An fNIRS Investigation of Masculinity, Femininity, and Sex on Nonparents’ Empathic Response to Infant Cries
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
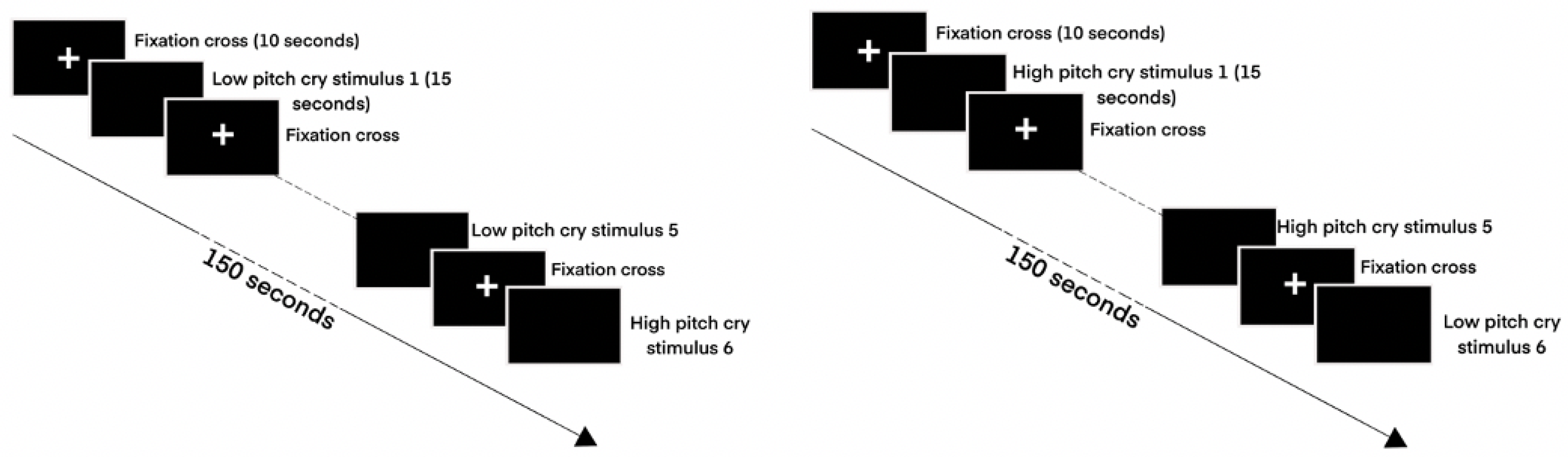
2.2. Procedure
2.3. Measures
2.3.1. Participant Demographic Survey
2.3.2. Toronto Empathy Questionnaire
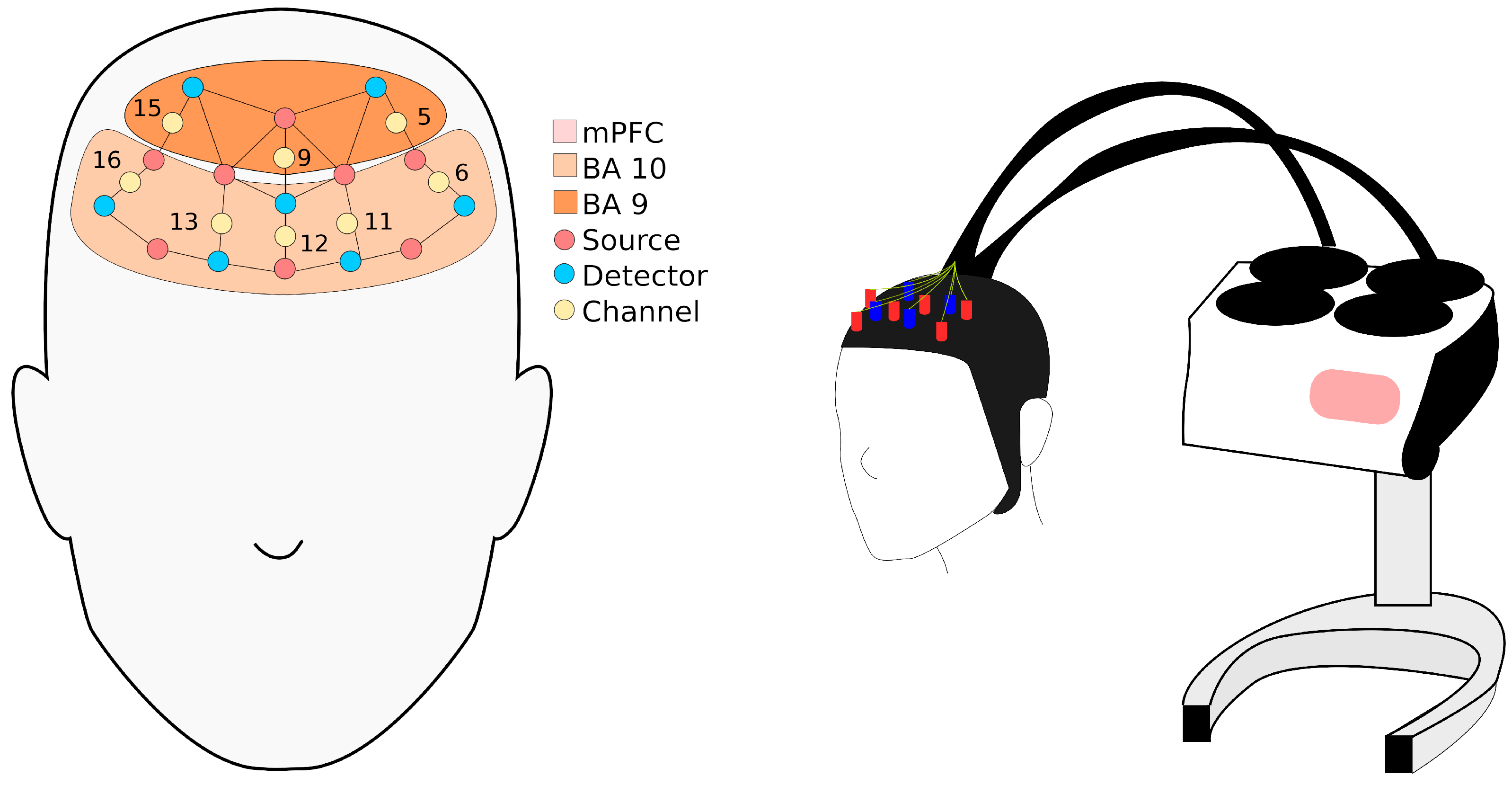
2.3.3. Near-Infrared Spectroscopy
2.3.4. Bem Sex-Role Inventory
2.4. Infant Cry Stimuli
2.5. Analytic Plan
3. Results
3.1. fNIRS Results
3.2. Sex Difference in Trait Empathy
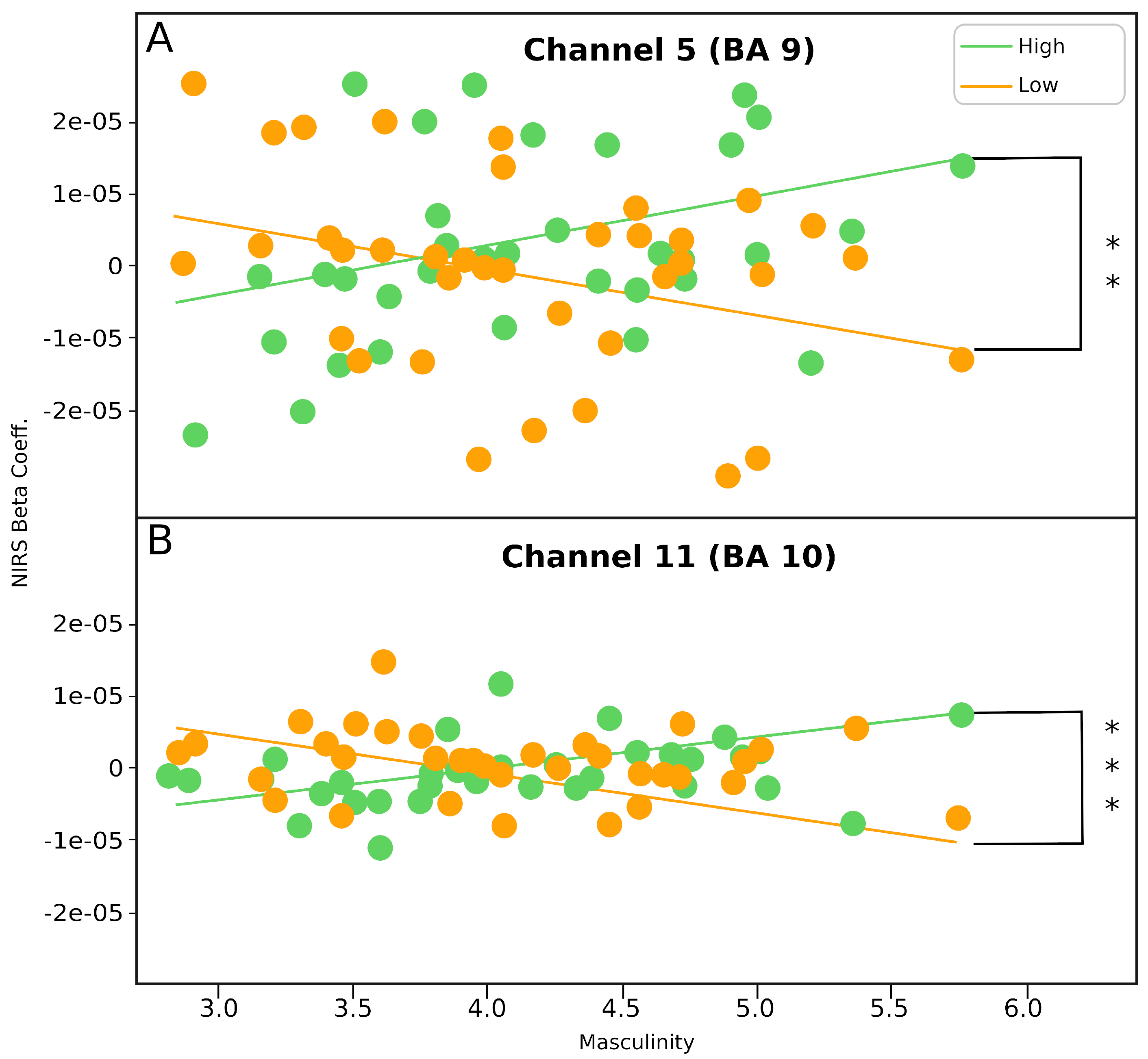
3.3. Correlation between Femininity and mPFC Activity
3.4. Correlation between Masculinity and mPFC Activity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basch, M.F. Empathic understanding: A review of the concept and some theoretical considerations. J. Am. Psychoanal. Assoc. 1983, 31, 101–126. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, N.; Lennon, R. Sex differences in empathy and related capacities. Psychol. Bull. 1983, 94, 100. [Google Scholar] [CrossRef]
- Mestre Escrivá, M.V.; Samper García, P.; Frías Navarro, M.D.; Tur Porcar, A.M. Are women more empathetic than men? A longitudinal study in adolescence. Span. J. Psychol. 2009, 12, 76–83. [Google Scholar] [CrossRef]
- Simner, M.L. Newborn’s response to the cry of another infant. Dev. Psychol. 1971, 5, 136. [Google Scholar] [CrossRef]
- Kemp, A.H.; Silberstein, R.B.; Armstrong, S.M.; Nathan, P.J. Gender differences in the cortical electrophysiological processing of visual emotional stimuli. NeuroImage 2004, 21, 632–646. [Google Scholar] [CrossRef]
- Kesler, M.L.; Andersen, A.H.; Smith, C.D.; Avison, M.J.; Davis, C.E.; Kryscio, R.J.; Blonder, L.X. Neural substrates of facial emotion processing using fMRI. Cogn. Brain Res. 2001, 11, 213–226. [Google Scholar] [CrossRef]
- Fukushima, H.; Hiraki, K. Perceiving an opponent’s loss: Gender-related differences in the medial-frontal negativity. Soc. Cogn. Affect. Neurosci. 2006, 1, 149–157. [Google Scholar] [CrossRef]
