Narrative Review: Quantitative EEG in Disorders of Consciousness
Abstract
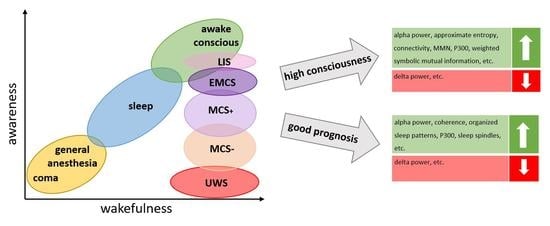
1. Introduction
2. Methods
- If sample sizes (n1, n2), means (mean1, mean2) and standard deviations (sd1, sd2) were available, we calculated d directly as
- If a t statistic and sample sizes were available, we used
- For F statistics with the first degree of freedom equal to one, resulting from a comparison between two groups, we first calculated a t statistic by taking the square root of F and then proceeded as above.
- For chi-squared statistics with one degree of freedom, we first transformed to a correlation viaand then to Cohen’s d via
- For an area under the curve (AUC), we usedwhere −1 is the inverse of the distribution function of the standard normal distribution.
- For 2 × 2 contingency tables, we performed Fisher’s exact test as implemented in R using the command fisher.test. The odds ratios (OR) and limits of their confidence intervals were then transformed using
- When confidence intervals were not given for the originally reported effect measure, we calculated confidence intervals for Cohen’s d using
3. Results
3.1. Diagnosis
3.1.1. Resting-State EEG
Spectral Power
Functional Connectivity
Dynamic Functional Connectivity
Graph Theory
Microstates
Nonlinear Measures
3.1.2. Sleep Patterns
3.1.3. Evoked Potentials
P300
Mismatch Negativity (MMN)
N100
N400
Late Positive Component (LPC)
Other Measures
3.2. Prognosis
3.2.1. Resting State
Spectral Power
Functional Connectivity
Graph Theory
Microstates
Nonlinear Measures
3.2.2. Sleep Patterns
3.2.3. Evoked Potentials
4. Conclusions and Discussion
4.1. Diagnosis
4.2. Prognosis
4.3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviation
| Abbreviation | Meaning |
| AUC | Area Under the Curve |
| CNC | Coma/Near Coma |
| CRS | Coma Recovery Scale |
| CRS-R | Coma Recovery Scale-Revised |
| CS | Conscious Subject |
| DOC | Disorders of Consciousness |
| DRS | Disability Rating Scale |
| EEG | Electroencephalogram |
| EMCS | Emerging Minimally Conscious State |
| fMRI | Functional Magnetic Resonance Imaging |
| GCS | Glasgow Coma Scale |
| GOS | Glasgow Outcome Scale |
| GOS-E | Glasgow Outcome Scale-Extended |
| GLS | Glasgow–Liège Scale |
| HC | Healthy Control |
| ICS | Innsbruck Coma Sale |
| LIS | Locked-In Syndrome |
| LPC | Late Positive Component |
| MCS | Minimally Conscious State |
| MCS+ | Minimally Conscious State Plus |
| MCS− | Minimally Conscious State Minus |
| MMN | Mismatch Negativity |
| Ms | Milliseconds |
| OFN | Other First Name |
| OR | Odds Ratio |
| PET | Positron Emission Tomography |
| QEEG | Quantitative Electroencephalogram |
| REM | Rapid Eye Movement |
| Sd | Standard Deviation |
| SND | Severe Neurocognitive Disorder |
| SON | Subject’s Own Name |
| TC | Tetraplegic Controls |
| TMS | Transcranial Magnetic Stimulation |
| UWS | Unresponsive Wakefulness Syndrome |
| WHIM | Wessex Head Injury Matrix |
References
- Siman-Tov, M.; Radomislensky, I.; Israel Trauma Group; Peleg, K. Reduction in trauma mortality in Israel during the last decade (2000–2010): The impact of changes in the trauma system. Injury 2013, 44, 1448–1452. [Google Scholar] [CrossRef]
- Plum, F.; Posner, J.B. Diagnosis of Stupor and Coma, 4th ed.; F. A. Davis Company: Philadelphia, PA, USA, 1966. [Google Scholar]
- Xie, Q.; Ni, X.; Yu, R.; Li, Y.; Huang, R. Chronic disorders of consciousness. Exp. Ther. Med. 2017, 14, 1277–1283. [Google Scholar] [CrossRef]
- Giacino, J.T.; Ashwal, S.; Childs, N.; Cranford, R.; Jennett, B.; Katz, D.I.; Kelly, J.P.; Rosenberg, J.H.; Whyte, J.; Zafonte, R.D.; et al. The minimally conscious state: Definition and diagnostic criteria. Neurology 2002, 58, 349–353. [Google Scholar] [CrossRef]
- Kretschmer, E. Das apallische Syndrom. Z. Für Gesamte Neurol. Psychiatr. 1940, 169, 576–579. [Google Scholar] [CrossRef]
- Gerstenbrand, F. Das Traumatische Apallische Syndrom: Klinik, Morphologie, Pathophysiologie und Behandlung; Springer: Wien, Austria, 1967; ISBN 978-3-7091-8168-3. [Google Scholar]
- Jennett, B.; Plum, F. Persistent vegetative state after brain damage. A syndrome in search of a name. Lancet Lond. Engl. 1972, 1, 734–737. [Google Scholar] [CrossRef]
- Von Wild, K.; Laureys, S.T.; Gerstenbrand, F.; Dolce, G.; Onose, G. The vegetative state—A syndrome in search of a name. J. Med. Life 2012, 5, 3–15. [Google Scholar]
- Bruno, M.-A.; Vanhaudenhuyse, A.; Thibaut, A.; Moonen, G.; Laureys, S. From unresponsive wakefulness to minimally conscious PLUS and functional locked-in syndromes: Recent advances in our understanding of disorders of consciousness. J. Neurol. 2011, 258, 1373–1384. [Google Scholar] [CrossRef]
- Bodart, O.; Laureys, S.; Gosseries, O. Coma and disorders of consciousness: Scientific advances and practical considerations for clinicians. Semin. Neurol. 2013, 33, 83–90. [Google Scholar] [CrossRef]
- Leon-Carrion, J.; Martin-Rodriguez, J.F.; Damas-Lopez, J.; Barroso y Martin, J.M.; Dominguez-Morales, M.R. Brain function in the minimally conscious state: A quantitative neurophysiological study. Clin. Neurophysiol. 2008, 119, 1506–1514. [Google Scholar] [CrossRef]
- Wu, D.-Y.; Cai, G.; Yuan, Y.; Liu, L.; Li, G.-Q.; Song, W.-Q.; Wang, M.-B. Application of nonlinear dynamics analysis in assessing unconsciousness: A preliminary study. Clin. Neurophysiol. 2011, 122, 490–498. [Google Scholar] [CrossRef]
- Seel, R.; Sherer, M.; Whyte, J.; Katz, D.; Giacino, J.; Rosenbaum, A.; Hammond, F.; Kalmar, K.; Pape, T.; Zafonte, R.; et al. Assessment Scales for Disorders of Consciousness: Evidence-Based Recommendations for Clinical Practice and Research. Arch. Phys. Med. Rehabil. 2010, 91, 1795–1813. [Google Scholar] [CrossRef]
- Teasdale, G.; Jennett, B. Assessment of coma and impaired consciousness. A practical scale. Lancet Lond. Engl. 1974, 2, 81–84. [Google Scholar] [CrossRef]
- Born, J.D. The Glasgow-Liège Scale. Acta Neurochir. (Wien.) 1988, 91, 1–11. [Google Scholar] [CrossRef]
- Benzer, A.; Mitterschiffthaler, G.; Marosi, M.; Luef, G.; Pühringer, F.; De La Renotiere, K.; Lehner, H.; Schmutzhard, E. Prediction of non-survival after trauma: Innsbruck Coma Scale. Lancet Lond. Engl. 1991, 338, 977–978. [Google Scholar] [CrossRef]
- Shiel, A.; Horn, S.A.; Wilson, B.A.; Watson, M.J.; Campbell, M.J.; McLellan, D.L. The Wessex Head Injury Matrix (WHIM) main scale: A preliminary report on a scale to assess and monitor patient recovery after severe head injury. Clin. Rehabil. 2000, 14, 408–416. [Google Scholar] [CrossRef]
- Giacino, J.T.; Kalmar, K.; Whyte, J. The JFK Coma Recovery Scale-Revised: Measurement characteristics and diagnostic utility. Arch. Phys. Med. Rehabil. 2004, 85, 2020–2029. [Google Scholar] [CrossRef]
- Rappaport, M.; Hall, K.M.; Hopkins, K.; Belleza, T.; Cope, D.N. Disability rating scale for severe head trauma: Coma to community. Arch. Phys. Med. Rehabil. 1982, 63, 118–123. [Google Scholar]
- Giacino, J.T.; Kezmarsky, M.A.; DeLuca, J.; Cicerone, K.D. Monitoring rate of recovery to predict outcome in minimally responsive patients. Arch. Phys. Med. Rehabil. 1991, 72, 897–901. [Google Scholar] [CrossRef]
- Rappaport, M. The Disability Rating and Coma/Near-Coma scales in evaluating severe head injury. Neuropsychol. Rehabil. 2005, 15, 442–453. [Google Scholar] [CrossRef]
- Nuwer, M. Assessment of digital EEG, quantitative EEG, and EEG brain mapping: Report of the American Academy of Neurology and the American Clinical Neurophysiology Society*. Neurology 1997, 49, 277–292. [Google Scholar] [CrossRef]
- Giacino, J.T.; Schnakers, C.; Rodriguez-Moreno, D.; Kalmar, K.; Schiff, N.; Hirsch, J. Behavioral assessment in patients with disorders of consciousness: Gold standard or fool’s gold? Prog. Brain Res. 2009, 177, 33–48. [Google Scholar]
- Song, M.; Yang, Y.; Yang, Z.; Cui, Y.; Yu, S.; He, J.; Jiang, T. Prognostic models for prolonged disorders of consciousness: An integrative review. Cell. Mol. Life Sci. CMLS 2020, 77, 3945–3961. [Google Scholar] [CrossRef]
- Bai, Y.; Xia, X.; Li, X. A Review of Resting-State Electroencephalography Analysis in Disorders of Consciousness. Front. Neurol. 2017, 8, 471. [Google Scholar] [CrossRef]
- Corchs, S.; Chioma, G.; Dondi, R.; Gasparini, F.; Manzoni, S.; Markowska-Kacznar, U.; Mauri, G.; Zoppis, I.; Morreale, A. Computational Methods for Resting-State EEG of Patients With Disorders of Consciousness. Front. Neurosci. 2019, 13, 807. [Google Scholar] [CrossRef] [PubMed]
- Annen, J.; Laureys, S.; Gosseries, O. Brain-computer interfaces for consciousness assessment and communication in severely brain-injured patients. Handb. Clin. Neurol. 2020, 168, 137–152. [Google Scholar]
- Bai, Y.; Lin, Y.; Ziemann, U. Managing disorders of consciousness: The role of electroencephalography. J. Neurol. 2020. [Google Scholar] [CrossRef]
- Comanducci, A.; Boly, M.; Claassen, J.; De Lucia, M.; Gibson, R.M.; Juan, E.; Laureys, S.; Naccache, L.; Owen, A.M.; Rosanova, M.; et al. Clinical and advanced neurophysiology in the prognostic and diagnostic evaluation of disorders of consciousness: Review of an IFCN-endorsed expert group. Clin. Neurophysiol. 2020, 131, 2736–2765. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Ramakrishnan, A.G. Electrophysiological and Neuroimaging Studies-During Resting State and Sensory Stimulation in Disorders of Consciousness: A Review. Front. Neurosci. 2020, 14, 555093. [Google Scholar] [CrossRef]
- Ragazzoni, A.; Cincotta, M.; Giovannelli, F.; Cruse, D.; Young, G.B.; Miniussi, C.; Rossi, S. Clinical neurophysiology of prolonged disorders of consciousness: From diagnostic stimulation to therapeutic neuromodulation. Clin. Neurophysiol. 2017, 128, 1629–1646. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Hu, N.; Wang, T. Music Interventions for Disorders of Consciousness: A Systematic Review and Meta-analysis. J. Neurosci. Nurs. J. Am. Assoc. Neurosci. Nurses 2020, 52, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Kotchoubey, B. Evoked and event-related potentials in disorders of consciousness: A quantitative review. Conscious. Cogn. 2017, 54, 155–167. [Google Scholar] [CrossRef]
- Bai, Y.; Xia, X.; Wang, Y.; He, J.; Li, X. Electroencephalography quadratic phase self-coupling correlates with consciousness states and restoration in patients with disorders of consciousness. Clin. Neurophysiol. 2019, 130, 1235–1242. [Google Scholar] [CrossRef]
- Noirhomme, Q.; Brecheisen, R.; Lesenfants, D.; Antonopoulos, G.; Laureys, S. “Look at my classifier’s result”: Disentangling unresponsive from (minimally) conscious patients. NeuroImage 2017, 145, 288–303. [Google Scholar] [CrossRef]
- Fidali, B.C.; Stevens, R.D.; Claassen, J. Novel approaches to prediction in severe brain injury. Curr. Opin. Neurol. 2020, 33, 669–675. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: Cambridge, MA, USA, 2013; ISBN 978-1-4832-7648-9. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 17 May 2021).
