Preparing to React: A Behavioral Study on the Interplay between Proactive and Reactive Action Inhibition
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
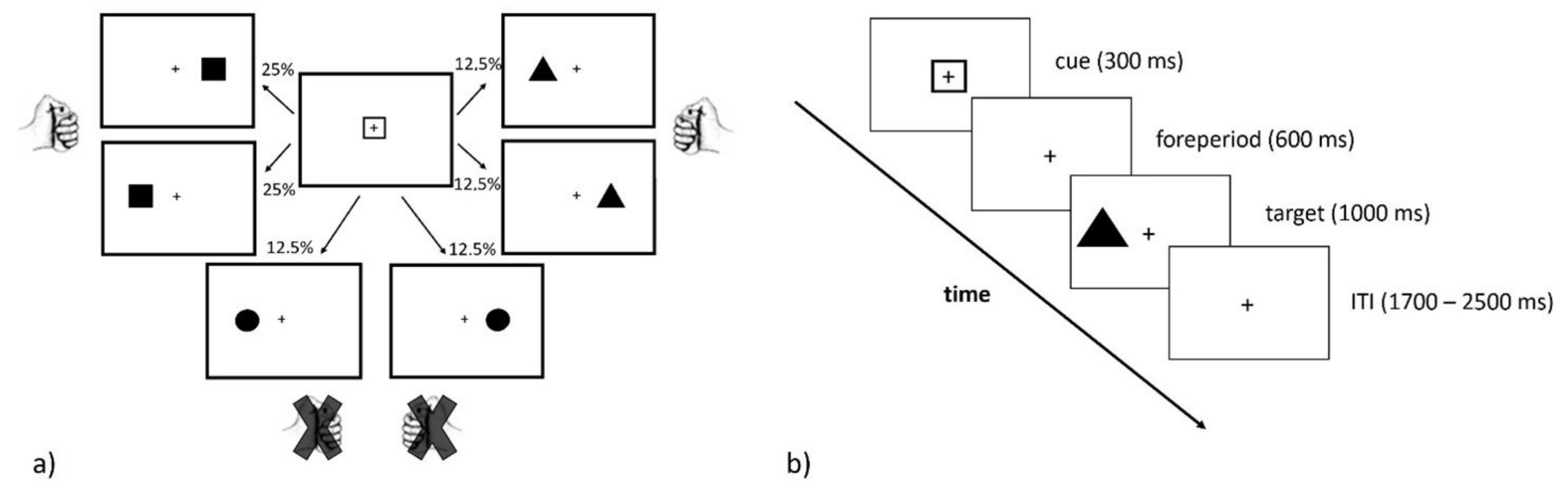
2.2. Stimuli and Procedure
2.3. EMG Recordings
2.4. EMG Signal Processing
2.5. Data Analysis
3. Results
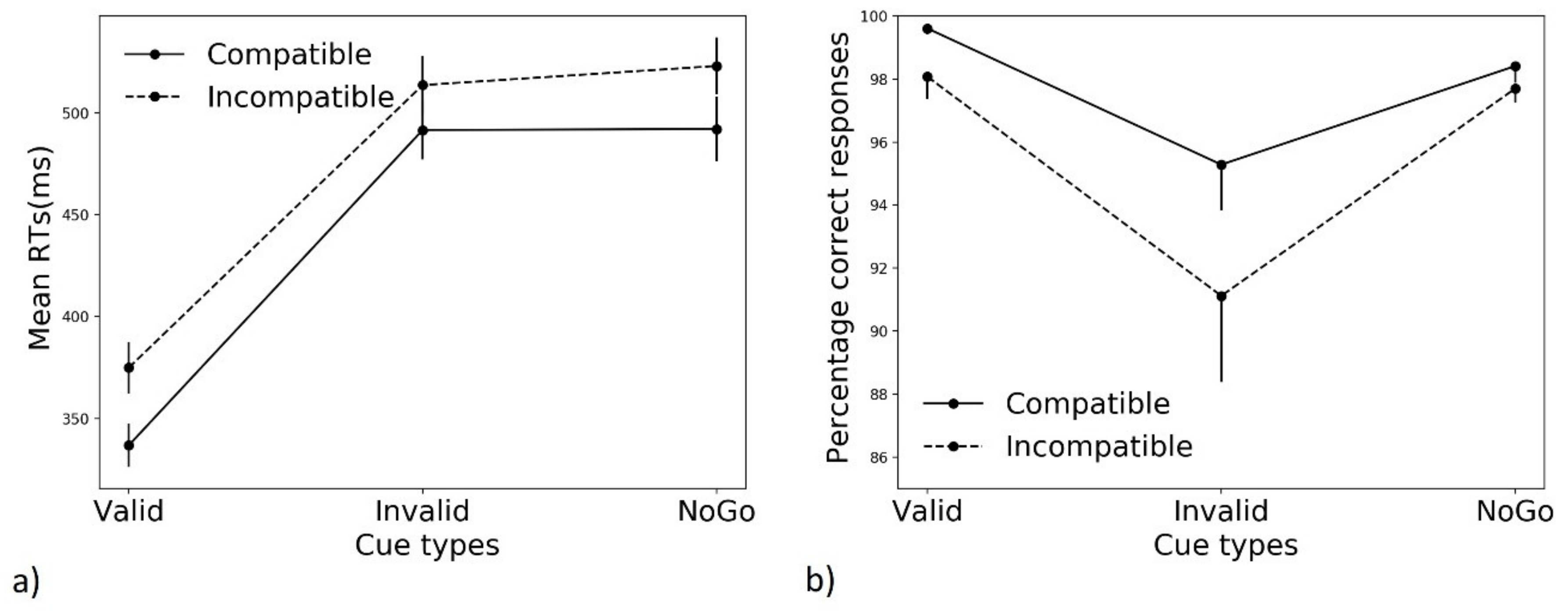
3.1. Mean Performance
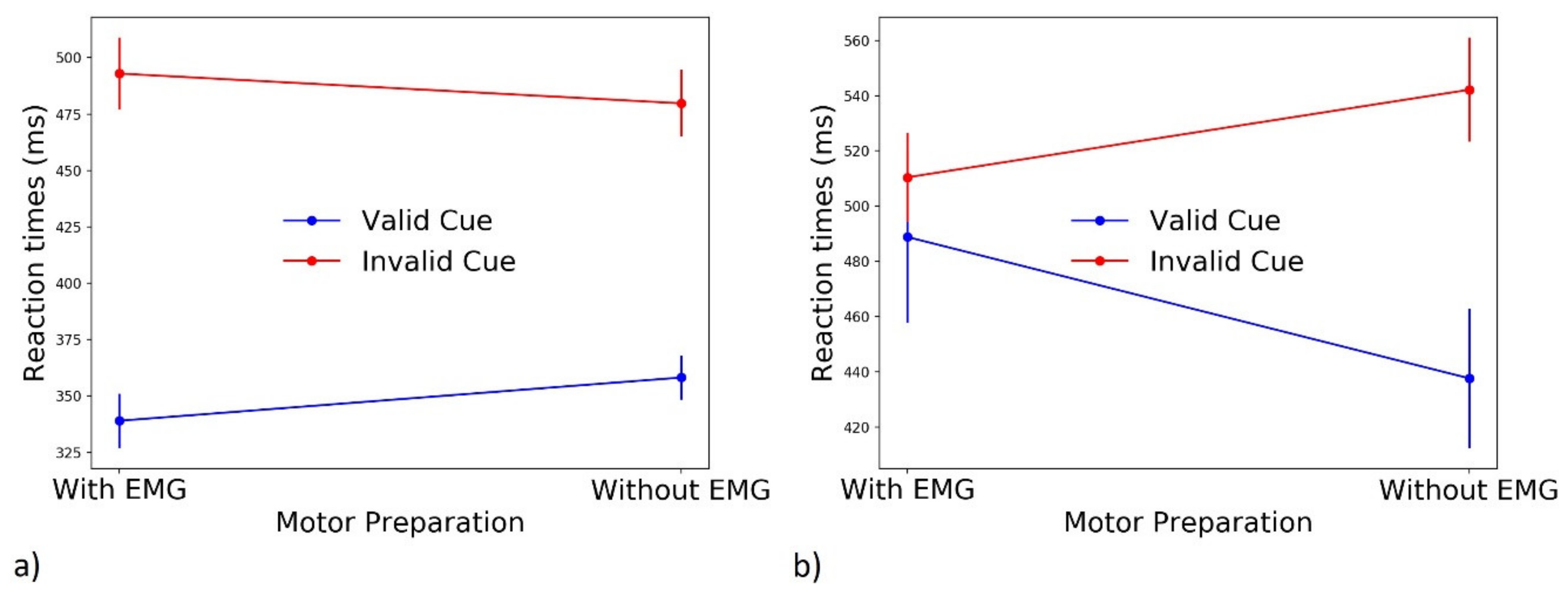
3.1.1. Reaction Times (RTs)
3.1.2. Accuracy
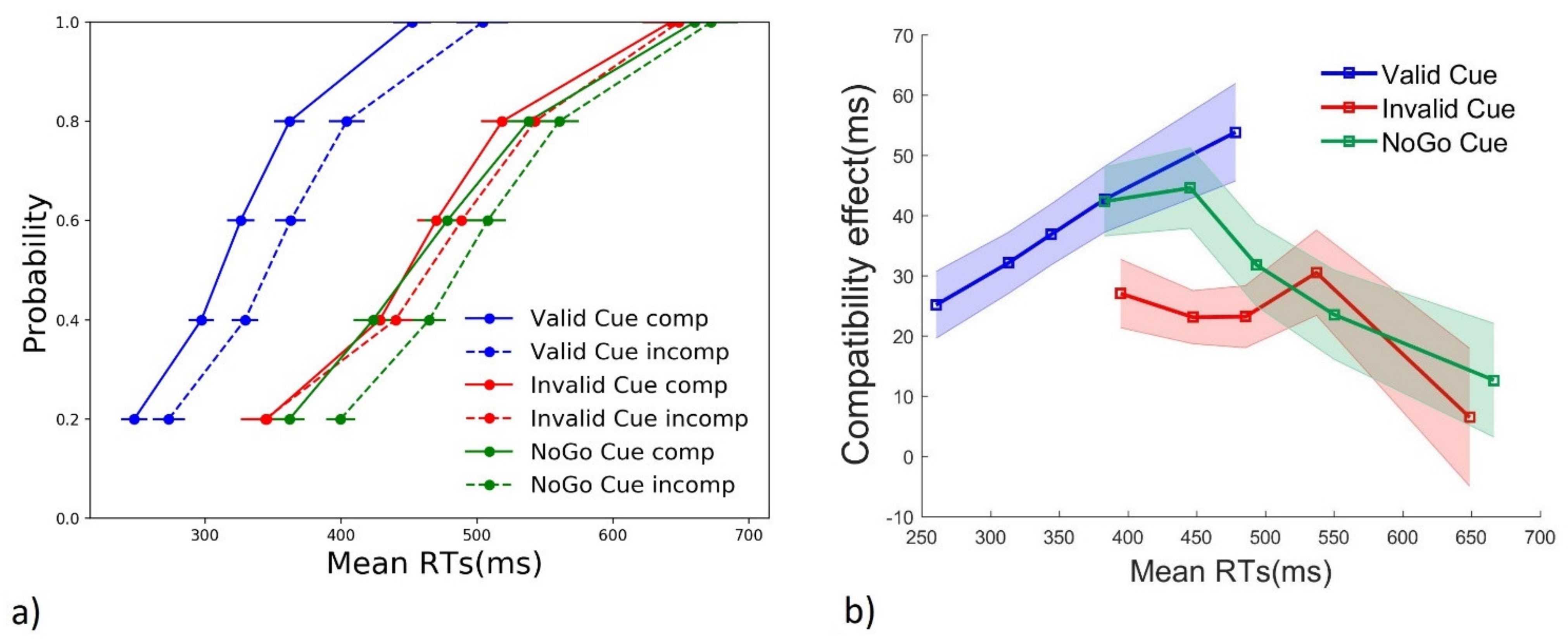
3.2. Distribution Analyses
3.2.1. RTs
3.2.2. Accuracy
3.3. EMG Analyses
3.3.1. Motor Preparation
3.3.2. RTs Based on EMG Categories
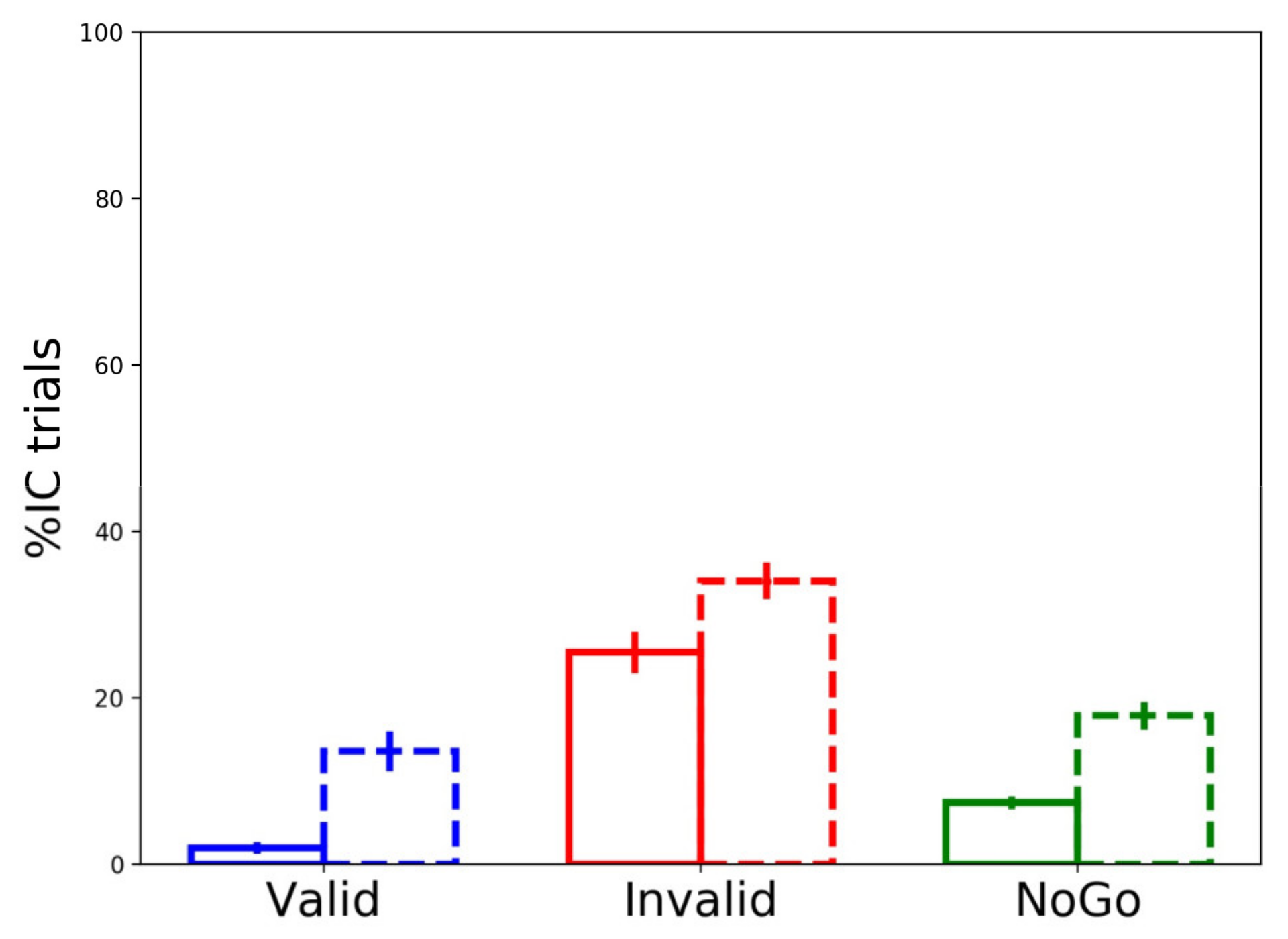
3.3.3. Partial Errors’ Frequency
3.3.4. EMG-Based Accuracy Dynamics
3.3.5. Effect of Motor Preparation on EMG Categories
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jaffard, M.; Benraiss, A.; Longcamp, M.; Velay, J.L.; Boulinguez, P. Cueing method biases in visual detection studies. Brain Res. 2007, 1179, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Wardak, C.; Ramanoël, S.; Guipponi, O.; Boulinguez, P.; Ben Hamed, S. Proactive inhibitory control varies with task context. Eur. J. Neurosci. 2012, 36, 3568–3579. [Google Scholar] [CrossRef] [PubMed]
- Chikazoe, J.; Jimura, K.; Hirose, S.; Yamashita, K.; Miyashita, Y.; Konishi, S. Preparation to inhibit a response complements response inhibition during performance of a stop-signal task. J. Neurosci. 2009, 29, 15870–15877. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Oldenkamp, C.L.; Aron, A. A proactive mechanism for selective suppression of response tendencies. J. Neurosci. 2011, 31, 5965–5969. [Google Scholar] [CrossRef]
- Aron, A. From reactive to proactive and selective control: Developing a richer model for stopping inappropriate responses. Biol. Psychiatry 2011, 69, e55–e68. [Google Scholar] [CrossRef]
