Neural Processing of Cognitive Control in an Emotionally Neutral Context in Anxiety Patients
Abstract
1. Introduction
1.1. Cognitive Control Impairments in Anxiety
1.2. Neural Mechanisms of Cognitive Control
1.3. Aims of the Study and Hypotheses
- 1.
- Higher task performance (higher accuracy rate, i.e., less errors as well as faster reaction times) in controls than anxiety patients is expected as anxiety is associated with distinct inhibition impairments;
- 2.
- With respect to ERPs we expect a larger N200 amplitude for incongruent compared to congruent stimuli as well as a larger P300 component for congruent compared to incongruent stimuli in healthy controls, indicating a higher degree of conflict and a larger task difficulty for incongruent stimuli;
- 3.
- Regarding the P300 component, a longer latency for incongruent compared to congruent stimuli in controls is expected reflecting more difficult stimulus evaluation processes;
- 4.
- If a general inhibition and stimulus evaluation deficit, extending beyond the emotional context, is present in anxiety patients, we expect differences between anxiety patients and controls regarding the N200 and P300 component. If, however, the deficits are restricted to emotional stimuli addressing the threatening events, a similar processing as in healthy controls will be expected;
- 5.
- Concerning fNIRS results we expect a higher activation in prefrontal areas for incongruent compared to congruent trials in healthy subjects indicating a stronger need for inhibitory control;
- 6.
- In anxiety patients a hypoactivation of frontal areas is assumed to be reflected in the fNIRS results.
2. Materials and Methods
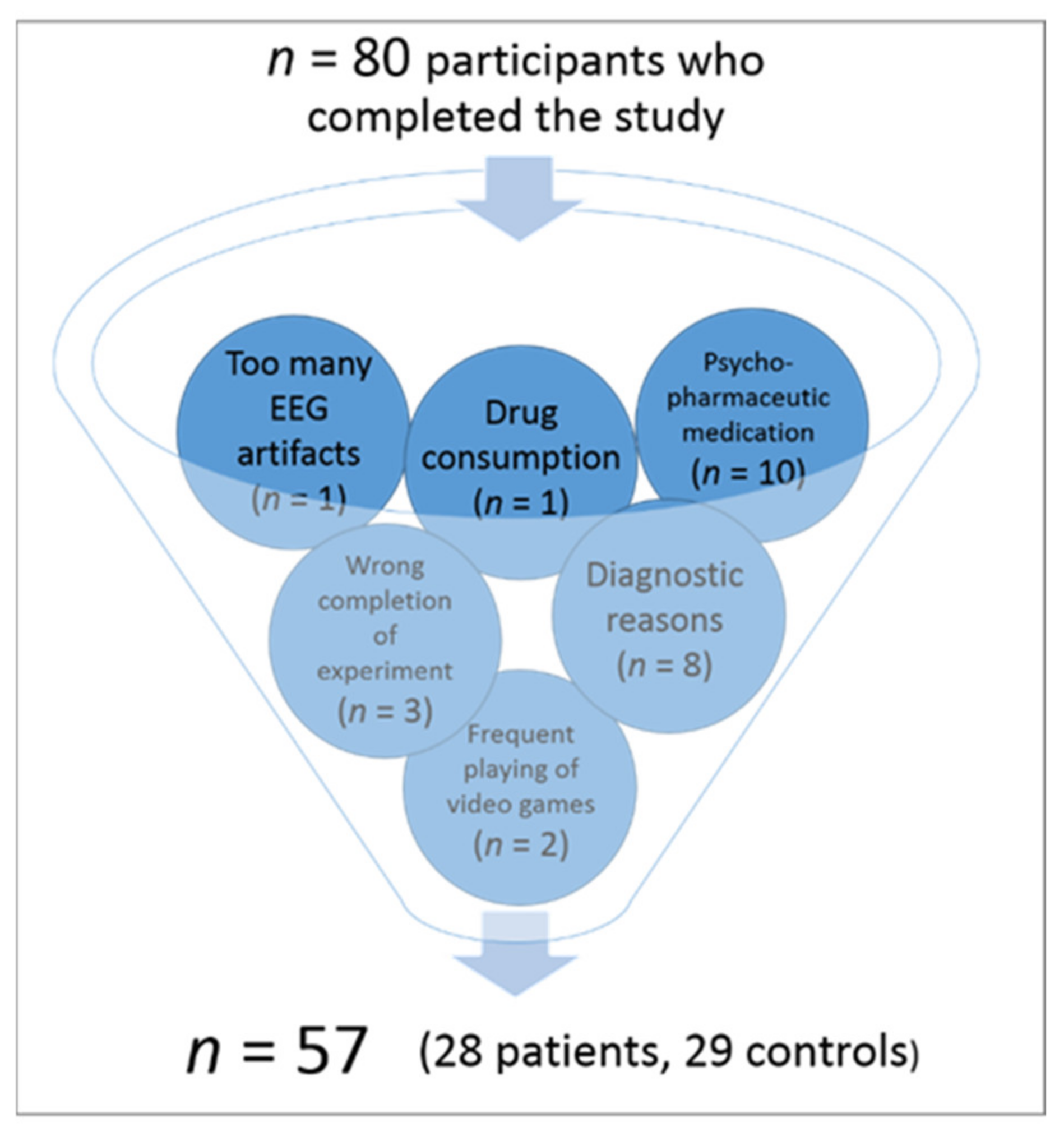
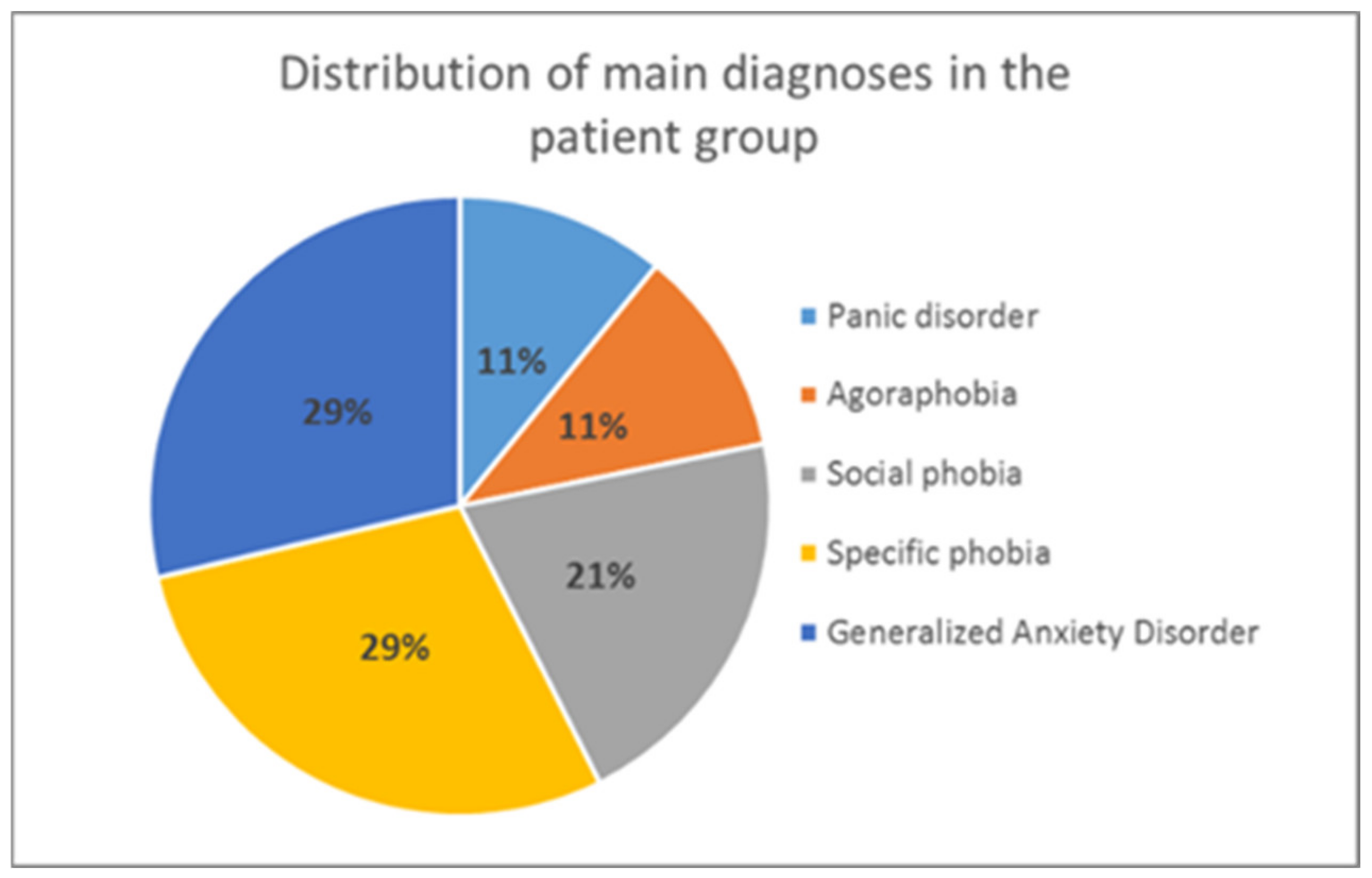
2.1. Participants
2.2. Materials
2.2.1. Flanker Task
2.2.2. Behavioral Measures: Standardized Psychological Tests, Response Times, and Accuracy Rates
2.3. Procedure
2.4. Electroencephalography (EEG) Recordings