- Lenhard, W.; Lenhard, A. Calculation of Effect Sizes. Dettelb. (Germany) Psychom. 2016. Available online: https://www.psychometrica.de/effect_size.html (accessed on 17 May 2021). [CrossRef]
- Salgado, J.F. Transforming the Area under the Normal Curve (AUC) into Cohen’s d, Pearson’s rpb, Odds-Ratio, and Natural Log Odds-Ratio: Two Conversion Tables. Eur. J. Psychol. Appl. Leg. Context 2018, 10, 35–47. [Google Scholar] [CrossRef]
- Cooper, H.; Hedges, L.V.; Hedges, P.L.V. The Handbook of Research Synthesis; Russell Sage Foundation: New York, NY, USA, 1994; ISBN 978-0-87154-226-7. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Schnakers, C.; Majerus, S.; Laureys, S. Bispectral analysis of electroencephalogram signals during recovery from coma: Preliminary findings. Neuropsychol. Rehabil. 2005, 15, 381–388. [Google Scholar] [CrossRef]
- Schnakers, C.; Ledoux, D.; Majerus, S.; Damas, P.; Damas, F.; Lambermont, B.; Lamy, M.; Boly, M.; Vanhaudenhuyse, A.; Moonen, G.; et al. Diagnostic and prognostic use of bispectral index in coma, vegetative state and related disorders. Brain Inj. 2008, 22, 926–931. [Google Scholar] [CrossRef]
- Babiloni, C.; Pistoia, F.; Sarà, M.; Vecchio, F.; Buffo, P.; Conson, M.; Onorati, P.; Albertini, G.; Rossini, P.M. Resting state eyes-closed cortical rhythms in patients with locked-in-syndrome: An EEG study. Clin. Neurophysiol. 2010, 121, 1816–1824. [Google Scholar] [CrossRef]
- Pollonini, L.; Pophale, S.; Situ, N.; Wu, M.-H.; Frye, R.E.; Leon-Carrion, J.; Zouridakis, G. Information communication networks in severe traumatic brain injury. Brain Topogr. 2010, 23, 221–226. [Google Scholar] [CrossRef]
- Sarà, M.; Pistoia, F. Complexity loss in physiological time series of patients in a vegetative state. Nonlinear Dyn. Psychol. Life Sci. 2010, 14, 1–13. [Google Scholar]
- Gosseries, O.; Schnakers, C.; Ledoux, D.; Vanhaudenhuyse, A.; Bruno, M.-A.; Demertzi, A.; Noirhomme, Q.; Lehembre, R.; Damas, P.; Goldman, S.; et al. Automated EEG entropy measurements in coma, vegetative state/unresponsive wakefulness syndrome and minimally conscious state. Funct. Neurol. 2011, 26, 25–30. [Google Scholar]
- Sarà, M.; Pistoia, F.; Pasqualetti, P.; Sebastiano, F.; Onorati, P.; Rossini, P.M. Functional isolation within the cerebral cortex in the vegetative state: A nonlinear method to predict clinical outcomes. Neurorehabil. Neural Repair 2011, 25, 35–42. [Google Scholar] [CrossRef]
- Wu, D.; Cai, G.; Zorowitz, R.D.; Yuan, Y.; Wang, J.; Song, W. Measuring interconnection of the residual cortical functional islands in persistent vegetative state and minimal conscious state with EEG nonlinear analysis. Clin. Neurophysiol. 2011, 122, 1956–1966. [Google Scholar] [CrossRef]
- Fingelkurts, A.A.; Fingelkurts, A.A.; Bagnato, S.; Boccagni, C.; Galardi, G. EEG oscillatory states as neuro-phenomenology of consciousness as revealed from patients in vegetative and minimally conscious states. Conscious. Cogn. 2012, 21, 149–169. [Google Scholar] [CrossRef]
- Lehembre, R.; Marie-Aurélie, B.; Vanhaudenhuyse, A.; Chatelle, C.; Cologan, V.; Leclercq, Y.; Soddu, A.; Macq, B.; Laureys, S.; Noirhomme, Q. Resting-state EEG study of comatose patients: A connectivity and frequency analysis to find differences between vegetative and minimally conscious states. Funct. Neurol. 2012, 27, 41–47. [Google Scholar]
- Leon-Carrion, J.; Leon-Dominguez, U.; Pollonini, L.; Wu, M.-H.; Frye, R.E.; Dominguez-Morales, M.R.; Zouridakis, G. Synchronization between the anterior and posterior cortex determines consciousness level in patients with traumatic brain injury (TBI). Brain Res. 2012, 1476, 22–30. [Google Scholar] [CrossRef]
- King, J.-R.; Sitt, J.D.; Faugeras, F.; Rohaut, B.; El Karoui, I.; Cohen, L.; Naccache, L.; Dehaene, S. Information sharing in the brain indexes consciousness in noncommunicative patients. Curr. Biol. CB 2013, 23, 1914–1919. [Google Scholar] [CrossRef]
- Lechinger, J.; Bothe, K.; Pichler, G.; Michitsch, G.; Donis, J.; Klimesch, W.; Schabus, M. CRS-R score in disorders of consciousness is strongly related to spectral EEG at rest. J. Neurol. 2013, 260, 2348–2356. [Google Scholar] [CrossRef]
- Chennu, S.; Finoia, P.; Kamau, E.; Allanson, J.; Williams, G.B.; Monti, M.M.; Noreika, V.; Arnatkeviciute, A.; Canales-Johnson, A.; Olivares, F.; et al. Spectral signatures of reorganised brain networks in disorders of consciousness. PLoS Comput. Biol. 2014, 10, e1003887. [Google Scholar] [CrossRef]
- Höller, Y.; Thomschewski, A.; Bergmann, J.; Kronbichler, M.; Crone, J.S.; Schmid, E.V.; Butz, K.; Höller, P.; Nardone, R.; Trinka, E. Connectivity biomarkers can differentiate patients with different levels of consciousness. Clin. Neurophysiol. 2014, 125, 1545–1555. [Google Scholar] [CrossRef]
- Marinazzo, D.; Gosseries, O.; Boly, M.; Ledoux, D.; Rosanova, M.; Massimini, M.; Noirhomme, Q.; Laureys, S. Directed information transfer in scalp electroencephalographic recordings: Insights on disorders of consciousness. Clin. EEG Neurosci. 2014, 45, 33–39. [Google Scholar] [CrossRef]
- Sitt, J.D.; King, J.-R.; El Karoui, I.; Rohaut, B.; Faugeras, F.; Gramfort, A.; Cohen, L.; Sigman, M.; Dehaene, S.; Naccache, L. Large scale screening of neural signatures of consciousness in patients in a vegetative or minimally conscious state. Brain J. Neurol. 2014, 137, 2258–2270. [Google Scholar] [CrossRef] [PubMed]
- Rossi Sebastiano, D.; Panzica, F.; Visani, E.; Rotondi, F.; Scaioli, V.; Leonardi, M.; Sattin, D.; D’Incerti, L.; Parati, E.; Ferini Strambi, L.; et al. Significance of multiple neurophysiological measures in patients with chronic disorders of consciousness. Clin. Neurophysiol. 2015, 126, 558–564. [Google Scholar] [CrossRef]
- Naro, A.; Bramanti, P.; Leo, A.; Cacciola, A.; Bramanti, A.; Manuli, A.; Calabrò, R.S. Towards a method to differentiate chronic disorder of consciousness patients’ awareness: The Low-Resolution Brain Electromagnetic Tomography Analysis. J. Neurol. Sci. 2016, 368, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Piarulli, A.; Bergamasco, M.; Thibaut, A.; Cologan, V.; Gosseries, O.; Laureys, S. EEG ultradian rhythmicity differences in disorders of consciousness during wakefulness. J. Neurol. 2016, 263, 1746–1760. [Google Scholar] [CrossRef]
- Schorr, B.; Schlee, W.; Arndt, M.; Bender, A. Coherence in resting-state EEG as a predictor for the recovery from unresponsive wakefulness syndrome. J. Neurol. 2016, 263, 937–953. [Google Scholar] [CrossRef]
- Thul, A.; Lechinger, J.; Donis, J.; Michitsch, G.; Pichler, G.; Kochs, E.F.; Jordan, D.; Ilg, R.; Schabus, M. EEG entropy measures indicate decrease of cortical information processing in Disorders of Consciousness. Clin. Neurophysiol. 2016, 127, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Naro, A.; Bramanti, A.; Leo, A.; Cacciola, A.; Manuli, A.; Bramanti, P.; Calabrò, R.S. Shedding new light on disorders of consciousness diagnosis: The dynamic functional connectivity. Cortex 2018, 103, 316–328. [Google Scholar] [CrossRef]
- Stefan, S.; Schorr, B.; Lopez-Rolon, A.; Kolassa, I.-T.; Shock, J.P.; Rosenfelder, M.; Heck, S.; Bender, A. Consciousness Indexing and Outcome Prediction with Resting-State EEG in Severe Disorders of Consciousness. Brain Topogr. 2018, 31, 848–862. [Google Scholar] [CrossRef] [PubMed]