- Munakata, Y.; Herd, S.A.; Chatham, C.H.; Depue, B.E.; Banich, M.T.; O’Reilly, R.C. A unified framework for inhibitory control. Trends Cogn. Sci. 2011, 15, 453–459. [Google Scholar] [CrossRef]
- Perri, R.L. Is there a proactive and a reactive mechanism of inhibition? Towards an executive account of the attentional inhibitory control model. Behav. Brain Res. 2020, 377, 112243. [Google Scholar] [CrossRef]
- Swann, N.C.; Cai, W.; Conner, C.R.; Pieters, T.A.; Claffey, M.P.; George, J.S.; Aron, A.R.; Tandon, N. Roles for the pre-supplementary motor area and the right inferior frontal gyrus in stopping action: Electrophysiological responses and functional and structural connectivity. Neuroimage 2012, 59, 2860–2870. [Google Scholar] [CrossRef]
- Mirabella, G.; Pani, P.; Ferraina, S. Context influences on the preparation and execution of reaching movements. Cogn. Neuropsychol 2008, 25, 996–1010. [Google Scholar] [CrossRef]
- Duque, J.; Ivry, R.B. Role of corticospinal suppression during motor preparation. Cereb. Cortex 2009, 19, 2013–2024. [Google Scholar] [CrossRef]
- Duque, J.; Lew, D.; Mazzocchio, R.; Olivier, E.; Ivry, R.B. Evidence for two concurrent inhibitory mechanisms during response preparation. J. Neurosci. 2010, 30, 3793–3802. [Google Scholar] [CrossRef] [PubMed]
- Hasbroucq, T.; Kaneko, H.; Akamatsu, M.; Possamai, C.A. Preparatory inhibition of cortico-spinal excitability: A transcranial magnetic stimulation study in man. Brain Res. Cogn. Brain Res. 1997, 5, 185–192. [Google Scholar] [CrossRef]
- Touge, T.; Taylor, J.L.; Rothwell, J.C. Reduced excitability of the corticospinal system during the warning period of a reaction time task. Electroencephalogr. Clin. Neurophysiol. 1998, 109, 489–495. [Google Scholar] [CrossRef]