2.5. Functional Near-Infrared Spectroscopy (fNIRS) Recordings
2.6. Data Analysis
2.6.1. Behavioral Data Analyses of the Flanker Task
2.6.2. EEG Data Analyses
2.6.3. fNIRS Data Analyses
3. Results
3.1. Results from Behavioral Data of the Flanker Task
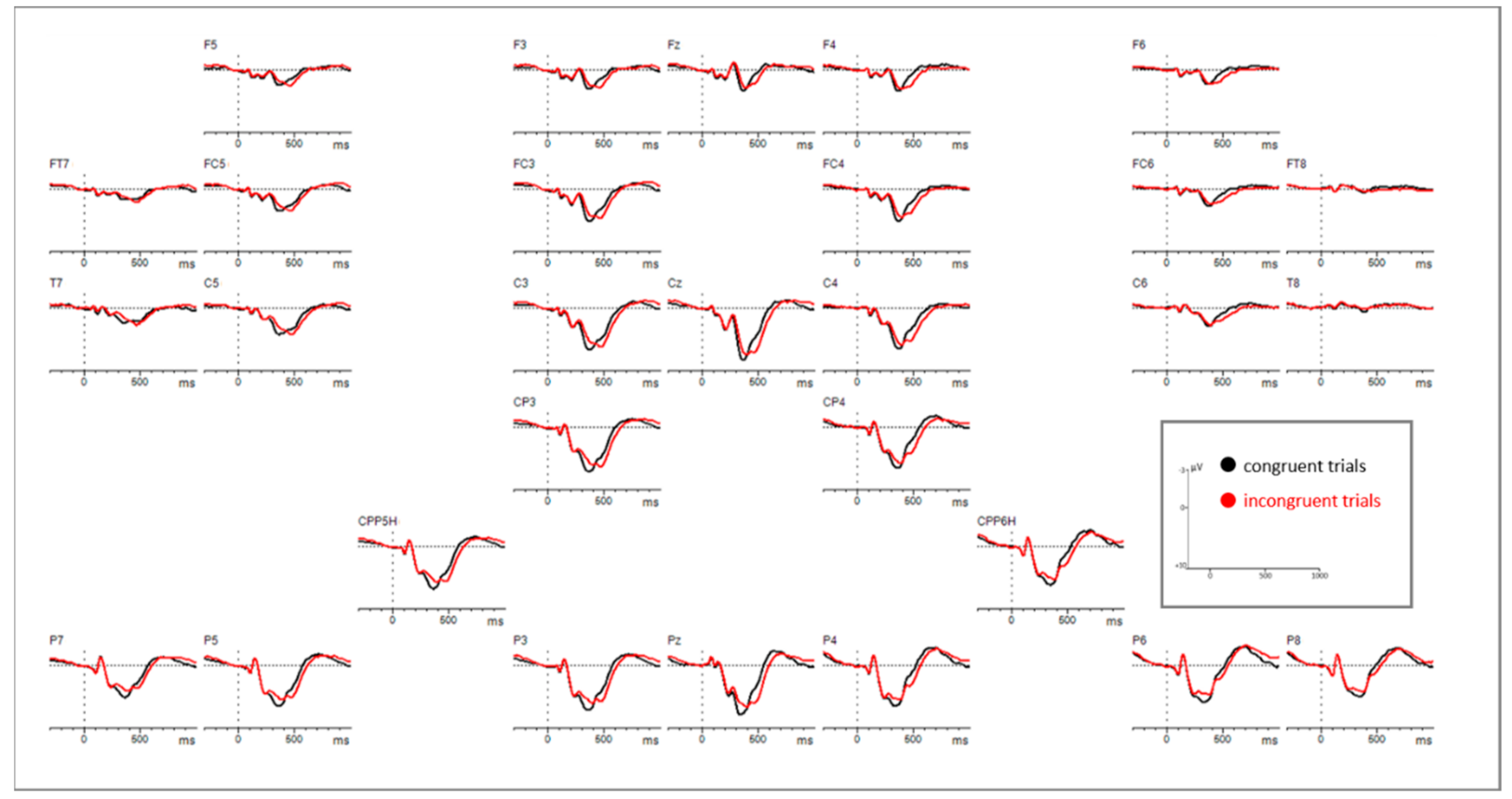
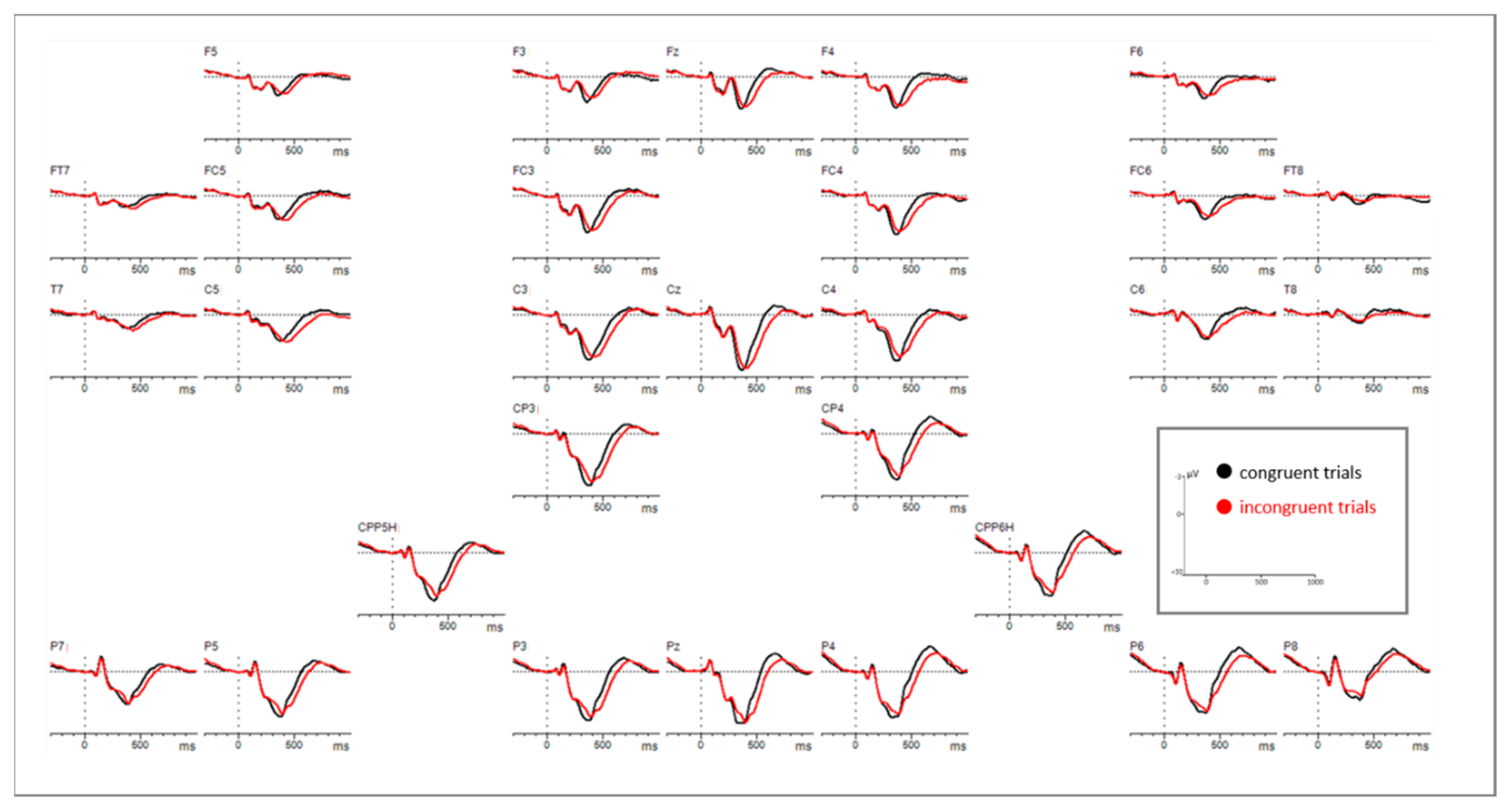
3.2. EEG Results
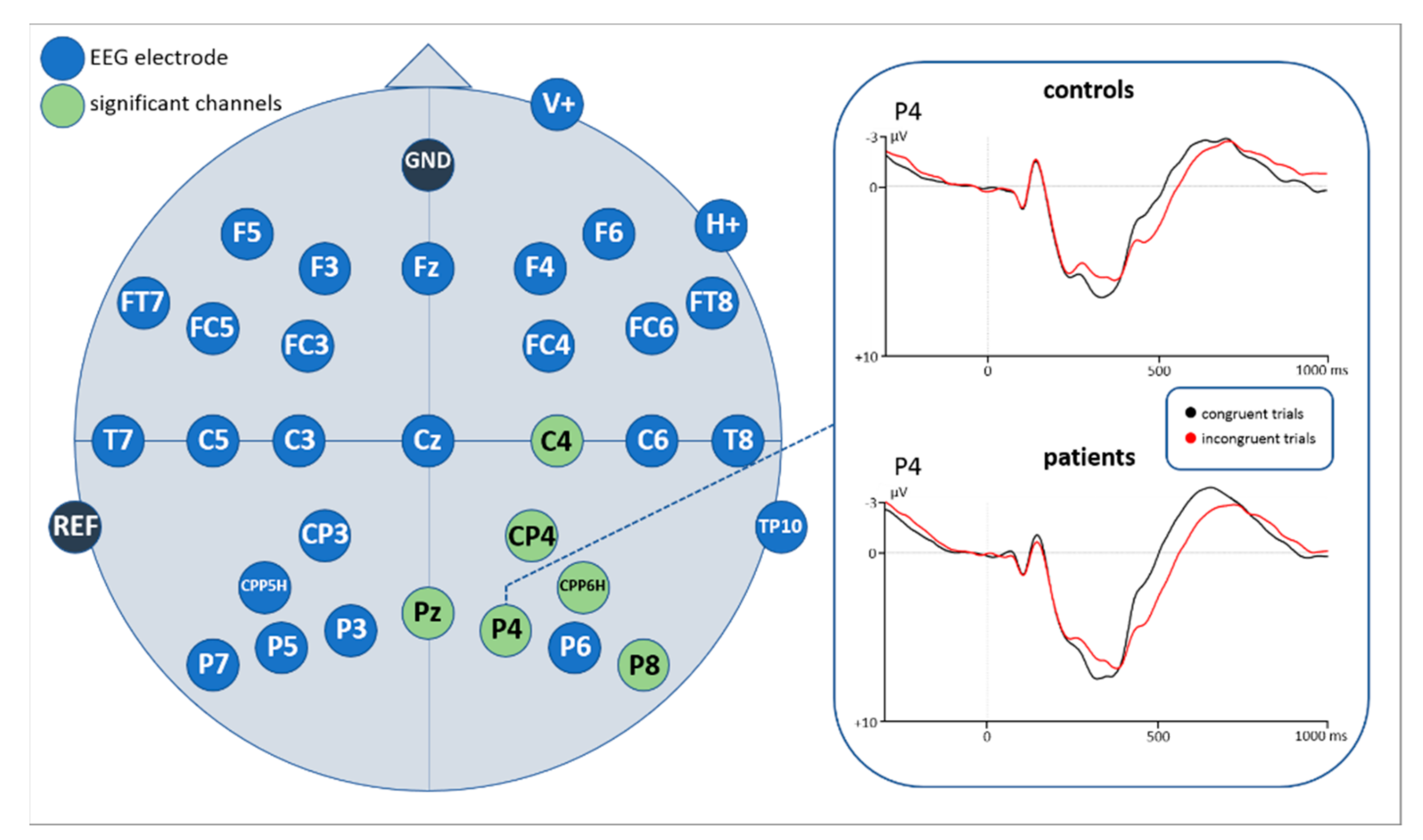
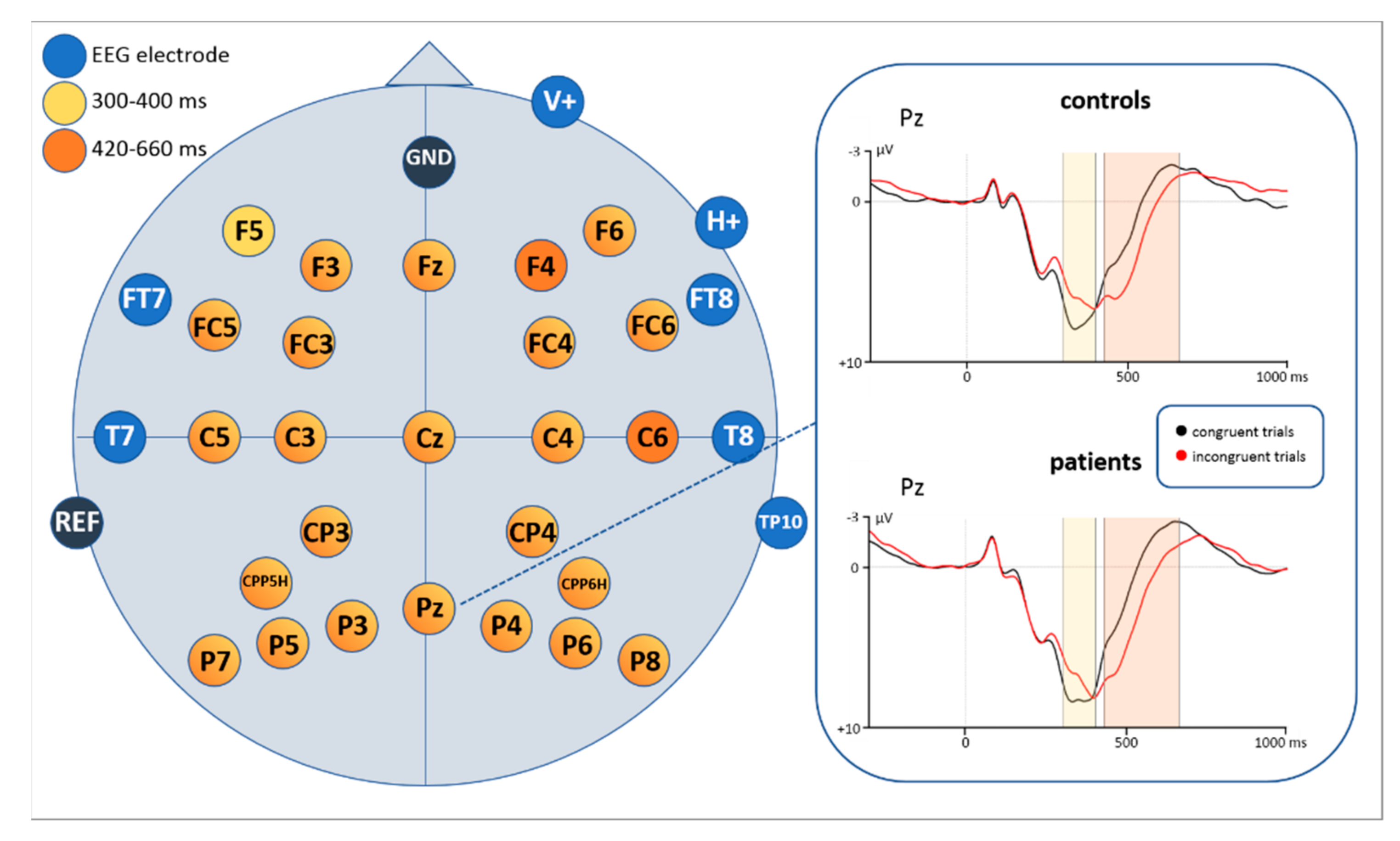
3.2.1. EEG Mean Amplitude Results
Time Window: 300–400 ms
Time Window: 300–400 ms
Time Window: 420–660 ms
3.2.2. EEG Peak-Latency Results
Time Window: 260–300 ms
Time Window: 300–660 ms
3.3. fNIRS Results
4. Discussion
4.1. Behavioral Results
4.2. N200
4.3. P300
4.4. Brain Activations Measured by fNIRS
4.5. Comparison of Anxiety Patients to Healthy Controls
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Electrode | df | t | p |
|---|---|---|---|
| F3 | 55 | 5.720 | <0.0001 |
| F5 | 55 | 2.968 | 0.004 |
| FC3 | 56 | 4.771 | <0.0001 |
| FC5 | 56 | 2.819 | 0.007 |
| C3 | 56 | 5.779 | <0.0001 |
| C5 | 56 | 3.207 | 0.002 |
| CP3 | 56 | 5.551 | <0.0001 |
| CPP5H | 56 | 5.551 | <0.0001 |
| P3 | 56 | 5.778 | <0.0001 |
| P5 | 56 | 5.007 | <0.0001 |
| P7 | 56 | 3.681 | 0.001 |
| F6 | 55 | 2,970 | 0.004 |
| FC4 | 56 | 5.471 | <0.0001 |
| FC6 | 56 | 4.298 | <0.0001 |
| C4 | 56 | 6.425 | <0.0001 |
| CP4 | 56 | 4.278 | <0.0001 |
| CPP6H | 56 | 4.202 | <0.0001 |
| P4 | 56 | 3.670 | 0.001 |
| P6 | 56 | 3.312 | 0.002 |
| P8 | 55 | 4.807 | <0.0001 |
| Fz | 54 | 4.214 | <0.0001 |
| Cz | 56 | 4.487 | <0.0001 |
| Pz | 56 | 5.101 | <0.0001 |
| Electrode | df | t | p |
|---|---|---|---|
| F3 | 55 | −3.313 | 0.002 |
| FC3 | 56 | −4.391 | <0.0001 |
| FC5 | 56 | −3.170 | 0.002 |
| C3 | 56 | −5.446 | <0.0001 |
| C5 | 56 | −3.568 | 0.001 |
| CP3 | 56 | −5.167 | <0.0001 |
| CPP5H | 56 | −5.634 | <0.0001 |
| P3 | 56 | −6.355 | <0.0001 |
| P5 | 56 | −5.676 | <0.0001 |
| P7 | 56 | −3.919 | <0.0001 |
| F4 | 56 | −4.086 | <0.0001 |
| F6 | 55 | −4.747 | <0.0001 |
