Postoperative Pneumocephalus on Computed Tomography Might Predict Post-Corpus Callosotomy Chemical Meningitis
Abstract
1. Introduction
2. Methods
2.1. Study Design and Ethics Approval
2.2. Clinical Information
2.3. Corpus Callosotomy
2.4. Diagnosis of Chemical Meningitis
2.5. Postoperative Pneumocephalus
2.6. Outcome Measurement
2.7. Statistical Analysis
3. Results
3.1. Clinical Information
3.2. Outcome Measurements
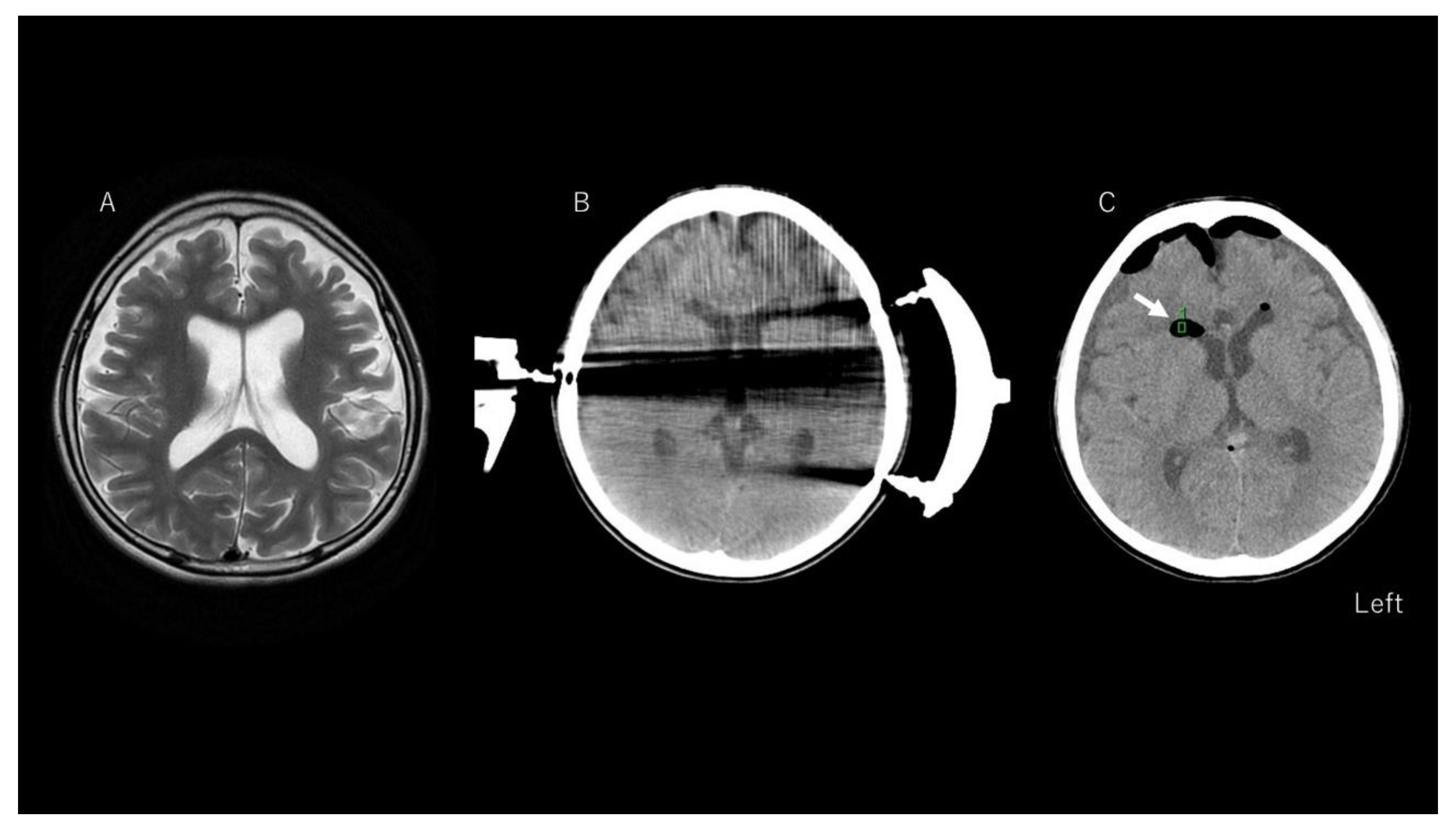
3.3. Representative Case of Chemical Meningitis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Wagenen, W.P.; Herren, R.Y. Surgical division of commissural pathways in the corpus callosum: Relation to spread of an epileptic attack. Arch. Neurol. Psychiatry 1940, 44, 740–759. [Google Scholar] [CrossRef]
- Mathews, M.S.; Linskey, M.E.; Binder, D.K.; van William, P. Wagenen and the first corpus callosotomies for epilepsy. J. Neurosurg. JNS 2008, 108, 608. [Google Scholar] [CrossRef] [PubMed]
- Malmgren, K.; Rydenhag, B.; Hallböök, T. Reappraisal of corpus callosotomy. Curr. Opin. Neurol. 2015, 28, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H. Our experience with pediatric epilepsy surgery focusing on corpus callosotomy and hemispherotomy. Epilepsia 2005, 46 (Suppl. 1), 30–31. [Google Scholar] [CrossRef] [PubMed]
- Asadi-Pooya, A.A.; Sharan, A.; Nei, M.; Sperling, M.R. Corpus callosotomy. Epilepsy Behav. 2008, 13, 271–278. [Google Scholar] [CrossRef]
- Honda, R.; Baba, H.; Adachi, K.; Koshimoto, R.; Ono, T.; Toda, K.; Tanaka, S.; Baba, S.; Yamasaki, K.; Yatsuhashi, H. Developmental outcome after corpus callosotomy for infants and young children with drug-resistant epilepsy. Epilepsy Behav. 2021, 117, 107799. [Google Scholar] [CrossRef]
- Ueda, R.; Matsuda, H.; Sato, N.; Iwasaki, M.; Sone, D.; Takeshita, E.; Shimizu-Motohashi, Y.; Ishiyama, A.; Saito, T.; Komaki, H.; et al. Alteration of the anatomical covariance network after corpus callosotomy in pediatric intractable epilepsy. PLoS ONE 2019, 14, e0222876. [Google Scholar] [CrossRef]
- Nozaki, T.; Fujimoto, A.; Ichikawa, N.; Baba, S.; Enoki, H.; Okanishi, T. Higher intelligence may be a risk factor for postoperative transient disturbance of consciousness after corpus callosotomy. Epilepsy Behav. 2021, 115, 107617. [Google Scholar] [CrossRef] [PubMed]
- Caruso, J.P.; Janjua, M.B.; Dolce, A.; Price, A.V. Retrospective analysis of open surgical versus laser interstitial thermal therapy callosotomy in pediatric patients with refractory epilepsy. J. Neurosurg. Pediatr. 2021, 1–9. [Google Scholar] [CrossRef]