- Davranche, K.; Tandonnet, C.; Burle, B.; Meynier, C.; Vidal, F.; Hasbroucq, T. The dual nature of time preparation: Neural activation and suppression revealed by transcranial magnetic stimulation of the motor cortex. Eur. J. Neurosci. 2007, 25, 3766–3774. [Google Scholar] [CrossRef]
- Greenhouse, I.; Sias, A.; Labruna, L.; Ivry, R.B. Nonspecific inhibition of the motor system during response preparation. J. Neurosci. 2015, 35, 10675–10684. [Google Scholar] [CrossRef] [PubMed]
- Labruna, L.; Lebon, F.; Duque, J.; Klein, P.A.; Cazares, C.; Ivry, R.B. Generic inhibition of the selected movement and constrained inhibition of nonselected movements during response preparation. J. Cogn. Neurosci. 2014, 26, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Lebon, F.; Greenhouse, I.; Labruna, L.; Vanderschelden, B.; Papaxanthis, C.; Ivry, R.B. Influence of Delay Period Duration on Inhibitory Processes for Response Preparation. Cereb. Cortex 2016, 26, 2461–2470. [Google Scholar] [CrossRef]
- Derosiere, G.; Vassiliadis, P.; Duque, J. Advanced TMS approaches to probe corticospinal excitability during action preparation. Neuroimage 2020, 213, 116746. [Google Scholar] [CrossRef]
- Duque, J.; Greenhouse, I.; Labruna, L.; Ivry, R.B. Physiological markers of motor inhibition during human behavior. Trends Neurosci. 2017, 40, 219–236. [Google Scholar] [CrossRef]
- Ficarella, S.C.; Battelli, L. Proactive inhibition activation depends on motor preparation: A single pulse TMS study. Front. Psychol. 2018, 9, 1891. [Google Scholar] [CrossRef]
- Ficarella, S.C.; Battelli, L. Motor Preparation for Action Inhibition: A Review of Single Pulse TMS Studies Using the Go/NoGo Paradigm. Front. Psychol. 2019, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Wessel, J.R. Prepotent motor activity and inhibitory control demands in different variants of the go/no-go paradigm. Psychophysiology 2017, 55, e12871. [Google Scholar] [CrossRef] [PubMed]