| FC4 | 56 | −6.454 | <0.0001 |
| FC6 | 56 | −5.186 | <0.0001 |
| C4 | 56 | −7.987 | <0.0001 |
| C6 | 54 | −4.656 | <0.0001 |
| CP4 | 56 | −6.041 | <0.0001 |
| CPP6H | 56 | −5.712 | <0.0001 |
| P4 | 56 | −5.397 | <0.0001 |
| P6 | 56 | −3.739 | <0.0001 |
| P8 | 55 | −4.380 | <0.0001 |
| Fz | 54 | −4.539 | <0.0001 |
| Cz | 56 | −5.957 | <0.0001 |
| Pz | 56 | −7.039 | <0.0001 |
Appendix B
References
- Kessler, R.C.; Angermeyer, M.; Anthony, J.C.; De Graaf, R.; Demyttenaere, K.; Gasquet, I.; de Girolamo, G.; Gluzman, S.; Gureje, O.; Haro, J.M.; et al. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization’s world mental health survey initiative. World Psychiatry 2007, 6, 168–176. [Google Scholar]
- Hu, T.W. Perspectives: An international review of the national cost estimates of mental illness, 1990–2003. J. Ment. Health Policy Econ. 2006, 9, 3–13. [Google Scholar]
- Gustavsson, A.; Svensson, M.; Jacobi, F.; Allgulander, C.; Alonso, J.; Beghi, E.; Dodel, R.; Ekman, M.; Faravelli, C.; Fratiglioni, L.; et al. Cost of disorders of the brain in Europe 2010. Eur. Neuropsychopharmacol. 2011, 21, 718–779. [Google Scholar] [CrossRef] [PubMed]
- Mihalopoulos, C.; Chatterton, M.L. Cost-effectiveness of interventions for anxiety and depressive disorders. In Mental Health Economics: The Costs and Benefits of Psychiatric Care; Razzouk, D., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 283–298. ISBN 978-3-319-55266-8. [Google Scholar]
- Hallion, L.S.; Ruscio, A.M. Should uncontrollable worry be removed from the definition of GAD? A test of incremental validity. J. Abnorm. Psychol. 2013, 122, 369–375. [Google Scholar] [CrossRef]
- Hallion, L.S.; Tolin, D.F.; Assaf, M.; Goethe, J.; Diefenbach, G.J. Cognitive control in generalized anxiety disorder: Relation of inhibition impairments to worry and anxiety severity. Cogn. Ther. Res. 2017, 41, 610–618. [Google Scholar] [CrossRef]
- Kalisch, R. The functional neuroanatomy of reappraisal: Time matters. Neurosci. Biobehav. Rev. 2009, 33, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef]