- Geary, D.C. Male, Female: The Evolution of Human Sex Differences; American Psychological Association: Washington, DC, USA, 2010. [Google Scholar]
- Zaidi, Z.F. Gender differences in human brain: A review. Open Anat. J. 2010, 2, 37–55. [Google Scholar] [CrossRef]
- McClure, I. The essential difference: Men, women and the extreme male brain. BMJ 2003, 327, 57. [Google Scholar] [CrossRef][Green Version]
- Wilson, E.O. Sociobiology: The New Synthesis; Harvard University Press: Cambridge, MA, USA, 2000. [Google Scholar]
- Cash, T.F. Cognitive-Behavioral Perspectives on Body Image. In Encyclopedia of Body Image and Human Appearance; Cash, T., Ed.; Elsevier: London, UK, 2012; pp. 334–342. [Google Scholar]
- Eagly, A.H.; Wood, W. Social role theory of sex differences. In The Wiley Blackwell Encyclopedia of Gender and Sexuality Studies; Wiley-Blackwell: Malden, MA, USA, 2016; pp. 1–3. [Google Scholar]
- Rueckert, L.; Naybar, N. Gender differences in empathy: The role of the right hemisphere. Brain Cogn. 2008, 67, 162–167. [Google Scholar] [CrossRef]
- Spreng, R.N.; McKinnon, M.C.; Mar, R.A.; Levine, B. The Toronto Empathy Questionnaire: Scale development and initial validation of a factor-analytic solution to multiple empathy measures. J. Personal. Assess. 2009, 91, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; McFatter, R. Empathy and distress: Two distinct but related emotions in response to infant crying. Infant Behav. Dev. 2012, 35, 887–897. [Google Scholar] [CrossRef]
- Bernhardt, B.C.; Klimecki, O.M.; Leiberg, S.; Singer, T. Structural covariance networks of the dorsal anterior insula predict females’ individual differences in empathic responding. Cereb. Cortex 2014, 24, 2189–2198. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Horta, M.; Mascaro, J.S.; Bijanki, K.; Arnal, L.H.; Adams, M.; Barr, R.G.; Rilling, J.K. Explaining individual variation in paternal brain responses to infant cries. Physiol. Behav. 