- Tripathi, M.; Maskara, P.; Rangan, V.S.; Mohindra, S.; De Salles, A.A.F.; Kumar, N. Radiosurgical Corpus Callosotomy: A Review of Literature. World Neurosurg. 2021, 145, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Sood, S.; Asano, E.; Altinok, D.; Luat, A. Endoscopic posterior interhemispheric complete corpus callosotomy. J. Neurosurg. Pediatr. 2016, 25, 689–692. [Google Scholar] [CrossRef]
- Chandra, S.P.; Kurwale, N.S.; Chibber, S.S.; Banerji, J.; Dwivedi, R.; Garg, A.; Bal, C.; Tripathi, M.; Sarkar, C.; Tripathi, M. Endoscopic-Assisted (Through a Mini Craniotomy) Corpus Callosotomy Combined With Anterior, Hippocampal, and Posterior Commissurotomy in Lennox-Gastaut Syndrome: A Pilot Study to Establish Its Safety and Efficacy. Neurosurgery 2016, 78, 743–751. [Google Scholar] [CrossRef]
- Mendenhall, S.K.; Ahluwalia, R.K.; Barbaro, N.M. Corpus Callosotomy. In Epilepsy Surgery and Intrinsic Brain Tumor Surgery: A Practical Atlas; Fountas, K., Kapsalaki, E.Z., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 189–196. [Google Scholar] [CrossRef]
- Reutens, D.C.; Bye, A.M.; Hopkins, I.J.; Danks, A.; Somerville, E.; Walsh, J.; Bleasel, A.; Ouvrier, R.; MacKenzie, R.A.; Manson, J.I.; et al. Corpus callosotomy for intractable epilepsy: Seizure outcome and prognostic factors. Epilepsia 1993, 34, 904–909. [Google Scholar] [CrossRef]
- Sorenson, J.M.; Wheless, J.W.; Baumgartner, J.E.; Thomas, A.; Brookshire, B.L.; Clifton, G.L.; Willmore, J. Corpus callosotomy for medically intractable seizures. Pediatric Neurosurg. 1997, 27, 260–267. [Google Scholar] [CrossRef]
- Hader, W.; Bezchlibnyk, I.; Pillay, N.; Wiebe, S. Corpus callosotomy, risks and benefits: A systematic review of the evidence. Epilepsy Curr. 2012, 12, 1. [Google Scholar]
- Burchiel, K.J. Corpus callosotomy for intractable epilepsy in children. West. J. Med. 1993, 158, 66–67. [Google Scholar] [PubMed]
- Wong, T.T.; Kwan, S.Y.; Chang, K.P.; Hsiu-Mei, W.; Yang, T.F.; Chen, Y.S.; Yi-Yen, L. Corpus callosotomy in children. Childs Nerv. Syst. 2006, 22, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- Kamath, A.A.; Limbrick, D.L.; Smyth, M.D. Characterization of postoperative fevers after hemispherotomy. Childs Nerv. Syst. 2015, 31, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, T.; Fujimoto, A.; Baba, S.; Enoki, H.; Okanishi, T. Postoperative persistent fever may be a risk factor for hydrocephalus in hemispherical disconnection surgery. Epilepsy Behav. 2020, 112, 107466. [Google Scholar] [CrossRef]