- Gratton, G.; Cooper, P.; Fabiani, M.; Carter, C.S.; Karayanidis, F. Dynamics of cognitive control: Theoretical bases, paradigms, and a view for the future. Psychophysiology 2018, 55, e13016. [Google Scholar] [CrossRef]
- Lee, J.K.; Orsillo, S.M. Investigating cognitive flexibility as a potential mechanism of mindfulness in generalized anxiety disorder. J. Behav. Ther. Exp. Psychiatry 2014, 45, 208–216. [Google Scholar] [CrossRef]
- Wright, L.; Lipszyc, J.; Dupuis, A.; Thayapararajah, S.W.; Schachar, R. Response inhibition and psychopathology: A meta-analysis of go/no-go task performance. J. Abnorm. Psychol. 2014, 123, 429–439. [Google Scholar] [CrossRef]
- Schmid, P.C.; Kleiman, T.; Amodio, D.M. Neural mechanisms of proactive and reactive cognitive control in social anxiety. Cortex 2015, 70, 137–145. [Google Scholar] [CrossRef]
- Gratton, G.; Coles, M.G.H.; Donchin, E. Optimizing the use of information: Strategic control of activation of responses. J. Exp. Psychol. Gen. 1992, 121, 480. [Google Scholar] [CrossRef] [PubMed]
- Righi, S.; Mecacci, L.; Viggiano, M.P. Anxiety, cognitive self-evaluation and performance: ERP correlates. J. Anxiety Disord. 2009, 23, 1132–1138. [Google Scholar] [CrossRef]
- Kanske, P.; Kotz, S.A. Effortful control, depression, and anxiety correlate with the influence of emotion on executive attentional control. Biol. Psychol. 2012, 91, 88–95. [Google Scholar] [CrossRef][Green Version]
- Cavanagh, J.F.; Meyer, A.; Hajcak, G. Error-specific cognitive control alterations in generalized anxiety disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2017, 2, 413–420. [Google Scholar] [CrossRef]
- Lampert, R. ECG signatures of psychological stress. J. Electrocardiol. 2015, 48, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Boucsein, W. Electrodermal Activity; Springer Science & Business Media: Berlin/Heidelberg Germany, 2012; ISBN 978-1-4614-1126-0. [Google Scholar]