2018, 193, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Lorberbaum, J.P.; Newman, J.D.; Dubno, J.R.; Horwitz, A.R.; Nahas, Z.; Teneback, C.C.; Bloomer, C.W.; Bohning, D.E.; Vincent, D.; Johnson, M.R.; et al. Feasibility of using fMRI to study mothers responding to infant cries. Depress. Anxiety 1999, 10, 99–104. [Google Scholar] [CrossRef]
- Montoya, J.L.; Landi, N.; Kober, H.; Worhunsky, P.D.; Rutherford, H.J.; Mencl, W.E.; Mayes, L.C.; Potenza, M.N. Regional brain responses in nulliparous women to emotional infant stimuli. PLoS ONE 2012, 7, e36270. [Google Scholar] [CrossRef]
- Farrow, T.F.; Zheng, Y.; Wilkinson, I.D.; Spence, S.A.; Deakin, J.W.; Tarrier, N.; Griffiths, P.D.; Woodruff, P.W. Investigating the functional anatomy of empathy and forgiveness. Neuroreport 2001, 12, 2433–2438. [Google Scholar] [CrossRef]
- Seitz, R.J.; Nickel, J.; Azari, N.P. Functional modularity of the medial prefrontal cortex: Involvement in human empathy. Neuropsychology 2006, 20, 743. [Google Scholar] [CrossRef]
- Masten, C.L.; Morelli, S.A.; Eisenberger, N.I. An fMRI investigation of empathy for ‘social pain’and subsequent prosocial behavior. Neuroimage 2011, 55, 381–388. [Google Scholar] [CrossRef]
- Völlm, B.A.; Taylor, A.N.; Richardson, P.; Corcoran, R.; Stirling, J.; McKie, S.; Deakin, J.F.; Elliott, R. Neuronal correlates of theory of mind and empathy: A functional magnetic resonance imaging study in a nonverbal task. Neuroimage 2006, 29, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Mathur, V.A.; Harada, T.; Lipke, T.; Chiao, J.Y. Neural basis of extraordinary empathy and altruistic motivation. Neuroimage 2010, 51, 1468–1475. [Google Scholar] [CrossRef] [PubMed]
- Ostwald, P. The sounds of infancy. Dev. Med. Child Neurol. 1972, 14, 350–361. [Google Scholar] [CrossRef]
- Choonara, I. Why do babies cry? We still know too little about what will ease babies’ pain. BMJ 1999, 319, 1381. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.D. Aversiveness is in the mind of the beholder. In Infant Crying; Springer: Berlin/Heidelberg, Germany, 1985; pp. 217–239. [Google Scholar]