- Cacciola, A.; Naro, A.; Milardi, D.; Bramanti, A.; Malatacca, L.; Spitaleri, M.; Leo, A.; Muscoloni, A.; Cannistraci, C.V.; Bramanti, P.; et al. Functional Brain Network Topology Discriminates between Patients with Minimally Conscious State and Unresponsive Wakefulness Syndrome. J. Clin. Med. 2019, 8, 306. [Google Scholar] [CrossRef] [PubMed]
- Rizkallah, J.; Annen, J.; Modolo, J.; Gosseries, O.; Benquet, P.; Mortaheb, S.; Amoud, H.; Cassol, H.; Mheich, A.; Thibaut, A.; et al. Decreased integration of EEG source-space networks in disorders of consciousness. NeuroImage Clin. 2019, 23, 101841. [Google Scholar] [CrossRef] [PubMed]
- Bareham, C.A.; Roberts, N.; Allanson, J.; Hutchinson, P.J.A.; Pickard, J.D.; Menon, D.K.; Chennu, S. Bedside EEG predicts longitudinal behavioural changes in disorders of consciousness. NeuroImage Clin. 2020, 28, 102372. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Wang, J.; Guo, Y.; Lu, M.; Dong, Y.; Wei, X. Altered inter-frequency dynamics of brain networks in disorder of consciousness. J. Neural Eng. 2020, 17, 036006. [Google Scholar] [CrossRef]
- Naro, A.; Maggio, M.G.; Leo, A.; Calabrò, R.S. Multiplex and Multilayer Network EEG Analyses: A Novel Strategy in the Differential Diagnosis of Patients with Chronic Disorders of Consciousness. Int. J. Neural Syst. 2020, 2050052. [Google Scholar] [CrossRef]
- Lutkenhoff, E.S.; Nigri, A.; Rossi Sebastiano, D.; Sattin, D.; Visani, E.; Rosazza, C.; D’Incerti, L.; Bruzzone, M.G.; Franceschetti, S.; Leonardi, M.; et al. EEG Power spectra and subcortical pathology in chronic disorders of consciousness. Psychol. Med. 2020, 1–10. [Google Scholar] [CrossRef]
- Coleman, M.R.; Menon, D.K.; Fryer, T.D.; Pickard, J.D. Neurometabolic coupling in the vegetative and minimally conscious states: Preliminary findings. J. Neurol. Neurosurg. Psychiatry 2005, 76, 432–434. [Google Scholar] [CrossRef] [PubMed]
- Saletu, B.; Anderer, P.; Saletu-Zyhlarz, G.M. EEG Topography and Tomography (LORETA) in the Classification and Evaluation of the Pharmacodynamics of Psychotropic Drugs. Clin. EEG Neurosci. 2006, 37, 66–80. [Google Scholar] [CrossRef]
- Bastos, A.M.; Schoffelen, J.-M. A Tutorial Review of Functional Connectivity Analysis Methods and Their Interpretational Pitfalls. Front. Syst. Neurosci. 2016, 9, 175. [Google Scholar] [CrossRef]
- Hutchison, R.M.; Womelsdorf, T.; Allen, E.A.; Bandettini, P.A.; Calhoun, V.D.; Corbetta, M.; Penna, S.D.; Duyn, J.H.; Glover, G.H.; Gonzalez-Castillo, J.; et al. Dynamic functional connectivity: Promise, issues, and interpretations. NeuroImage 2013, 80, 360–378. [Google Scholar] [CrossRef] [PubMed]
- Sporns, O. Networks of the Brain; MIT Press: Cambridge, MA, USA, 2010; ISBN 978-0-262-28892-7. [Google Scholar]
- Kaminski, M.; Blinowska, K.J. Is Graph Theoretical Analysis a Useful Tool for Quantification of Connectivity Obtained by Means of EEG/MEG Techniques? Front. Neural Circuits 2018, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Michel, C.M.; Koenig, T. EEG microstates as a tool for studying the temporal dynamics of whole-brain neuronal networks: A review. NeuroImage 2018, 180, 577–593. [Google Scholar] [CrossRef] [PubMed]
- Oksenberg, A.; Gordon, C.; Arons, E.; Sazbon, L. Phasic activities of rapid eye movement sleep in vegetative state patients. Sleep 2001, 24, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Landsness, E.; Bruno, M.-A.; Noirhomme, Q.; Riedner, B.; Gosseries, O.; Schnakers, C.; Massimini, M.; Laureys, S.; Tononi, G.; Boly, M. Electrophysiological correlates of behavioural changes in vigilance in vegetative state and minimally conscious state. Brain J. Neurol. 2011, 134, 2222–2232. [Google Scholar] [CrossRef] [PubMed]
- Mertel, I.; Pavlov, Y.G.; Barner, C.; Müller, F.; Diekelmann, S.; Kotchoubey, B. Sleep in disorders of consciousness: Behavioral and polysomnographic recording. BMC Med. 2020, 18, 350. [Google Scholar] [CrossRef]
- Rossi Sebastiano, D.; Visani, E.; Panzica, F.; Sattin, D.; Bersano, A.; Nigri, A.; Ferraro, S.; Parati, E.; Leonardi, M.; Franceschetti, S. Sleep patterns associated with the severity of impairment in a large cohort of patients with chronic disorders of consciousness. Clin. Neurophysiol. 2018, 129, 687–693. [Google Scholar] [CrossRef]
- Cologan, V.; Drouot, X.; Parapatics, S.; Delorme, A.; Gruber, G.; Moonen, G.; Laureys, S. Sleep in the unresponsive wakefulness syndrome and minimally conscious state. J. Neurotrauma 2013, 30, 339–346. [Google Scholar] [CrossRef]
- Malinowska, U.; Chatelle, C.; Bruno, M.-A.; Noirhomme, Q.; Laureys, S.; Durka, P.J. Electroencephalographic profiles for differentiation of disorders of consciousness. Biomed. Eng. Online 2013, 12, 109. [Google Scholar] [CrossRef] [PubMed]
- De Biase, S.; Gigli, G.L.; Lorenzut, S.; Bianconi, C.; Sfreddo, P.; Rossato, G.; Basaldella, F.; Fuccaro, M.; Corica, A.; Tonon, D.; et al. The importance of polysomnography in the evaluation of prolonged disorders of consciousness: Sleep recordings more adequately correlate than stimulus-related evoked potentials with patients’ clinical status. Sleep Med. 2014, 15, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Forgacs, P.B.; Conte, M.M.; Fridman, E.A.; Voss, H.U.; Victor, J.D.; Schiff, N.D. Preservation of electroencephalographic organization in patients with impaired consciousness and imaging-based evidence of command-following. Ann. Neurol. 2014, 76, 869–879. [Google Scholar] [CrossRef]
- Mouthon, A.-L.; van Hedel, H.J.A.; Meyer-Heim, A.; Kurth, S.; Ringli, M.; Pugin, F.; Huber, R. High-density electroencephalographic recordings during sleep in children with disorders of consciousness. NeuroImage Clin. 2016, 11, 468–475. [Google Scholar] [CrossRef][Green Version]
- Wislowska, M.; Del Giudice, R.; Lechinger, J.; Wielek, T.; Heib, D.P.J.; Pitiot, A.; Pichler, G.; Michitsch, G.; Donis, J.; Schabus, M. Night and day variations of sleep in patients with disorders of consciousness. Sci. Rep. 2017, 7, 266. [Google Scholar] [CrossRef]
- Zieleniewska, M.; Duszyk, A.; Różański, P.; Pietrzak, M.; Bogotko, M.; Durka, P. Parametric Description of EEG Profiles for Assessment of Sleep Architecture in Disorders of Consciousness. Int. J. Neural Syst. 2019, 29, 1850049. [Google Scholar] [CrossRef]
- Chiappa, K.H. Evoked Potentials in Clinical Medicine; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1997; ISBN 978-0-397-51659-9. [Google Scholar]
- Sloan, T.B.; Jameson, L.; Janik, D. Chapter 7-EVOKED POTENTIALS. In Cottrell and Young’s Neuroanesthesia, 5th ed.; Cottrell, J.E., Young, W.L., Eds.; Mosby: Philadelphia, PA, USA, 2010; pp. 115–130. ISBN 978-0-323-05908-4. [Google Scholar]
- Munte, T.F.; Urbach, T.P.; Duzel, E.; Kutas, M. Event-related brain potentials in the study of human cognition and neuropsychology; Elsevier Science Publishers B.V.: Amsterdam, The Netherlands, 2000; p. 97. [Google Scholar]
- Schoenle, P.W.; Witzke, W. How vegetative is the vegetative state? Preserved semantic processing in VS patients—Evidence from N 400 event-related potentials. NeuroRehabilitation 2004, 19, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Kotchoubey, B.; Lang, S.; Mezger, G.; Schmalohr, D.; Schneck, M.; Semmler, A.; Bostanov, V.; Birbaumer, N. Information processing in severe disorders of consciousness: Vegetative state and minimally conscious state. Clin. Neurophysiol. 2005, 116, 2441–2453. [Google Scholar] [CrossRef]
- Perrin, F.; Schnakers, C.; Schabus, M.; Degueldre, C.; Goldman, S.; Brédart, S.; Faymonville, M.-E.; Lamy, M.; Moonen, G.; Luxen, A.; et al. Brain response to one’s own name in vegetative state, minimally conscious state, and locked-in syndrome. Arch. Neurol. 2006, 63, 562–569. [Google Scholar] [CrossRef]