- Chalmers, J.A.; Quintana, D.S.; Abbott, M.J.-A.; Kemp, A.H. Anxiety disorders are associated with reduced heart rate variability: A meta-analysis. Front. Psychiatry 2014, 5. [Google Scholar] [CrossRef]
- Paszkiel, S.; Dobrakowski, P.; Łysiak, A. The impact of different sounds on stress level in the context of eeg, cardiac measures and subjective stress level: A pilot study. Brain Sci. 2020, 10, 728. [Google Scholar] [CrossRef] [PubMed]
- McDermott, T.J.; Wiesman, A.I.; Proskovec, A.L.; Heinrichs-Graham, E.; Wilson, T.W. Spatiotemporal oscillatory dynamics of visual selective attention during a flanker task. NeuroImage 2017, 156, 277–285. [Google Scholar] [CrossRef]
- Niendam, T.A.; Laird, A.R.; Ray, K.L.; Dean, Y.M.; Glahn, D.C.; Carter, C.S. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn. Affect. Behav. Neurosci. 2012, 12, 241–268. [Google Scholar] [CrossRef]
- Mehnert, J.; Akhrif, A.; Telkemeyer, S.; Rossi, S.; Schmitz, C.H.; Steinbrink, J.; Wartenburger, I.; Obrig, H.; Neufang, S. Developmental changes in brain activation and functional connectivity during response inhibition in the early childhood brain. Brain Dev. 2013, 35, 894–904. [Google Scholar] [CrossRef]
- Nishimura, Y.; Tanii, H.; Fukuda, M.; Kajiki, N.; Inoue, K.; Kaiya, H.; Nishida, A.; Okada, M.; Okazaki, Y. Frontal dysfunction during a cognitive task in drug-naive patients with panic disorder as investigated by multi-channel near-infrared spectroscopy imaging. Neurosci. Res. 2007, 59, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Bishop, S.J. Trait anxiety and impoverished prefrontal control of attention. Nat. Neurosci. 2009, 12, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Ohta, H.; Yamagata, B.; Tomioka, H.; Takahashi, T.; Yano, M.; Nakagome, K.; Mimura, M. Hypofrontality in panic disorder and major depressive disorder assessed by multi-channel near-infrared spectroscopy. Depress. Anxiety 2008, 25, 1053–1059. [Google Scholar] [CrossRef]