- Out, D.; Pieper, S.; Bakermans-Kranenburg, M.J.; Zeskind, P.S.; van IJzendoorn, M.H. Intended sensitive and harsh caregiving responses to infant crying: The role of cry pitch and perceived urgency in an adult twin sample. Child Abus. Negl. 2010, 34, 863–873. [Google Scholar] [CrossRef]
- Zeskind, P.S.; Shingler, E.A. Child abusers’ perceptual responses to newborn infant cries varying in pitch. Infant Behav. Dev. 1991, 14, 335–347. [Google Scholar] [CrossRef]
- Batson, C.D.; Shaw, L.L. Evidence for altruism: Toward a pluralism of prosocial motives. Psychol. Inq. 1991, 2, 107–122. [Google Scholar] [CrossRef]
- Schroeder, D.A.; Dovidio, J.F.; Sibicky, M.E.; Matthews, L.L.; Allen, J.L. Empathic concern and helping behavior: Egoism or altruism? J. Exp. Soc. Psychol. 1988, 24, 333–353. [Google Scholar] [CrossRef]
- Holmgren, R.A.; Eisenberg, N.; Fabes, R.A. The relations of children’s situational empathy-related emotions to dispositional prosocial behaviour. Int. J. Behav. Dev. 1998, 22, 169–193. [Google Scholar] [CrossRef]
- Cialdini, R.B.; Brown, S.L.; Lewis, B.P.; Luce, C.; Neuberg, S.L. Reinterpreting the empathy–altruism relationship: When one into one equals oneness. J. Personal. Soc. Psychol. 1997, 73, 481. [Google Scholar] [CrossRef]
- Davis, M.H. A Multidimensional Approach to Individual Differences in Empathy. PJSAS Cat. Sel. Doc. Psychol. 1980, 10, 85. [Google Scholar]
- Smith, A. Cognitive empathy and emotional empathy in human behavior and evolution. Psychol. Rec. 2006, 56, 3–21. [Google Scholar] [CrossRef]
- Lockwood, P.L.; Seara-Cardoso, A.; Viding, E. Emotion regulation moderates the association between empathy and prosocial behavior. PLoS ONE 2014, 9, e96555. [Google Scholar] [CrossRef] [PubMed]
- Kourmousi, N.; Amanaki, E.; Tzavara, C.; Merakou, K.; Barbouni, A.; Koutras, V. The toronto empathy questionnaire: Reliability and validity in a nationwide sample of greek teachers. Soc. Sci. 2017, 6, 62. [Google Scholar] [CrossRef]