- Schnakers, C.; Perrin, F.; Schabus, M.; Majerus, S.; Ledoux, D.; Damas, P.; Boly, M.; Vanhaudenhuyse, A.; Bruno, M.-A.; Moonen, G.; et al. Voluntary brain processing in disorders of consciousness. Neurology 2008, 71, 1614–1620. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Di, H.; Yan, X.; Yu, S.; Yu, D.; Laureys, S.; Weng, X. Mismatch negativity to the patient’s own name in chronic disorders of consciousness. Neurosci. Lett. 2008, 448, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Luaute, J.; Morlet, D. Event-related potentials (MMN and novelty P3) in permanent vegetative or minimally conscious states. Clin. Neurophysiol. 2010, 121, 1032–1042. [Google Scholar] [CrossRef] [PubMed]
- Boly, M. Measuring the fading consciousness in the human brain. Curr. Opin. Neurol. 2011, 24, 394–400. [Google Scholar] [CrossRef]
- Cavinato, M.; Volpato, C.; Silvoni, S.; Sacchetto, M.; Merico, A.; Piccione, F. Event-related brain potential modulation in patients with severe brain damage. Clin. Neurophysiol. 2011, 122, 719–724. [Google Scholar] [CrossRef]
- Faugeras, F.; Rohaut, B.; Weiss, N.; Bekinschtein, T.; Galanaud, D.; Puybasset, L.; Bolgert, F.; Sergent, C.; Cohen, L.; Dehaene, S.; et al. Event related potentials elicited by violations of auditory regularities in patients with impaired consciousness. Neuropsychologia 2012, 50, 403–418. [Google Scholar] [CrossRef]
- Balconi, M.; Arangio, R.; Guarnerio, C. Disorders of consciousness and N400 ERP measures in response to a semantic task. J. Neuropsychiatry Clin. Neurosci. 2013, 25, 237–243. [Google Scholar] [CrossRef]
- Chennu, S.; Finoia, P.; Kamau, E.; Monti, M.M.; Allanson, J.; Pickard, J.D.; Owen, A.M.; Bekinschtein, T.A. Dissociable endogenous and exogenous attention in disorders of consciousness. NeuroImage Clin. 2013, 3, 450–461. [Google Scholar] [CrossRef]
- Risetti, M.; Formisano, R.; Toppi, J.; Quitadamo, L.R.; Bianchi, L.; Astolfi, L.; Cincotti, F.; Mattia, D. On ERPs detection in disorders of consciousness rehabilitation. Front. Hum. Neurosci. 2013, 7, 775. [Google Scholar] [CrossRef]
- Wijnen, V.J.M.; Eilander, H.J.; de Gelder, B.; van Boxtel, G.J.M. Visual processing during recovery from vegetative state to consciousness: Comparing behavioral indices to brain responses. Neurophysiol. Clin. 2014, 44, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Balconi, M.; Arangio, R. The relationship between coma near coma, disability ratings, and event-related potentials in patients with disorders of consciousness: A semantic association task. Appl. Psychophysiol. Biofeedback 2015, 40, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Hauger, S.L.; Schnakers, C.; Andersson, S.; Becker, F.; Moberget, T.; Giacino, J.T.; Schanke, A.-K.; Løvstad, M. Neurophysiological Indicators of Residual Cognitive Capacity in the Minimally Conscious State. Behav. Neurol. 2015, 2015, 145913. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Song, W.-Q.; Du, J.-B.; Huo, S.; Shan, G.-X. Connecting the P300 to the diagnosis and prognosis of unconscious patients. Neural Regen. Res. 2015, 10, 473–480. [Google Scholar]
- Rohaut, B.; Faugeras, F.; Chausson, N.; King, J.-R.; Karoui, I.E.; Cohen, L.; Naccache, L. Probing ERP correlates of verbal semantic processing in patients with impaired consciousness. Neuropsychologia 2015, 66, 279–292. [Google Scholar] [CrossRef]
- Schnakers, C.; Giacino, J.T.; Løvstad, M.; Habbal, D.; Boly, M.; Di, H.; Majerus, S.; Laureys, S. Preserved covert cognition in noncommunicative patients with severe brain injury? Neurorehabil. Neural Repair 2015, 29, 308–317. [Google Scholar] [CrossRef]
- Beukema, S.; Gonzalez-Lara, L.E.; Finoia, P.; Kamau, E.; Allanson, J.; Chennu, S.; Gibson, R.M.; Pickard, J.D.; Owen, A.M.; Cruse, D. A hierarchy of event-related potential markers of auditory processing in disorders of consciousness. NeuroImage Clin. 2016, 12, 359–371. [Google Scholar] [CrossRef]
- Gibson, R.M.; Chennu, S.; Fernández-Espejo, D.; Naci, L.; Owen, A.M.; Cruse, D. Somatosensory attention identifies both overt and covert awareness in disorders of consciousness. Ann. Neurol. 2016, 80, 412–423. [Google Scholar] [CrossRef]
- Real, R.G.L.; Veser, S.; Erlbeck, H.; Risetti, M.; Vogel, D.; Müller, F.; Kotchoubey, B.; Mattia, D.; Kübler, A. Information processing in patients in vegetative and minimally conscious states. Clin. Neurophysiol. 2016, 127, 1395–1402. [Google Scholar] [CrossRef]
- Erlbeck, H.; Real, R.G.L.; Kotchoubey, B.; Mattia, D.; Bargak, J.; Kübler, A. Basic discriminative and semantic processing in patients in the vegetative and minimally conscious state. Int. J. Psychophysiol. 2017, 113, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Sergent, C.; Faugeras, F.; Rohaut, B.; Perrin, F.; Valente, M.; Tallon-Baudry, C.; Cohen, L.; Naccache, L. Multidimensional cognitive evaluation of patients with disorders of consciousness using EEG: A proof of concept study. NeuroImage Clin. 2017, 13, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Y.; Wu, H.-Y.; Lu, H.-T.; Huang, T.-T.; Zhang, H.; Zhang, T. Assessment of mismatch negativity and P300 response in patients with disorders of consciousness. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4896–4906. [Google Scholar] [PubMed]
- Kempny, A.M.; James, L.; Yelden, K.; Duport, S.; Farmer, S.F.; Diane Playford, E.; Leff, A.P. Patients with a severe prolonged Disorder of Consciousness can show classical EEG responses to their own name compared with others’ names. NeuroImage Clin. 2018, 19, 311–319. [Google Scholar] [CrossRef]
- Rivera-Lillo, G.; Rojas-Líbano, D.; Burgos, P.; Egaña, J.I.; Chennu, S.; Maldonado, P.E. Reduced delta-band modulation underlies the loss of P300 responses in disorders of consciousness. Clin. Neurophysiol. 2018, 129, 2613–2622. [Google Scholar] [CrossRef]
- Annen, J.; Mertel, I.; Xu, R.; Chatelle, C.; Lesenfants, D.; Ortner, R.; Bonin, E.A.C.; Guger, C.; Laureys, S.; Müller, F. Auditory and Somatosensory P3 Are Complementary for the Assessment of Patients with Disorders of Consciousness. Brain Sci. 2020, 10, 748. [Google Scholar] [CrossRef]
- Wu, M.; Li, F.; Wu, Y.; Zhang, T.; Gao, J.; Xu, P.; Luo, B. Impaired Frontoparietal Connectivity in Traumatic Individuals with Disorders of Consciousness: A Dynamic Brain Network Analysis. Aging Dis. 2020, 11, 301–314. [Google Scholar] [CrossRef]
- Garrido, M.I.; Kilner, J.M.; Stephan, K.E.; Friston, K.J. The mismatch negativity: A review of underlying mechanisms. Clin. Neurophysiol. 2009, 120, 453–463. [Google Scholar] [CrossRef]
- Kutas, M.; Federmeier, K.D. Thirty years and counting: Finding meaning in the N400 component of the event related brain potential (ERP). Annu. Rev. Psychol. 2011, 62, 621–647. [Google Scholar] [CrossRef]
- Friedman, D.; Johnson, R. Event-related potential (ERP) studies of memory encoding and retrieval: A selective review. Microsc. Res. Tech. 2000, 51, 6–28. [Google Scholar] [CrossRef]
- Babiloni, C.; Sarà, M.; Vecchio, F.; Pistoia, F.; Sebastiano, F.; Onorati, P.; Albertini, G.; Pasqualetti, P.; Cibelli, G.; Buffo, P.; et al. Cortical sources of resting-state alpha rhythms are abnormal in persistent vegetative state patients. Clin. Neurophysiol. 2009, 120, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Fingelkurts, A.A.; Fingelkurts, A.A.; Bagnato, S.; Boccagni, C.; Galardi, G. Life or death: Prognostic value of a resting EEG with regards to survival in patients in vegetative and minimally conscious States. PLoS ONE 2011, 6, e25967. [Google Scholar] [CrossRef] [PubMed]