- Finucane, T.E.; Bynum, J.P. Use of tube feeding to prevent aspiration pneumonia. Lancet 1996, 348, 1421–1424. [Google Scholar] [CrossRef]
- Forgacs, P.; Geyer, C.A.; Freidberg, S.R. Characterization of Chemical Meningitis after Neurological Surgery. Clin. Infect. Dis. 2001, 32, 179–185. [Google Scholar] [CrossRef]
- Association, K.N. Clinical Practice Guidelines for the Management of Bacterial Meningitis in Adults in Korea. Infect. Chemother. 2012, 44, 140–163. [Google Scholar]
- Amano, Y.; Fujimoto, A.; Ichikawa, N.; Sato, K.; Baba, S.; Nishimura, M.; Enoki, H.; Okanishi, T. Cranioplasty with Titanium Might Be Suitable for Adult Epilepsy Surgery After Subdural Placement Surgery To Avoid Surgical Site Infection. World Neurosurg. 2019, 131, e503–e507. [Google Scholar] [CrossRef]
- Fujimoto, A.; Masuda, Y.; Ichikawa, N.; Sato, K.; Baba, S.; Itamura, S.; Nishimura, M.; Enoki, H.; Okanishi, T. Side Slit Guide Pipe for Precise Placement of Depth Electrodes. World Neurosurg. 2019, 126, 291–295. [Google Scholar] [CrossRef]
- Concato, J.; Peduzzi, P.; Holford, T.R.; Feinstein, A.R. Importance of events per independent variable in proportional hazards analysis. I. Background, goals, and general strategy. J. Clin. Epidemiol. 1995, 48, 1495–1501. [Google Scholar] [CrossRef]
- Peduzzi, P.; Concato, J.; Feinstein, A.R.; Holford, T.R. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J. Clin. Epidemiol. 1995, 48, 1503–1510. [Google Scholar] [CrossRef]
- Peduzzi, P.; Concato, J.; Kemper, E.; Holford, T.R.; Feinstein, A.R. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 1996, 49, 1373–1379. [Google Scholar] [CrossRef]
- Borni, M.; Abdelhedi, A.; Kammoun, B.; Kolsi, F.; Boudawara, M.Z. Ruptured Central Nervous System Dermoid Cyst of Suprasellar Region Manifesting as Unusual Epileptic Seizure. World Neurosurg. 2019, 122, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Satoh, H.; Uozumi, T.; Arita, K.; Kurisu, K.; Hotta, T.; Kiya, K.; Ikawa, F.; Goishi, J.; Sogabe, T. Spontaneous rupture of craniopharyngioma cysts. A report of five cases and review of the literature. Surg. Neurol. 1993, 40, 414–419. [Google Scholar] [CrossRef]
- Pettorini, B.L.; Inzitari, R.; Massimi, L.; Tamburrini, G.; Caldarelli, M.; Fanali, C.; Cabras, T.; Messana, I.; Castagnola, M.; Di Rocco, C. The role of inflammation in the genesis of the cystic component of craniopharyngiomas. Childs Nerv. Syst. 2010, 26, 1779–1784. [Google Scholar] [CrossRef]