- Morelli, S.A.; Lieberman, M.D. The role of automaticity and attention in neural processes underlying empathy for happiness, sadness, and anxiety. Front. Hum. Neurosci. 2013, 7, 160. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Steele, S.C.; Becerra, L.; Borsook, D. Brodmann area 10: Collating, integrating and high level processing of nociception and pain. Prog. Neurobiol. 2018, 161, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Bem, S.L. The measurement of psychological androgyny. J. Consult. Clin. Psychol. 1974, 42, 155. [Google Scholar] [CrossRef]
- Auster, C.J.; Ohm, S.C. Masculinity and femininity in contemporary American society: A reevaluation using the Bem Sex-Role Inventory. Sex Roles 2000, 43, 499–528. [Google Scholar] [CrossRef]
- Green, J.A.; Whitney, P.G.; Potegal, M. Screaming, yelling, whining, and crying: Categorical and intensity differences in vocal expressions of anger and sadness in children’s tantrums. Emotion 2011, 11, 1124. [Google Scholar] [CrossRef]
- Gabrieli, G.; Leck, W.Q.; Bizzego, A.; Esposito, G. Are praat’s default settings optimal for infant cry analysis. In Proceedings of the 2019 CCRMA Linux Audio Conference, LAC, Stanford, LA, USA, 23–26 March 2019; pp. 23–26. [Google Scholar]
- Bornstein, M.H.; Putnick, D.L.; Rigo, P.; Esposito, G.; Swain, J.E.; Suwalsky, J.T.; Su, X.; Du, X.; Zhang, K.; Cote, L.R.; et al. Neurobiology of culturally common maternal responses to infant cry. Proc. Natl. Acad. Sci. USA 2017, 114, E9465–E9473. [Google Scholar] [CrossRef]
- Alabas, O.; Tashani, O.; Tabasam, G.; Johnson, M. Gender role affects experimental pain responses: A systematic review with meta-analysis. Eur. J. Pain 2012, 16, 1211–1223. [Google Scholar] [CrossRef]
- Mogil, J.S.; Bailey, A.L. Sex and gender differences in pain and analgesia. Prog. Brain Res. 2010, 186, 140–157. [Google Scholar]