- Eriksen, B.A.; Eriksen, C.W. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept. Psychophys. 1974, 16, 143–149. [Google Scholar] [CrossRef]
- Fan, J.; Flombaum, J.I.; McCandliss, B.D.; Thomas, K.M.; Posner, M.I. Cognitive and brain consequences of conflict. NeuroImage 2003, 18, 42–57. [Google Scholar] [CrossRef]
- Kanske, P.; Kotz, S.A. Emotion triggers executive attention: Anterior cingulate cortex and amygdala responses to emotional words in a conflict task. Hum. Brain Mapp. 2011, 32, 198–208. [Google Scholar] [CrossRef]
- Yuan, Z.; Lin, X. The hemodynamic changes in the human prefrontal cortex during the flanker and simon tasks: A FNIRS study. In Proceedings of the Clinical and Translational Neurophotonics; Neural Imaging and Sensing; and Optogenetics and Optical Manipulation, International Society for Optics and Photonics, San Francisco, CA, USA, 9 March 2016; Volume 9690, p. 96901V. [Google Scholar]
- Rihs, T.A.; Michel, C.M.; Thut, G. Mechanisms of selective inhibition in visual spatial attention are indexed by α-band EEG synchronization. Eur. J. Neurosci. 2007, 25, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.T.; Smith, S.L.; Kraus, B.T.; Allen, A.V.; Moses, M.A.; Simon-Dack, S.L. Alpha band frequency differences between low-trait and high-trait anxious individuals. NeuroReport 2018, 29, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Heil, M.; Osman, A.; Wiegelmann, J.; Rolke, B.; Hennighausen, E. N200 in the Eriksen-Task: Inhibitory executive process? J. Psychophysiol. 2000, 14, 218–225. [Google Scholar] [CrossRef]
- Folstein, J.R.; Petten, C.V. Influence of cognitive control and mismatch on the N2 component of the ERP: A review. Psychophysiology 2008, 45, 152–170. [Google Scholar] [CrossRef]
- Groom, M.J.; Cragg, L. Differential modulation of the N2 and P3 event-related potentials by response conflict and inhibition. Brain Cogn. 2015, 97, 1–9. [Google Scholar] [CrossRef]