- Raud, L.; Huster, R.J.; Ivry, R.B.; Labruna, L.; Messel, M.S.; Greenhouse, I. A Single Mechanism for Global and Selective Response Inhibition under the Influence of Motor Preparation. J. Neurosci. 2020, 40, 7921–7935. [Google Scholar] [CrossRef]
- Swick, D.; Ashley, V.; Turken, U. Are the neural correlates of stopping and not going identical? quantitative meta-analysis of two response inhibition tasks. NeuroImage 2011, 56, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Dambacher, F.; Sack, A.T.; Lobbestael, J.; Arntz, A.; Brugman, S.; Schuhmann, T. A network approach to response inhibition: Dissociating functional connectivity of neural components involved in action restraint and action cancellation. Eur. J. Neurosci. 2014, 39, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Criaud, M.; Wardak, C.; Ben Hamed, S.; Ballanger, B.; Boulinguez, P. Proactive inhibitory control of response as the default state of executive control. Front. Psychol. 2012, 3, 59. [Google Scholar] [CrossRef]
- Liebrand, M.; Pein, I.; Tzvi, E.; Krämer, U.M. Temporal Dynamics of Proactive and Reactive Motor Inhibition. Front. Hum. Neurosci. 2017, 11, 204. [Google Scholar] [CrossRef]
- Lavallee, C.F.; Meemken, M.T.; Herrmann, C.S.; Huster, R.J. When holding your horses meets the deer in the headlights: Time-frequency characteristics of global and selective stopping under conditions of proactive and reactive control. Front. Hum. Neurosci. 2014, 8, 994. [Google Scholar] [CrossRef]
- Boulinguez, P.; Ballanger, B.; Granjon, L.; Benraiss, A. The paradoxical effect of warning on reaction time: Demonstrating proactive response inhibition with event-related potentials. Clin. Neurophysiol. 2009, 120, 730–737. [Google Scholar] [CrossRef]