- Schulte-Rüther, M.; Markowitsch, H.J.; Fink, G.R.; Piefke, M. Mirror neuron and theory of mind mechanisms involved in face-to-face interactions: A functional magnetic resonance imaging approach to empathy. J. Cogn. Neurosci. 2007, 19, 1354–1372. [Google Scholar] [CrossRef]
- Pfeifer, J.H.; Lieberman, M.D.; Dapretto, M. “I know you are but what am I?!”: Neural bases of self-and social knowledge retrieval in children and adults. J. Cogn. Neurosci. 2007, 19, 1323–1337. [Google Scholar] [CrossRef]
- Calderon, L.E.; Carney, L.D.; Kavanagh, K.T. The cry of the child and its relationship to hearing loss in parental guardians and health care providers. J. Evid. Inf. Soc. Work 2016, 13, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, G.; Santhanam, P.; Hu, X. Instantaneous and causal connectivity in resting state brain networks derived from functional MRI data. Neuroimage 2011, 54, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Raichle, M.E. The brain’s default mode network. Annu. Rev. Neurosci. 2015, 38, 433–447. [Google Scholar] [CrossRef]
- Greicius, M.D.; Menon, V. Default-mode activity during a passive sensory task: Uncoupled from deactivation but impacting activation. J. Cogn. Neurosci. 2004, 16, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Berke, D.S.; Reidy, D.E.; Miller, J.D.; Zeichner, A. Take it like a man: Gender-threatened men’s experience of gender role discrepancy, emotion activation, and pain tolerance. Psychol. Men Masculinity 2017, 18, 62. [Google Scholar] [CrossRef]
- Schaller, M.; Cialdini, R.B. The economics of empathic helping: Support for a mood management motive. J. Exp. Soc. Psychol. 1988, 24, 163–181. [Google Scholar] [CrossRef]
- Chew, Y.R.; Cheng, M.H.; Goh, M.C.; Shen, L.; Wong, P.C.; Ganapathy, S. Five-Year Review of Patients Presenting with Non-Accidental Injury to a Children’s Emergency Unit in Singapore. Ann. Acad. Med. Singap. 2018, 47, 413–419. [Google Scholar] [PubMed]
- US Department of Health and Human Services. Child Maltreatment 2012; US Department of Health and Human Services: Washington, DC, USA, 2013.