- Pan, F.; Ou, Y.; Zhang, L.; Zhang, X. Cognitive conflict could facilitate negative stimulus processing: Evidence from trait anxiety in the flanker paradigm. NeuroReport 2019, 30, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Cao, B.; Li, Z.; Li, F. Neural dynamics of cognitive control in various types of incongruence. Front. Hum. Neurosci. 2020, 14. [Google Scholar] [CrossRef]
- Nieuwenhuis, S.; Yeung, N.; van den Wildenberg, W.; Ridderinkhof, K.R. Electrophysiological correlates of anterior cingulate function in a go/no-go task: Effects of response conflict and trial type frequency. Cogn. Affect. Behav. Neurosci. 2003, 3, 17–26. [Google Scholar] [CrossRef]
- Vanveen, V.; Carter, C. The anterior cingulate as a conflict monitor: FMRI and ERP studies. Physiol. Behav. 2002, 77, 477–482. [Google Scholar] [CrossRef]
- McKay, C.C.; van den Berg, B.; Woldorff, M.G. Neural cascade of conflict processing: Not just time-on-task. Neuropsychologia 2017, 96, 184–191. [Google Scholar] [CrossRef]
- Bartholow, B.D.; Pearson, M.A.; Dickter, C.L.; Sher, K.J.; Fabiani, M.; Gratton, G. Strategic control and medial frontal negativity: Beyond errors and response conflict. Psychophysiology 2005, 42, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Kopp, B.; Rist, F.; Mattler, U. N200 in the flanker task as a neurobehavioral tool for investigating executive control. Psychophysiology 1996, 33, 282–294. [Google Scholar] [CrossRef]
- Clayson, P.E.; Larson, M.J. Conflict adaptation and sequential trial effects: Support for the conflict monitoring theory. Neuropsychologia 2011, 49, 1953–1961. [Google Scholar] [CrossRef] [PubMed]
- Botvinick, M.M.; Braver, T.S.; Barch, D.M.; Carter, C.S.; Cohen, J.D. Conflict monitoring and cognitive control. Psychol. Rev. 2001, 108, 624–652. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, Y.; Zhou, R. Examination Stress Results in Attentional Bias and Altered Neural Reactivity in Test-Anxious Individuals. Available online: https://www.hindawi.com/journals/np/2018/3281040 (accessed on 11 March 2021).