- Jaffard, M.; Longcamp, M.; Velay, J.L.; Anton, J.L.; Roth, M.; Nazarian, B.; Boulinguez, P. Proactive inhibitory control of movement assessed by event-related fMRI. Neuroimage 2008, 42, 1196–1206. [Google Scholar] [CrossRef]
- Allain, S.; Carbonnell, L.; Burle, B.; Hasbroucq, T.; Vidal, F. Online executive control: An electromyographic study. Psychophysiology 2004, 41, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Śmigasiewicz, K.; Ambrosi, S.; Blaye, A.; Burle, B. Inhibiting errors while they are produced: Direct evidence for error monitoring and inhibitory control in children. Dev. Cogn. Neurosci. 2020, 41, 100742. [Google Scholar] [CrossRef] [PubMed]
- Burle, B.; Possamaı, C.-A.; Vidal, F.; Bonnet, M.; Hasbroucq, T. Executive control in the Simon effect: An electromyographic and distributional analysis. Psychol. Res. 2002, 66, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, C.W.; Coles, M.G.H.; Morris, L.R.; O’Hara, W.P. An electromyographic examination of response competition. Bull. Psychon. Soc. 1985, 23, 165–168. [Google Scholar] [CrossRef]
- Smid, H.G.O.M.; Mulder, G.; Mulder, L.J.M. Selective response activation can begin before stimulus recognition is complete: A psychophysiological and error analysis of continuous flow. Acta Psychol. 1990, 74, 169–201. [Google Scholar] [CrossRef]
- Rochet, N.; Spieser, L.; Casini, L.; Hasbroucq, T.; Burle, B. Detecting and correcting partial errors: Evidence for efficient control without conscious access. Cogn. Affect. Behav. Neurosci. 2014, 14, 970–982. [Google Scholar] [CrossRef] [PubMed]
- Ficarella, S.C.; Rochet, N.; Burle, B. Becoming aware of subliminal responses: An EEG/EMG study on partial error detection and correction in humans. Cortex 2019, 120, 443–456. [Google Scholar] [CrossRef]