- Fingelkurts, A.A.; Fingelkurts, A.A.; Bagnato, S.; Boccagni, C.; Galardi, G. Prognostic value of resting-state electroencephalography structure in disentangling vegetative and minimally conscious states: A preliminary study. Neurorehabil. Neural Repair 2013, 27, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Chennu, S.; Annen, J.; Wannez, S.; Thibaut, A.; Chatelle, C.; Cassol, H.; Martens, G.; Schnakers, C.; Gosseries, O.; Menon, D.; et al. Brain networks predict metabolism, diagnosis and prognosis at the bedside in disorders of consciousness. Brain J. Neurol. 2017, 140, 2120–2132. [Google Scholar] [CrossRef]
- Kustermann, T.; Ata Nguepnjo Nguissi, N.; Pfeiffer, C.; Haenggi, M.; Kurmann, R.; Zubler, F.; Oddo, M.; Rossetti, A.O.; De Lucia, M. Brain functional connectivity during the first day of coma reflects long-term outcome. NeuroImage Clin. 2020, 27, 102295. [Google Scholar] [CrossRef]
- Valente, M.; Placidi, F.; Oliveira, A.J.; Bigagli, A.; Morghen, I.; Proietti, R.; Gigli, G.L. Sleep organization pattern as a prognostic marker at the subacute stage of post-traumatic coma. Clin. Neurophysiol. 2002, 113, 1798–1805. [Google Scholar] [CrossRef]
- Arnaldi, D.; Terzaghi, M.; Cremascoli, R.; De Carli, F.; Maggioni, G.; Pistarini, C.; Nobili, F.; Moglia, A.; Manni, R. The prognostic value of sleep patterns in disorders of consciousness in the sub-acute phase. Clin. Neurophysiol. 2016, 127, 1445–1451. [Google Scholar] [CrossRef]
- Yang, X.; Song, C.; Yuan, F.; Zhao, J.; Jiang, Y.; Yang, F.; Kang, X.; Jiang, W. Prognostic roles of sleep electroencephalography pattern and circadian rhythm biomarkers in the recovery of consciousness in patients with coma: A prospective cohort study. Sleep Med. 2020, 69, 204–212. [Google Scholar] [CrossRef]
- Fischer, C.; Dailler, F.; Morlet, D. Novelty P3 elicited by the subject’s own name in comatose patients. Clin. Neurophysiol. 2008, 119, 2224–2230. [Google Scholar] [CrossRef] [PubMed]
- Cavinato, M.; Freo, U.; Ori, C.; Zorzi, M.; Tonin, P.; Piccione, F.; Merico, A. Post-acute P300 predicts recovery of consciousness from traumatic vegetative state. Brain Inj. 2009, 23, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Steppacher, I.; Eickhoff, S.; Jordanov, T.; Kaps, M.; Witzke, W.; Kissler, J. N400 predicts recovery from disorders of consciousness. Ann. Neurol. 2013, 73, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Estraneo, A.; Fiorenza, S.; Magliacano, A.; Formisano, R.; Mattia, D.; Grippo, A.; Romoli, A.M.; Angelakis, E.; Cassol, H.; Thibaut, A.; et al. Multicenter prospective study on predictors of short-term outcome in disorders of consciousness. Neurology 2020, 95, e1488–e1499. [Google Scholar] [CrossRef]
- Bekinschtein, T.A.; Dehaene, S.; Rohaut, B.; Tadel, F.; Cohen, L.; Naccache, L. Neural signature of the conscious processing of auditory regularities. Proc. Natl. Acad. Sci. USA 2009, 106, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- Faugeras, F.; Rohaut, B.; Weiss, N.; Bekinschtein, T.A.; Galanaud, D.; Puybasset, L.; Bolgert, F.; Sergent, C.; Cohen, L.; Dehaene, S.; et al. Probing consciousness with event-related potentials in the vegetative state. Neurology 2011, 77, 264–268. [Google Scholar] [CrossRef]
- Xu, W.; Jiang, G.; Chen, Y.; Wang, X.; Jiang, X. Prediction of minimally conscious state with somatosensory evoked potentials in long-term unconscious patients after traumatic brain injury. J. Trauma Acute Care Surg. 2012, 72, 1024–1029. [Google Scholar] [CrossRef]
| Authors and Reference | Patient Sample | Finding |
|---|---|---|
| Schnakers et al. [43] | 11 Coma 32 UWS 42 MCS 21 EMCS | Nonlinear measures
|
| Leon-Carrion et al. [11] | 7 MCS 9 SND | Spectral power
|
| Schnakers et al. [44] | 16 Coma 13 UWS 30 MCS 13 EMCS | Nonlinear measures
|
| Babiloni et al. [45] | 13 LIS 15 HC | Spectral power
|
| Pollonini et al. [46] | 7 MCS 9 SND | Functional connectivity
|
| Sarà and Pistoia [47] | 10 UWS 10 HC | Nonlinear measures
|
| Gosseries et al. [48] | 6 Coma 24 UWS 26 MCS | Nonlinear measures
|
| Sarà et al. [49] | 38 UWS 40 HC | Nonlinear measures
|
| Wu et al. [12] | 21 UWS 16 MCS 30 CS | Nonlinear measures
|
| Wu et al. [50] | 30 UWS 20 MCS 30 CS | Functional connectivity
|
| Fingelkurts et al. [51] | 21 UWS 16 MCS 5 HC | Microstates
|
| Lehembre et al. [52] | 10 UWS 21 MCS | Spectral power
|
| Leon-Carrion et al. [53] | 7 MCS 9 SND | Functional connectivity
|
| King et al. [54] | 75 VS 68 MCS 24 CS | Functional connectivity
|
| Lechinger et al. [55] | 8 UWS 9 MCS 14 HC | Spectral power
|
| Chennu et al. [56] | 13 UWS 19 MCS 26 HC | Spectral power
|
| Höller et al. [57] | 27 UWS 22 MCS 23 HC | Functional connectivity
|
| Marinazzo et al. [58] | 11 UWS 10 MCS 5 EMCS 10 HC | Functional connectivity
|
| Sitt et al. [59] | 75 UWS 68 MCS 24 CS 14 HC | Spectral power
|
| Rossi Sebastiano et al. [60] | 85 UWS 57 MCS | Spectral power
|
| Naro et al. [61] | 6 UWS 7 MCS 10 HC | Spectral power
|
| Piarulli et al. [62] | 6 UWS 6 MCS | Spectral power
|
| Schorr et al. [63] | 58 UWS 15 MCS 24 HC | Spectral power
|
| Thul et al. [64] | UWS 8 MCS 7 HC 24 | Functional connectivity
|
| Naro et al. [65] | 17 UWS 15 MCS | Spectral power
|
| Stefan et al. [66] | 51 UWS 11 MCS | Spectral power
|
| Bai et al. [34] | 31 UWS 20 MCS 20 HC | Functional connectivity
|
| Cacciola et al. [67] | 12 UWS 13 MCS | Graph theory
|
| Rizkallah et al. [68] | 9 UWS 17 MCS− 29 MCS+ 6 EMCS 21 HC | Graph theory
|
| Bareham et al. [69] | 16 UWS 15 MCS− 7 MCS+ 1 EMCS | Spectral power
|
| Cai et al. [70] | 35 UWS 19 MCS 23 HC | Graph theory
|
| Naro et al. [71] | 17 UWS 15 MCS | Graph theory
|
| Lutkenhoff et al. [72] | 37 UWS 17 MCS− 7 MCS+ | Spectral power
|
| Authors and Reference | Patient Sample | Finding |
|---|---|---|
| Oksenberg et al. [80] | 11 UWS 6 HC |
|
| Landsness et al. [81] | 5 UWS 6 MCS |
|
| Cologan et al. [84] | 10 UWS 10 MCS |
|
| Malinowska et al. [85] | 11 UWS 20 MCS 1 LIS 5 HC |
|
| de Biase et al. [86] | 27 UWS 5 MCS |
|
| Forgacs et al. [87] | 8 UWS 23 MCS 13 EMCS |
|
| Mouthon et al. [88] | 4 MCS 1 EMCS 5 CS 10 HC |
|
| Wislowska et al. [89] | 18 UWS 17 MCS 26 HC |
|
| Rossi Sebastiano et al. [83] | 49 UWS 36 MCS |
|
| Zieleniewska et al. [90] | 8 UWS 4 MCS− 2 MCS+ 5 EMCS |
|
| Mertel et al. [82] | 16 UWS 16 MCS 10 TC |
|
| Authors and Reference | Patient Sample | Finding |
|---|---|---|
| Schoenle and Witzke [94] | 43 UWS 23 near UWS 45 non UWS | N400
|
| Kotchoubey et al. [95] | 38 UWS 38 MCS 22 CS |
|
| Perrin et al. [96] | 5 UWS 6 MCS 4 LIS 5 HC | P300
|
| Schnakers et al. [97] | 8 UWS 14 MCS 12 HC | P300
|
| Qin et al. [98] | 4 Coma 6 UWS 2 MCS | MMN
|
| Fischer et al. [99] | 16 UWS 11 MCS | P300
|
| Boly [100] | 8 UWS 13 MCS 22 HC | MMN
|
| Cavinato et al. [101] | 6 UWS 11 MCS 10 HC | P300
|
| Faugeras et al. [102] | 22 UWS 19 MCS 8 CS 10 HC | MMN
|
| Balconi et al. [103] | 10 UWS 8 MCS 20 HC | N400
|
| Chennu et al. [104] | 9 UWS 12 MCS 8 HC | P300
|
| Risetti et al. [105] | 8 UWS 3 MCS | P300
|
| Sitt et al. [59] | 75 UWS 68 MCS 24 CS 14 HC |
|
| Wijnen et al. [106] | 11 UWS 22 HC |
|
| Balconi and Arangio [107] | 7 UWS 11 MCS | N400
|
| Hauger et al. [108] | 11 MCS− 9 MCS+ 20 HC | P300
|
| Li et al. [109] | 2 Coma 6 UWS 5 MCS 17 HC | P300
|