- Seifritz, E.; Esposito, F.; Neuhoff, J.G.; Lüthi, A.; Mustovic, H.; Dammann, G.; Von Bardeleben, U.; Radue, E.W.; Cirillo, S.; Tedeschi, G.; et al. Differential sex-independent amygdala response to infant crying and laughing in parents versus nonparents. Biol. Psychiatry 2003, 54, 1367–1375. [Google Scholar] [CrossRef]
- Ferreri, L.; Bigand, E.; Perrey, S.; Bugaiska, A. The promise of Near-Infrared Spectroscopy (NIRS) for psychological research: A brief review. LAnnee Psychol. 2014, 114, 537–569. [Google Scholar] [CrossRef]
- Zeskind, P.S.; Marshall, T.R. The relation between variations in pitch and maternal perceptions of infant crying. Child Dev. 1988, 59, 193–196. [Google Scholar] [CrossRef]
| Low-Pitch Cry × Masculinity Scores | High-Pitch Cry × Masculinity SCORES | Low-Pitch Cry Masculinity Scores × High-Pitch Cry Masculinity Scores | ||||
|---|---|---|---|---|---|---|
| N | r | N | r | Z | p | |
| Channel 5 | 38 | −0.34 | 38 | 0.35 | −2.97 | 0.0015 ** |
| Channel 11 | 38 | −0.32 | 38 | 0.41 | −3.16 | 0.0008 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, X.; Ng, L.Y.; Gabrieli, G.; Azhari, A.; Neoh, M.J.Y.; Esposito, G. An fNIRS Investigation of Masculinity, Femininity, and Sex on Nonparents’ Empathic Response to Infant Cries. Brain Sci. 2021, 11, 635. https://doi.org/10.3390/brainsci11050635
Ng X, Ng LY, Gabrieli G, Azhari A, Neoh MJY, Esposito G. An fNIRS Investigation of Masculinity, Femininity, and Sex on Nonparents’ Empathic Response to Infant Cries. Brain Sciences. 2021; 11(5):635. https://doi.org/10.3390/brainsci11050635
Chicago/Turabian StyleNg, Xinyao, Li Ying Ng, Giulio Gabrieli, Atiqah Azhari, Michelle Jin Yee Neoh, and Gianluca Esposito. 2021. "An fNIRS Investigation of Masculinity, Femininity, and Sex on Nonparents’ Empathic Response to Infant Cries" Brain Sciences 11, no. 5: 635. https://doi.org/10.3390/brainsci11050635
APA StyleNg, X., Ng, L. Y., Gabrieli, G., Azhari, A., Neoh, M. J. Y., & Esposito, G. (2021). An fNIRS Investigation of Masculinity, Femininity, and Sex on Nonparents’ Empathic Response to Infant Cries. Brain Sciences, 11(5), 635. https://doi.org/10.3390/brainsci11050635