- Simon, J.R. The Effect of an Irrelevant Directional Cue on Human Information Processing. In Stimulus-Response Compatibility: An Integrated Perspective; Proctor, R.W., Reeve, T.G., Eds.; North-Holland: Amsterdam, The Netherlands, 1990; pp. 31–88. [Google Scholar]
- Ridderinkhof, K.R. Activation and Suppression in Conflict Tasks: Empirical Clarification through Distributional Analyses. In Attention and Performance, Common Mechanisms in Perception and Action; Prinz, W., Hommel, B., Eds.; Oxford University Press: Oxford, UK, 2002; Volume 19, pp. 494–519. [Google Scholar]
- Mathôt, S.; Schreij, D.; Theeuwes, J. OpenSesame: An open-source, graphical experiment builder for the social sciences. Behav. Res. Methods 2012, 44, 314–324. [Google Scholar] [CrossRef]
- Hodges, P.W.; Bui, B.H. A comparison of computer-based methods for the determination of onset of muscle contraction using electromyography. Electroencephalogr. Clin. Neurophysiol. 1996, 101, 511–519. [Google Scholar]
- Santello, M.; McDonagh, M.J. The control of timing and amplitude of EMG activity in landing movements in humans. Exp. Physiol. 1998, 83, 857–874. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Q. Use of the integrated profile for voluntary muscle activity detection using EMG signals with spurious background spikes: A study with incomplete spinal cord injury. Biomed. Signal Process. Control 2016, 24, 19–24. [Google Scholar] [CrossRef]
- Picton, T.W.; Bentin, S.; Berg, P.; Donchin, E.; Hillyard, S.A.; Johnson, R., Jr.; Miller, G.A.; Ritter, W.; Ruchkin, D.S.; Rugg, M.D.; et al. Guidelines for using human event-related potentials to study cognition: Recording standards and publication criteria. Psychophysiology 2000, 37, 127–152. [Google Scholar] [CrossRef] [PubMed]