| Rohaut et al. [110] | 15 UWS 14 MCS 19 HC | N400
|
| Schnakers et al. [111] | 10 UWS 8 MCS− 8 MCS+ 14 HC | P300
|
| Beukema et al. [112] | 8 UWS 8 MCS 17 HC | N400
|
| Gibson et al. [113] | 7 UWS 4 MCS 2 EMCS 18 HC | P300
|
| Real et al. [114] | 29 UWS 16 MCS 14 HC | P300
|
| Erlbeck et al. [115] | 13 UWS 3 MCS 3 EMCS | MMN
|
| Sergent et al. [116] | 4 UWS 8 MCS 1 CS 15 HC | P300
|
| Wang et al. [117] | 6 UWS 5 MCS 5 HC | P300
|
| Kempny et al. [118] | 5 UWS 11 MCS 12 HC | P300
|
| Rivera-Lillo et al. [119] | 10 UWS 3 MCS 10 HC |
|
| Annen et al. [120] | 15 UWS 23 MCS 2 EMCS 12 HC | P300
|
| Wu et al. [121] | 20 UWS 22 MCS | P300
|
| Authors and Reference | Patient Sample | Follow-Up | Finding |
|---|---|---|---|
| Schnakers et al. [44] | 16 Coma 13 UWS 30 MCS 13 EMCS | 12 months | Nonlinear measures
|
| Babiloni et al. [125] | 50 UWS 30 HC | 3 months | Spectral power
|
| Fingelkurts et al. [126] | 14 UWS 7 MCS | 6 months | Spectral power
|
| Sarà et al. [49] | 23 UWS 40 HC | 6 months | Nonlinear measures
|
| Fingelkurts et al. [127] | 14 UWS | 3 months | Functional connectivity
|
| Sitt et al. [59] | 75 UWS 68 MCS 24 CS 14 HC | <42 days | Spectral power
|
| Schorr et al. [63] | 58 UWS 15 MCS 24 HC | 12 months | Functional connectivity
|
| Chennu et al. [128] | 23 UWS 17 MCS− 49 MCS+ 11 EMCS 4 LIS 26 HC | 12 months | Functional connectivity
|
| Stefan et al. [66] | 51 UWS 11 MCS | 589.26 ± 1125.32 days | Spectral power
|
| Bai et al. [34] | 31 UWS 20 MCS 20 HC | 3 months | Functional connectivity
|
| Bareham et al. [69] | 16 UWS 15 MCS− 7 MCS+ 1 EMCS | 3 months | Spectral power
|
| Kustermann et al. [129] | 98 Coma | 3 months | Graph theory
|
| Authors and Reference | Patient Sample | Follow-Up | Finding |
|---|---|---|---|
| Oksenberg et al. [80] | 11 UWS 6 HC | 6 months |
|
| Valente et al. [130] | 24 Coma | 12–34 months |
|
| Cologan et al. [84] | 10 UWS 10 MCS | 6 months |
|
| Mouthon et al. [88] | 4 MCS 1 EMCS 5 CS 10 HC | 1.5–16.1 months |
|
| Arnaldi et al. [131] | 27 Coma | 18.5 ± 9.9 months |
|
| Wislowska et al. [89] | 18 UWS 17 MCS 26 HC | 1–150 months |
|
| Yang et al. [132] | 75 Coma | 1 month |
|
| Authors and Reference | Patient Sample | Follow-Up | Finding |
|---|---|---|---|
| Kotchoubey et al. [95] | 38 UWS 38 MCS 22 CS | 6 months | MMN
|
| Fischer et al. [133] | 50 Coma | 3 months | P300
|
| Qin et al. [98] | 4 Coma 6 UWS 2 MCS | 3 months | MMN
|
| Cavinato et al. [134] | 34 UWS | 12 months | P300
|
| Faugeras et al. [138] | 22 UWS | 3–4 days | Bekinschtein protocol [137]
|
| Faugeras et al. [102] | 22 UWS 19 MCS 8 CS 10 HC | 3–4 days | Global effect
|
| Xu et al. [139] | 58 UWS | 1 year |
|
| Steppacher et al. [135] | 53 UWS 39 MCS | 2–14 years | P300
|
| Wijnen et al. [106] | 11 UWS 22 HC | 2–3 years | Visual stimuli
|
| Li et al. [109] | 2 Coma 6 UWS 5 MCS 17 HC | 1, 2, 3, 6, 9, and 12 months | P300
|
| Estraneo et al. [136] | 71 UWS 76 MCS | 6 months | P300
|
| Value | Ref | Comparison | Comment | Cohen’s d | Confidence Interval |
|---|---|---|---|---|---|
| alpha power | [52] | MCS vs. UWS | Frontal | 0.60 | (−0.19, 1.40) |
| Posterior | 0.85 | (0.04, 1.66) | |||
| Left hemisphere | 0.70 | (−0.10, 1.50) | |||
| Right hemisphere | 1.00 | (0.18, 1.82) | |||
| [55] | HC vs. MCS | 1.50 | (0.50, 2.50) | ||
| HC vs. UWS | 1.79 | (0.71, 2.88) | |||
| [56] | HC vs. DOC | 2.64 | (1.92, 3.36) | ||
| [59] | CS vs. UWS | 1.47 | (1.19, 1.81) | ||
| MCS vs. UWS | 0.82 | (0.66, 1.00) | |||
| [62] | MCS vs. UWS | Fz | 2.81 | (0.98, 4.65) | |
| Cz | 2.31 | (0.65, 3.97) | |||
| Pz | 1.83 | (0.31, 3.35) | |||
| [66] | MCS vs. UWS | 0.14 | (0.04, 0.25) | ||
| approximate entropy | [12] | HC, CS vs. MCS | Eyes closed | 1.71 | (0.99, 2.43) |
| Auditory, Verbal | 1.49 | (0.80, 2.19) | |||
| Auditory, Music | 1.96 | (1.22, 2.71) | |||
| HC, CS vs. UWS | Eyes closed | 3.50 | (2.60, 4.4) | ||
| Auditory, Visual | 2.70 | (1.92, 3.48) | |||
| Auditory, Music | 3.23 | (2.37, 4.09) | |||
| MCS vs. UWS | Eyes closed | 2.1 | (1.27, 2.93) | ||
| Auditory, Verbal | 1.41 | (0.67, 2.16) | |||
| Auditory, Music | 1.33 | (0.59, 2.07) | |||
| [49] | HC vs. DOC | 2.83 | (2.19, 3.47) | ||
| [66] | MCS vs. UWS | 0.25 | (0.07, 0.43) | ||
| average clustering coefficient | [67] | MCS vs. UWS | −1.00 | (−1.39, −0.61) | |
| characteristic path length | [66] | MCS vs. UWS | Alpha | 0.54 | (0.40, 0.70) |
| Beta | 0.54 | (0.36, 0.74) | |||
| clustering coefficient | [56] | HC vs. DOC | 1.27 | (0.7, 1.84) | |
| [66] | MCS vs. UWS | 0.51 | (0.47, 0.54) | ||
| [68] | HC vs. MCS+ | Delta | −1.08 | (−1.28, −0.89) | |
| Theta | −0.98 | (−1.17, −0.79) | |||
| HC vs. MCS− | Delta | −1.69 | (−1.99, −1.39) | ||
| Theta | −1.61 | (−1.90, −1.31) | |||
| HC vs. UWS | Delta | −1.03 | (−1.41, −0.66) | ||
| Theta | −1.06 | (−1.43, −0.68) | |||
| coherence | [66] | MCS vs. UWS | Alpha | 0.51 | (0.36, 0.7) |
| Beta | 0.40 | (0.32, 0.47) | |||
| delta power | [52] | MCS vs. UWS | Frontal | −0.77 | (−1.58, 0.03) |
| Posterior | −0.97 | (−1.79, −0.15) | |||
| Left | −0.77 | (−1.58, 0.03) | |||
| Right | −0.93 | (−1.75, −0.12) | |||
| [55] | HC vs. UWS | Pz | −1.21 | (−2.2, −0.23) | |
| Oz | −1.34 | (−2.34, −0.33) | |||
| [56] | HC vs. DOC | −2.63 | (−3.35, −1,.91) | ||
| [59] | CS vs. UWS | −1.24 | (−1.47, −1.04) | ||
| MCS vs. UWS | −0.70 | (−0.87, −0.54) | |||
| [62] | MCS vs. UWS | Fz | −2.99 | (−4.94, −1.09) | |
| Cz | −2.61 | (−4.38, −0.85) | |||
| Pz | −2.52 | (−4.25, −0.79) | |||
| [66] | MCS vs. UWS | −0.29 | (−0.54, −0.04) | ||
| dynamic functional connectivity | [65] | MCS vs. UWS | Alpha spectral connectivity | 0.84 | (0.1, 1.59) |
| Gamma spectral connectivity | 0.99 | (0.23, 1.75) | |||
| entropy | [48] | HC vs. MCS | 1.06 | (0.38, 1.74) | |
| HC vs. UWS | 2.02 | (1.22, 2.81) | |||
| HC vs. Coma | 3.85 | (2.28, 5.42) | |||
| MCS vs. UWS | 1.18 | (0.56, 1.79) | |||
| MCS vs. Coma | 1.83 | (0.8, 2.86) | |||
| UWS vs. Coma | 0.36 | (−0.57, 1.29) | |||
| global effect | [59] | CS vs. UWS | 1.24 | (1.11, 1.37) | |
| MCS vs. UWS | 0.43 | (0.37, 0.49) | |||
| imaginary part coherence | [52] | MCS vs. UWS | Inter-hemisphere delta | −0.55 | (−1.34, 0.24) |
| Inter-hemisphere theta | 0.35 | (−0.43, 1.13) | |||
| Inter-hemisphere alpha | 0.83 | (0.02, 1.64) | |||
| Frontal to posterior delta | 0.85 | (0.04, 1.66) | |||
| Frontal to posterior theta | 1.10 | (0.27, 1.93) | |||
| Frontal to Posterior alpha | 0.59 | (−0.20, 1.38) | |||
| Left delta | 0.64 | (−0.16, 1.43) | |||
| Left theta | 0.73 | (−0.07, 1.53) | |||
| Left alpha | 0.71 | (−0.09, 1.51) | |||
| Right delta | 0.50 | (−0.29, 1.29) | |||
| Right theta | 0.50 | (−0.29, 1.29) | |||
| Right alpha | 0.32 | (−0.46, 1.10) | |||
| Kolmogorov–Chitain complexity | [59] | CS vs. MCS | Mean | 0.87 | (0.62, 1.14) |
| Fluctuation | −0.47 | (−0.7, −0.25) | |||
| CS vs. UWS | Mean | 1.29 | (1.00, 1.67) | ||
| Fluctuation | −0.62 | (−0.87, −0.4) | |||
| MCS vs. UWS | Mean | 0.43 | (0.25, 0.62) | ||
| Fluctuation | −0.14 | (−0.32, 0.04) | |||
| LPC | [110] | MCS vs. UWS | Presence | 1.13 | (−0.23, 3.29) |
| Lempel–Ziv complexity | [12] | HC, CS vs. MCS | Eyes closed | 2.59 | (1.76, 3.4) |
| Auditory, Verbal | 1.48 | (0.79, 2.18) | |||
| Auditory, Music | 1.54 | (0.84, 2.25) | |||
| HC, CS vs. UWS | Eyes closed | 4.17 | (3.16, 5.18) | ||
| Auditory, Verbal | 2.84 | (2.04, 3.65) | |||
| Auditory, Music | 2.48 | (1.73, 3.23) | |||
| MCS vs. UWS | Eyes closed | 2.00 | (1.18, 2.82) | ||
| Auditory, Verbal | 1.75 | (0.96, 2.54) | |||