- Feitosa, T.; Pereira, L.; Brilhante, D.; Brasil, P.; Leitão, F.; Tavora, D.; Macedo, C. Ventriculitis and its Different Patterns: An Imaging Aprouch. In Proceedings of the European Society of Radiology, Vienna, Austria, 1–5 March 2017. [Google Scholar]
- Kaufman, B.A.; Tunkel, A.R.; Pryor, J.C.; Dacey, R.G., Jr. Meningitis in the neurosurgical patient. Infect. Dis. Clin. N. Am. 1990, 4, 677–701. [Google Scholar] [CrossRef]
- Katzenelbogen, S. The Cerebrospinal Fluid and Its Relation to the Blood: A Physiological and Clinical Study; John Hopkins Press: Baltimore, MD, USA, 1935. [Google Scholar]
| c-Group (n = 11; 17%) | nc-Group (n = 54; 83%) | p-Value | |
|---|---|---|---|
| Age, y | mean 11.2, SD 12.3, median 8 | mean 16.6, SD 15.2, median 12 | 0.244 |
| Sex | seven females, four males | 18 females, 36 males | 0.016 * |
| Body mass index, kg/m2 | mean 16.3, SD 3.23, median 15.5 | mean 19.1, SD 5.11, median 17.6 | 0.07 |
| Operation time, mins | mean 177.9, SD 75, median 153 | mean 208.1, SD 77.6, median 173.5 | 0.159 |
| Blood loss, mL | mean 31.2, SD 40.6, median 20.0 | mean 66.2, SD 75.0, median 30.0 | 0.041 * |
| Pneumocephalus | 8 (73%) | 13 (24%) | 0.002 * |
| Surgery (total CC) | 3 (27%) | 25 (44%) | 0.556 |
| Coefficient | Standard Error | p-Value | |
|---|---|---|---|
| Age | −0.003 | 0.003 | 0.270 |
| Sex | 0.180 | 0.090 | 0.061 |
| Operation time | −0.001 | 0.001 | 0.242 |
| Blood loss | −0.001 | 0.001 | 0.139 |
| Pneumocephalus | 0.313 | 0.093 | 0.001 * |
| Total vs. Anterior CC | 0.109 | 0.094 | 0.252 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujimoto, A.; Hatano, K.; Nozaki, T.; Sato, K.; Enoki, H.; Okanishi, T. Postoperative Pneumocephalus on Computed Tomography Might Predict Post-Corpus Callosotomy Chemical Meningitis. Brain Sci. 2021, 11, 638. https://doi.org/10.3390/brainsci11050638
Fujimoto A, Hatano K, Nozaki T, Sato K, Enoki H, Okanishi T. Postoperative Pneumocephalus on Computed Tomography Might Predict Post-Corpus Callosotomy Chemical Meningitis. Brain Sciences. 2021; 11(5):638. https://doi.org/10.3390/brainsci11050638
Chicago/Turabian StyleFujimoto, Ayataka, Keisuke Hatano, Toshiki Nozaki, Keishiro Sato, Hideo Enoki, and Tohru Okanishi. 2021. "Postoperative Pneumocephalus on Computed Tomography Might Predict Post-Corpus Callosotomy Chemical Meningitis" Brain Sciences 11, no. 5: 638. https://doi.org/10.3390/brainsci11050638
APA StyleFujimoto, A., Hatano, K., Nozaki, T., Sato, K., Enoki, H., & Okanishi, T. (2021). Postoperative Pneumocephalus on Computed Tomography Might Predict Post-Corpus Callosotomy Chemical Meningitis. Brain Sciences, 11(5), 638. https://doi.org/10.3390/brainsci11050638