- De Jong, R.; Liang, C.-C.; Lauber, E. Conditional and unconditional automaticity: A dual-process model of effects of spatial stimulus response correspondence. J. Exp. Psychol. Hum. Percept. Perform. 1994, 20, 731–750. [Google Scholar] [CrossRef]
- Ratcliff, R. Group reaction time distributions and an analysis of distribution statistics. Psychol. Bull. 1979, 86, 446–461. [Google Scholar] [CrossRef]
- Vincent, S.B. The function of vibrissae in the behavior of the white rat. Behav. Monogr. 1912, 1, 1–82. [Google Scholar]
- Pratte, M.S.; Rouder, J.N.; Morey, R.D.; Feng, C. Exploring the differences in distributional properties between Stroop and Simon effects using delta plots. Atten. Percept. Psycho. 2010, 72, 2013–2025. [Google Scholar] [CrossRef] [PubMed]
- Ridderinkhof, K.R.; van den Wildenberg, W.P.M.; Wijnen, J.; Burle, B. Response Inhibition in Conflict Tasks is Revealed in Delta Plots. In Cognitive Neuroscience of Attention; Posner, M.I., Ed.; The Guilford Press: New York, NY, USA, 2004; pp. 369–377. [Google Scholar]
- Suarez, I.; Burle, B.; Tobon, C.; Pineda, D.; Lopera, F.; Hasbroucq, T.; Casini, L. Deciphering interference control in adults with ADHD by using distribution analyses and electromyographic activity. Acta Psychol. 2015, 159, 85–92. [Google Scholar] [CrossRef]
- Van den Wildenberg, W.P.; Wylie, S.A.; Forstmann, B.U.; Burle, B.; Hasbroucq, T.; Ridderinkhof, K.R. To head or to heed? Beyond the surface of selective action inhibition: A review. Front. Hum. Neurosci. 2010, 4, 222. [Google Scholar] [CrossRef] [PubMed]
