A NIRS Study. Brain Sciences. 2021; 11(6):730. https://doi.org/10.3390/brainsci11060730
Chicago/Turabian StyleMohammadi, Hanieh, Christine Gagnon, Thomas Vincent, Ali Kassab, Sarah Fraser, Anil Nigam, Frédéric Lesage, and Louis Bherer. 2021. "Longitudinal Impact of Physical Activity on Brain Pulsatility Index and Cognition in Older Adults with Cardiovascular Risk Factors: A NIRS Study" Brain Sciences 11, no. 6: 730. https://doi.org/10.3390/brainsci11060730
APA StyleMohammadi, H., Gagnon, C., Vincent, T., Kassab, A., Fraser, S., Nigam, A., Lesage, F., & Bherer, L. (2021). Longitudinal Impact of Physical Activity on Brain Pulsatility Index and Cognition in Older Adults with Cardiovascular Risk Factors: A NIRS Study. Brain Sciences, 11(6), 730. https://doi.org/10.3390/brainsci11060730