| Auditory, Music | 1.26 | (0.52, 1.99) | |||
| local-community paradigm correlation | [67] | MCS vs. UWS | −0.954 | (−1.34, −0.57) | |
| local efficiency | [67] | MCS vs. UWS | −1.19 | (−1.60, −0.78) | |
| microstates | [51] | HC vs. MCS | Total number of ms | 5.34 | (2.49, 8.20) |
| Posterior delta | −15.86 | (−23.58, −8.14) | |||
| Posterior theta | −19.96 | (−29.63, −10.30) | |||
| Posterior slow alpha | −3.22 | (−5.20, −1.23) | |||
| Posterior fast alpha | 29.93 | (15.50, 44.35) | |||
| Anterior delta | −5.41 | (−8.30, −2.52) | |||
| Anterior theta | −8.73 | (−13.11, −4.36) | |||
| Anterior slow alpha | −0.56 | (−1.85, 0.72) | |||
| Anterior fast alpha | 10.70 | (5.41, 15.99) | |||
| HC vs. UWS | Total number of ms | 7.22 | (4.43, 10.00) | ||
| Posterior delta | −19.78 | (−26.89, −12.67) | |||
| Posterior theta | −12.56 | (−17.16, −7.97) | |||
| Posterior slow alpha | −5.89 | (−8.25, −3.54) | |||
| Posterior fast alpha | 40.72 | (26.21, 55.22) | |||
| Anterior delta | −6.16 | (−8.60, −3.72) | |||
| Anterior theta | −9.33 | (−12.820, −5.85) | |||
| Anterior slow alpha | −1.83 | (−3.09, −0.57) | |||
| Anterior fast alpha | 13.95 | (8.88, 19.02) | |||
| MCS vs. UWS | Total number of ms | −1.19 | (−2.23, −0.16) | ||
| Posterior delta | −2.72 | (−4.04, −1.40) | |||
| Posterior theta | −0.52 | (−1.48, 0.45) | |||
| Posterior slow alpha | −3.00 | (−4.36, −1.63) | |||
| Posterior fast alpha | 8.54 | (5.53, 11.55) | |||
| Anterior delta | −0.05 | (−1.00, 0.90) | |||
| Anterior theta | −0.62 | (−1.59, 0.36) | |||
| Anterior slow alpha | −0.46 | (−1.43, 0.51) | |||
| N100 | [95] | MCS vs. UWS | 0.48 | (0.37, 0.59) | |
| [96] | HC vs. LIS | Latency | −0.35 | (−1.68, 0.97) | |
| HC vs. MCS | Latency | −2.67 | (−4.30, −1.04) | ||
| HC vs. UWS | Latency | −1.78 | (−3.24, 0.31) | ||
| LIS vs. MCS | Latency | −2.13 | (−3.71, −0.56) | ||
| LIS vs. UWS | Latency | −1.53 | (−3.02, −0.04) | ||
| MCS vs. UWS | Latency | −0.51 | (−0.69, 1.70) | ||
| [101] | HC vs. MCS | Sine tone | −0.77 | (−1.82, 0.27) | |
| SON | −0.48 | (−1.51, 0.55) | |||
| OFN | −0.77 | (−1.82, 0.28) | |||
| HC vs. UWS | Sine tone | −1.85 | (−2.87, −0.83) | ||
| SON | 0.059 | (−0.80, 0.91) | |||
| OFN | 0.07 | (−0.78, 0.93) | |||
| MCS vs. UWS | Sine tone | −0.92 | (−0.12, −1.90) | ||
| SON | 0.51 | (−0.50, 1.52) | |||
| OFN | 0.61 | (−0.41, 1.62) | |||
| N200 | [96] | HC vs. LIS | Latency | 0.44 | (−0.88, 1.78) |
| HC vs. MCS | Latency | −3.60 | (−5.52, −1.69) | ||
| HC vs. UWS | Latency | −6.31 | (−9.34, −3.28) | ||
| LIS vs. MCS | Latency | −4.18 | (−6.41, −1.96) | ||
| LIS vs. UWS | Latency | −7.84 | (−11.71, −3.99) | ||
| MCS vs. UWS | Latency | −1.61 | (−0.248, −2.98) | ||
| [101] | HC vs. MCS | Sine tone | 0.19 | (−0.83, 1.20) | |
| SON | −0.25 | (−1.26, 0.77) | |||
| OFN | 0.55 | (−0.48, 1.58) | |||
| HC vs. UWS | Sine tone | −0.88 | (−1.78, 0.02) | ||
| SON | 0.16 | (−0.70, 1.02) | |||
| OFN | 0.34 | (−0.52, 1.20) | |||
| MCS vs. UWS | Sine tone | −0.71 | (−1.74, 0.31) | ||
| SON | 0.51 | (−0.50, 1.52) | |||
| OFN | −0.21 | (−1.21, 0.79) | |||
| N400 | [94] | no UWS vs. near UWS | Presence | 0.54 | (−0.33, 1.42) |
| no UWS vs. UWS | Presence | 1.47 | (0.84, 2.22) | ||
| near UWS vs. UWS | Pressence | 0.93 | (0.24, 1.71) | ||
| [107] | MCS vs. UWS | Amplitude, congruous fronto-central | 0.09 | (−0.91, 1.10) | |
| Amplitude, incongruous fronto-central | −0.08 | (−0.93, 1.08) | |||
| Amplitude, congruous temporo-parietal | −0.15 | (−1.15, 0.86) | |||
| Amplitude, incongruous temporo-parietal | −0.07 | (−1.08, 0.94) | |||
| Amplitude, congruous occipital | 0.17 | (−0.83, 1.18) | |||
| Amplitude, incongruous occipital | −0.01 | (−1.03, 0.98) | |||
| Latency, congruous fronto-central | −4.88 | (−6.89, −2.87) | |||
| Latency, incongruous fronto-central | −26.83 | (−36.45, −17.21) | |||
| Latency, congruous temporo-parietal | −12.55 | (−17.14, −7.97) | |||
| Latency, inconcgruous temporo-parietal | −10.45 | (−14.32, −6.59) | |||
| Latency, congruous occipital | −8.21 | (−11.3, −5.12) | |||
| Latency, incongruou occipital | −10.14 | (−13.9, −6.39) | |||
| P200 | [95] | MCS vs. UWS | 0.48 | (0.37, 0.59) | |
| [96] | HC vs. LIS | Latency | 1.90 | (0.32, 3.48) | |
| HC vs. MCS | Latency | −2.11 | (−3.59, −0.635) | ||
| HC vs. UWS | Latency | −3.87 | (−6.10, −1.83) | ||
| LIS vs. MCS | Latency | −3.49 | (−5.47, −1.50) | ||
| LIS vs. UWS | Latency | −5.52 | (−8.39, −2.65) | ||
| MCS vs. UWS | Latency | −1.55 | (−0.20, −2.91) | ||
| [101] | HC vs. MCS | Sine tone | 0.24 | (−0.77, 1.26) | |
| SON | 0.15 | (−0.87, 1.16) | |||
| OFN | 0.16 | (−0.85, 1.18) | |||
| HC vs. UWS | Sine tone | 0.57 | (−0.31, 1.44) | ||
| SON | 0.25 | (−0.83,0.88) | |||
| OFN | 0.00 | (−0.86, 0.86) | |||
| MCS vs. UWS | Sine tone | 0.23 | (−0.77, 1.23) | ||
| SON | −0.08 | (−1.07, 0.92) | |||
| OFN | −0.96 | (−2.01, 0.8) | |||
| P300 | [95] | MCS vs. UWS | 0.46 | (0.35, 0.56) | |
| [96] | HC vs. LIS | Latency | −1.64 | (−3.16, −0.12) | |
| HC vs. MCS | Latency | −5.16 | (−7.62, −2.70) | ||
| HC vs. UWS | Latency | −8.76 | (−12.79, −4.73) | ||
| LIS vs. MCS | Latency | −3.22 | (−5.12, −1.33) | ||
| LIS vs. UWS | Latency | −5.31 | (−8.10. −2.53) | ||
| MCS vs. UWS | Latency | −1.04 | (−2.31, 0.22) | ||
| [101] | HC vs. MCS | Sine tone | −0.38 | (−1.40, 0.64) | |
| SON | −0.72 | (−1.76, 0.32) | |||
| OFN | −0.50 | (−1.53, 0.53) | |||
| HC vs. UWS | Sine tone | 0.28 | (−0.58, 1.14) | ||
| SON | 0.11 | (−0.75, 0.96) | |||
| OFN | 0.49 | (−0.38, 1.36) | |||
| MCS vs. UWS | Sine tone | 1.40 | (0.30, 2.50) | ||
| SON | 0.98 | (−0.149, 1.93) | |||
| OFN | 1.07 | (0.02, 2.13) | |||
| [116] | HC vs. MCS | Occurance SON | 0.35 | (−0.04, 0.74) | |
| HC vs. UWS | Occurance SON | 0.99 | (0.23, 1.75) | ||
| MCS vs. UWS | Occurance SON | 0.64 | (−0.24, 1.52) | ||
| [117] | HC vs. MCS | Test run 1 Cz latency SON | −0.08 | (−1.32, 1.16) | |
| Test run 1 Cz amplitude SON | 0.13 | (−1.11, 1.37) | |||
| Test run 1 Cz latency OFN | −0.56 | (−1.83, 0.70) | |||
| Test run 1 Cz amplitude OFN | 0.47 | (−0.79, 1.73) | |||
| HC vs. UWS | Test run 1 Cz latency SON | −1.88 | (−3.30, −0.45) | ||
| Test run 1 Cz amplitude SON | 0.21 | (−0.99, 1.40) | |||
| Test run 1 Cz latency OFN | −0.41 | (−1.6, 0.79) | |||
| Test run 1 Cz amplitude OFN | 0.61 | (−0.61, 1.82) | |||
| MCS vs. UWS | Test run 1 Cz latency SON | −1.81 | (−3.22, −0.40) | ||
| Test run 1 Cz amplitude SON | 0.08 | (−1.11, 1.26) | |||
| Test run 1 Cz latency OFN | 0.43 | (−0.77, 1.63) | |||
| Test run 1 Cz amplitude OFN | 0.09 | (−1.10, 1.27) | |||
| permutation entropy | [59] | CS vs. MCS | Theta mean | 0.54 | (0.25, 0.82) |
| Alpha mean | 0.74 | (0.47, 1.04) | |||
| Beta mean | 0.51 | (0.25, 0.78) | |||
| Gamma mean | 0.43 | (0.18, 0.7) | |||
| Theta fluctuation | −0.5 | (0.7, −0.25) | |||
| Alpha fluctuation | −0.54 | (−0.78, −0.32) | |||
| Beta fluctuation | −0.54 | (−0.78, −0.32) | |||
| Gamma fluctuation | −0.54 | (−0.78, −0.32) | |||
| CS vs. UWS | Theta mean | 1.35 | (1.09, 1.66) | ||
| Alpha mean | 0.95 | (0.70, 1.24) | |||
| Beta mean | 0.36 | (0.11, 0.62) | |||
| Gamma mean | 0.29 | (0.04, 0.54) | |||
| Theta fluctuation | −1.14 | (−1.41, −0.91) | |||
| Alpha fluctuation | −1.00 | (−1.24, −0.78) | |||
| Beta fluctuation | −0.43 | (−0.66, −0.21) | |||
| Gamma fluctuation | −0.32 | (−0.54, −0.11) | |||
| MCS vs. UWS | Theta mean | 0.82 | (0.66, 1.00) | ||
| Alpha mean | 0.40 | (0.21, 0.58) | |||
| Beta mean | −0.11 | (−0.29, 0.07) | |||