- Fluchère, F.; Burle, B.; Vidal, F.; van den Wildenberg, W.; Witjas, T.; Eusebio, A.; Azulay, J.P.; Hasbroucq, T. Subthalamic nucleus stimulation, dopaminergic treatment and impulsivity in Parkinson’s disease. Neuropsychologia 2018, 117, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.C.; Bucci, D.J. Neural and behavioral mechanisms of proactive and reactive inhibition. Learn. Mem. 2016, 23, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Tandonnet, C.; Davranche, K.; Meynier, C.; Burle, B.; Vidal, F.; Hasbroucq, T. How does temporal preparation speed up response implementation in choice tasks? Evidence for an early cortical activation. Psychophysiology 2012, 49, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Korolczuk, I.; Burle, B.; Coull, J.T. The costs and benefits of temporal predictability: Impaired inhibition of prepotent responses accompanies increased activation of task-relevant responses. Cognition 2018, 179, 102–110. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ficarella, S.C.; Desantis, A.; Zénon, A.; Burle, B. Preparing to React: A Behavioral Study on the Interplay between Proactive and Reactive Action Inhibition. Brain Sci. 2021, 11, 680. https://doi.org/10.3390/brainsci11060680
Ficarella SC, Desantis A, Zénon A, Burle B. Preparing to React: A Behavioral Study on the Interplay between Proactive and Reactive Action Inhibition. Brain Sciences. 2021; 11(6):680. https://doi.org/10.3390/brainsci11060680
Chicago/Turabian StyleFicarella, Stefania C., Andrea Desantis, Alexandre Zénon, and Boris Burle. 2021. "Preparing to React: A Behavioral Study on the Interplay between Proactive and Reactive Action Inhibition" Brain Sciences 11, no. 6: 680. https://doi.org/10.3390/brainsci11060680
APA StyleFicarella, S. C., Desantis, A., Zénon, A., & Burle, B. (2021). Preparing to React: A Behavioral Study on the Interplay between Proactive and Reactive Action Inhibition. Brain Sciences, 11(6), 680. https://doi.org/10.3390/brainsci11060680