| Gamma mean | −0.11 | (−0.29, −0.07) | |||
| Theta fluctuation | −0.70 | (−0.87, −0.54) | |||
| Alpha fluctuation | −0.54 | (−0.74, −0.36) | |||
| Beta fluctuation | 0.11 | (−0.07, 0.29) | |||
| Gamma fluctuation | 0.18 | (0.00, 0.36) | |||
| [66] | MCS vs. UWS | Alpha | 0.40 | (0.32, 0.47) | |
| phase lag index | [52] | MCS vs. UWS | Inter-hemisphere delta | −0.65 | (−1.44, 0.15) |
| Inter-hemisphere theta | 0.00 | (−0.78, 0.78) | |||
| Inter-hemisphere alpha | 1.30 | (0.45, 2.15) | |||
| Frontal to posterior delta | 0.47 | (−0.32, 1.25) | |||
| Frontal to posterior theta | 0.80 | (−0.01, 1.61) | |||
| Frontal to posterior alpha | 0.39 | (−0.39, 1.18) | |||
| Left delta | 0.00 | (−0.78, 0.78) | |||
| Left theta | 0.84 | (0.03, 1.65) | |||
| Left alpha | 0.70 | (−0.1, 1.5) | |||
| Right delta | 0.03 | (−0.75, 0.81) | |||
| Right theta | 0.37 | (−0.42, 1.15) | |||
| Right alpha | 0.42 | (−0.36, 1.21) | |||
| phase locking index | [59] | CS vs. MCS | Mean, delta | −0.07 | (−0.32, 0.18) |
| Fluctatuion, delta | −0.11 | (−0.36, 0.14) | |||
| CS vs. UWS | Mean, delta | −0.47 | (−0.7, −0.25) | ||
| Fluctatuion, delta | −0.54 | (−0.78, −0.32) | |||
| MCS vs. UWS | Mean, delta | −0.43 | (−0.62, −0.25) | ||
| Fluctatuion, delta | −0.43 | (−0.62, −0.25) | |||
| quadratic self-coupling | [34] | HC vs. MCS | Alpha | 0.46 | (−0.12, 1.04) |
| HC vs. UWS | Alpha | 1.02 | (0.34, 1.70) | ||
| MCS vs. UWS | Alpha | 0.40 | (−0.18, 0.98) | ||
| quadratic self-coupling | [34] | HC vs. MCS | Theta | 1.67 | (1.01, 2.33) |
| HC vs. UWS | Theta | 2.07 | (1.28, 2.87) | ||
| MCS vs. UWS | Theta | 0.91 | (0.30, 1.51) | ||
| REM | [80] | HC vs. DOC | Duration | 1.64 | (0.41, 2.87) |
| [81] | MCS vs. UWS | Presence | Inf | (0.17, Inf) | |
| [82] | HC vs. UWS | Time in REM | 1.92 | (1.50, 2.34) | |
| MCS vs. UWS | Time in REM | 0.76 | (0.53, 0.99) | ||
| sleep spindels | [82] | Hc vs. MCS | Inf | (−0.25, Inf) | |
| HC vs. UWS | Inf | (0.48, Inf) | |||
| MCS vs. UWS | 0.69 | (−0.2, 1.66) | |||
| [84] | MCS vs. UWS | 0.934 | (0.45, 1.42) | ||
| [85] | MCS vs. UWS | 1.10 | (0.10, 2.27) | ||
| slow-wave sleep | [81] | MCS vs. UWS | % power of waking vs. sleep (MCS) and eyes open vs. closed (UWS) | 6.33 | (2.78, 9.87) |
| Presence | Inf | (0.58, Inf) | |||
| [84] | MCS vs. UWS | Presence | 1.16 | (−0.07, 2.67) | |
| small-worldness omega | [67] | MCS vs. UWS | 1.24 | (0.83, 1.65) | |
| small-worldness omega efficiency | [67] | MCS vs. UWS | 1.09 | (0.69, 1.49) | |
| spectral entropy | [62] | MCS vs. UWS | Mean Fz | 2.51 | (0.78, 4.24) |
| Mean Cz | 1.97 | (0.41, 3.53) | |||
| Mean Pz | 1.86 | (0.33, 3.39) | |||
| Sd Fz | 2.42 | (0.72, 4.12) | |||
| Sd Cz | 1.89 | (0.35, 3.43) | |||
| Sd Pz | 1.53 | (0.08, 2.97) | |||
| Cov Fz | 2.32 | (0.65, 3.99) | |||
| Cov Cz | 1.62 | (0.16, 3.08) | |||
| Cov Pz | 1.26 | (−012, 2.63) | |||
| theta power | [59] | CS vs. MCS | Normalized | 0.14 | (0.03, 0.25) |
| CS vs. UWS | Normalized | 0.70 | (0.59, 0.82) | ||
| MCS vs. UWS | Normalized | 0.51 | (0.45, 0.56) | ||
| [62] | MCS vs. UWS | Fz | 1.87 | (0.51, 3.22) | |
| Cz | 2.38 | (0.9, 3.86) | |||
| Pz | 2.12 | (0.70, 3.53) | |||
| transfer entropy | [66] | MCS vs. UWS | Alpha | 0.62 | (0.51, 0.74) |
| weighted symbolic mutual information | [54] | MCS vs. UWS | Anoxia | 1.59 | (1.18, 2.00) |
| Traumatic | 1.09 | (0.89, 1.29) | |||
| Stroke | 0.82 | (0.58, 1.06) | |||
| [59] | CS vs. UWS | Theta | 1.09 | (0.97, 1.21) | |
| MCS vs. UWS | Theta | 0.91 | (0.85, 0.97) | ||
| [66] | MCS vs. UWS | Theta | 0.358 | (0.13, 0.58) | |
| Delta | 0.701 | (0.47, 0.93) | |||
| Alpha | 0.213 | (−0.01, 0.44) |
| Value | Ref | Comment | Cohen’s d | Confidence Interval |
|---|---|---|---|---|
| alpha power | [66] | 0.51 | (0.22, 0.79) | |
| [125] | Occipital | 5.40 | (4.41, 6.39) | |
| approximate entropy | [66] | 0.62 | (0.33, 0.91) | |
| bispectral index | [44] | 0.73 | (0.51, 0.95) | |
| clustering coefficient | [66] | Beta | 1.30 | (0.97, 1.62) |
| Alpha | 1.30 | (0.97, 1.62) | ||
| Theta | 0.83 | (0.53, 1.13) | ||
| [129] | −0.88 | (−0.97, −0.79) | ||
| coherence | [63] | Partial, theta | 0.95 | (0.29, 2.09) |
| Partial, delta | 0.87 | (0.25, 1.74) | ||
| fronto-parietal, alpha | 0.78 | (0.14, 1.74) | ||
| fronto-parietal, theta | 0.87 | (0.25, 1.81) | ||
| [66] | Theta | 1.09 | (0.78, 1.40) | |
| Alpha | 0.43 | (0.15, 0.71) | ||
| Beta | 0.62 | (0.33, 0.91) | ||
| delta power | [66] | −0.66 | (−0.37, −0.95) | |
| global effect | [102] | Inf | (−0.01, Inf) | |
| imaginary part of coherence | [66] | Beta | 0.95 | (0.65, 1.26) |
| mesoscale modularity | [128] | Delta, non-traumatic | 1.08 | (0.73, 1.43) |
| microscale clustering coefficient | [128] | Delta, traumatic | 1.09 | (0.71, 1.48) |
| microstate A | [66] | Duration, delta | 0.95 | (0.65, 1.26) |
| Frequency, theta | 0.95 | (0.65, 1.26) | ||
| Time in A, theta | 1.47 | (1.13, 1.80) | ||
| Frequency, 2–20Hz | 0.87 | (0.57, 1.17) | ||
| MMN | [95] | 0.76 | (0.56, 0.95) | |
| [98] | Inf | (0.29, Inf) | ||
| modified Valente’s grade | [132] | 0.45 | (0.12, 0.78) | |
| modularity | [129] | 0.61 | (0.52, 0.70) | |
| N400 | [135] | Wavelet | 0.91 | (0.79, 1.03) |
| Human | 2.15 | (1.99, 2.30) | ||
| organized sleep patterns | [130] | 1.31 | (0.91, 1.72) | |
| P300 | [109] | Inf | (0.11, Inf) | |
| [134] | 2.20 | (1.19, 3.29) | ||
| [135] | Wavelet | 0.25 | (0.15, 0.36) | |
| Human | 0.44 | (0.33, 0.55) | ||
| permutation entropy | [66] | Delta | 0.78 | (0.49, 1.08) |
| Theta | 1.35 | (1.02, 1.68) | ||
| [89] | 1.00 | (0.25, 1.75) | ||
| quadratic phase self-coupling | [34] | Theta, frontal | −0.84 | (−1.63, −0.05) |
| sleep spindles | [84] | Inf | (0.89, Inf) | |
| [89] | Density, MCS/MCS+ vs. death | 1.13 | (0.50, 1.76) | |
| Density, UWS/SD- vs. death | 0.96 | (0.35, 1.57) | ||
| somatosensory evoked potentials | [139] | 1.74 | (1.18, 3.03) | |
| theta normalized power | [59] | 0.78 | (0.51, 1.09) | |
| transfer entropy | [66] | Delta | 0.74 | (0.45, 1.03) |
| Alpha | 1.09 | (0.78, 1.40) | ||
| variance of path length | [129] | 0.75 | (0.66, 0.84) | |
| weighted symbolic mutual information | [66] | Alpha, 32s | 0.87 | (0.57, 1.17) |
| Alpha, 8s | 0.78 | (0.49, 1.08) | ||
| Delta, 8s | 0.70 | (0.41, 0.99) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wutzl, B.; Golaszewski, S.M.; Leibnitz, K.; Langthaler, P.B.; Kunz, A.B.; Leis, S.; Schwenker, K.; Thomschewski, A.; Bergmann, J.; Trinka, E. Narrative Review: Quantitative EEG in Disorders of Consciousness. Brain Sci. 2021, 11, 697. https://doi.org/10.3390/brainsci11060697
Wutzl B, Golaszewski SM, Leibnitz K, Langthaler PB, Kunz AB, Leis S, Schwenker K, Thomschewski A, Bergmann J, Trinka E. Narrative Review: Quantitative EEG in Disorders of Consciousness. Brain Sciences. 2021; 11(6):697. https://doi.org/10.3390/brainsci11060697
Chicago/Turabian StyleWutzl, Betty, Stefan M. Golaszewski, Kenji Leibnitz, Patrick B. Langthaler, Alexander B. Kunz, Stefan Leis, Kerstin Schwenker, Aljoscha Thomschewski, Jürgen Bergmann, and Eugen Trinka. 2021. "Narrative Review: Quantitative EEG in Disorders of Consciousness" Brain Sciences 11, no. 6: 697. https://doi.org/10.3390/brainsci11060697
APA StyleWutzl, B., Golaszewski, S. M., Leibnitz, K., Langthaler, P. B., Kunz, A. B., Leis, S., Schwenker, K., Thomschewski, A., Bergmann, J., & Trinka, E. (2021). Narrative Review: Quantitative EEG in Disorders of Consciousness. Brain Sciences, 11(6), 697. https://doi.org/10.3390/brainsci11060697