- Courchesne, E.; Hillyard, S.A.; Galambos, R. Stimulus novelty, task relevance and the visual evoked potential in man. Electroencephalogr. Clin. Neurophysiol. 1975, 39, 131–143. [Google Scholar] [CrossRef]
- Debener, S.; Makeig, S.; Delorme, A.; Engel, A.K. What is novel in the novelty oddball paradigm? Functional significance of the novelty P3 event-related potential as revealed by independent component analysis. Cogn. Brain Res. 2005, 22, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Donchin, E. Surprise!… Surprise? Psychophysiology 1981, 18, 493–513. [Google Scholar] [CrossRef]
- Friedman, D.; Cycowicz, Y.M.; Gaeta, H. The novelty P3: An event-related brain potential (ERP) sign of the brain’s evaluation of novelty. Neurosci. Biobehav. Rev. 2001, 25, 355–373. [Google Scholar] [CrossRef]
- Polich, J.; Comerchero, M.D. P3a from visual stimuli: Typicality, task, and topography. Brain Topogr. 2003, 15, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, A.; Spencer, K.M.; Donchin, E. The influence of stimulus deviance and novelty on the P300 and novelty P3. Psychophysiology 2002, 39, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Näätänen, R.; Gaillard, A.W.K. 5 The orienting reflex and the N2 deflection of the event-related potential (ERP). In Advances in Psychology; Gaillard, A.W.K., Ritter, W., Eds.; Tutorials in Event Related Potential Research: Endogenous Components; North-Holland: Amsterdam, The Netherlands, 1983; Volume 10, pp. 119–141. [Google Scholar]
- Spencer, K.M.; Dien, J.; Donchin, E. A componential analysis of the ERP Elicited by novel events using a dense electrode array. Psychophysiology 1999, 36, 409–414. [Google Scholar] [CrossRef]
- Spencer, K.M.; Dien, J.; Donchin, E. Spatiotemporal analysis of the late ERP responses to deviant stimuli. Psychophysiology 2001, 38, 343–358. [Google Scholar] [CrossRef]
- Enriquez-Geppert, S.; Konrad, C.; Pantev, C.; Huster, R.J. Conflict and inhibition differentially affect the N200/P300 complex in a combined go/nogo and stop-signal task. NeuroImage 2010, 51, 877–887. [Google Scholar] [CrossRef]
- Polich, J. Clinical application of the P300 event-related brain potential. Phys. Med. Rehabil. Clin. N. Am. 2004, 15, 133–161. [Google Scholar] [CrossRef]
- Kirino, E.; Belger, A.; Goldman-Rakic, P.; McCarthy, G. Prefrontal activation evoked by infrequent target and novel stimuli in a visual target detection task: An event-related functional magnetic resonance imaging study. J. Neurosci. 2000, 20, 6612–6618. [Google Scholar] [CrossRef]
- Neuhaus, A.H.; Koehler, S.; Opgen-Rhein, C.; Urbanek, C.; Hahn, E.; Dettling, M. Selective anterior cingulate cortex deficit during conflict solution in schizophrenia: An event-related potential study. J. Psychiatr. Res. 2007, 41, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, A.H.; Trempler, N.R.; Hahn, E.; Luborzewski, A.; Karl, C.; Hahn, C.; Opgen-Rhein, C.; Urbanek, C.; Schaub, R.; Dettling, M. Evidence of specificity of a visual P3 amplitude modulation deficit in schizophrenia. Schizophr. Res. 2010, 124, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Hagen, G.F.; Gatherwright, J.R.; Lopez, B.A.; Polich, J. P3a from visual stimuli: Task difficulty effects. Int. J. Psychophysiol. 2006, 59, 8–14. [Google Scholar] [CrossRef]
- Polich, J. Task difficulty, probability, and inter-stimulus interval as determinants of P300 from auditory stimuli. Electroencephalogr. Clin. Neurophysiol. Evoked Potentials Sect. 1987, 68, 311–320. [Google Scholar] [CrossRef]
- Donchin, E.; Coles, M.G.H. Is the P300 component a manifestation of context updating? Behav. Brain Sci. 1988, 11, 357. [Google Scholar] [CrossRef]
- Smid, H.G.O.M.; Mulder, G.; Mulder, L.J.M. selective response activation can begin before stimulus recognition is complete: A psychophysiological and error analysis of continuous flow. Acta Psychol. 1990, 74, 169–210. [Google Scholar] [CrossRef]
- Ridderinkhof, K.R.; van der Molen, M.W. When global information and local information collide: A brain potential analysis of the locus of interference effects. Biol. Psychol. 1995, 41, 29–53. [Google Scholar] [CrossRef]
- Van ’t Ent, D. Perceptual and motor contributions to performance and ERP components after incorrect motor activation in a flanker reaction task. Clin. Neurophysiol. 2002, 113, 270–283. [Google Scholar] [CrossRef]
- Umebayashi, K.; Okita, T. An ERP investigation of task switching using a flanker paradigm. Brain Res. 2010, 1346, 165–173. [Google Scholar] [CrossRef]
- Mückschel, M.; Chmielewski, W.; Ziemssen, T.; Beste, C. The norepinephrine system shows information-content specific properties during cognitive control—Evidence from eeg and pupillary responses. NeuroImage 2017, 149, 44–52. [Google Scholar] [CrossRef]
- Valakos, D.; d’Avossa, G.; Mylonas, D.; Butler, J.; Klein, C.; Smyrnis, N. P300 response modulation reflects breaches of non-probabilistic expectations. Sci. Rep. 2020, 10, 10254. [Google Scholar] [CrossRef]
- Quaglia, J.T.; Zeidan, F.; Grossenbacher, P.G.; Freeman, S.P.; Braun, S.E.; Martelli, A.; Goodman, R.J.; Brown, K.W. Brief mindfulness training enhances cognitive control in socioemotional contexts: Behavioral and neural evidence. PLoS ONE 2019, 14, e0219862. [Google Scholar] [CrossRef] [PubMed]
- Sass, S.M.; Heller, W.; Stewart, J.L.; Silton, R.L.; Edgar, J.C.; Fisher, J.E.; Miller, G.A. Time course of attentional bias in anxiety: Emotion and gender specificity. Psychophysiology 2010, 47, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Stevens, E.S.; Weinberg, A.; Nelson, B.D.; Meissel, E.E.E.; Shankman, S.A. The effect of panic disorder versus anxiety sensitivity on event-related potentials during anticipation of threat. J. Anxiety Disord 2018, 54, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sachs, G.; Anderer, P.; Margreiter, N.; Semlitsch, H.; Saletu, B.; Katschnig, H. P300 event-related potentials and cognitive function in social phobia. Psychiatry Res. Neuroimaging 2004, 131, 249–261. [Google Scholar] [CrossRef]
- Klawohn, J.; Santopetro, N.J.; Meyer, A.; Hajcak, G. Reduced P300 in depression: Evidence from a Flanker Task and impact on ERN, CRN, and Pe. Psychophysiology 2020, 57, e13520. [Google Scholar] [CrossRef]
- Yu, Y.; Jiang, C.; Xu, H.; Yang, Q.; Li, J.; Xu, Y.; Xiang, W.; Peng, L.; Liu, B.; Lv, F.; et al. impaired cognitive control of emotional conflict in trait anxiety: A preliminary study based on clinical and non-clinical individuals. Front. Psychiatry 2018, 9. [Google Scholar] [CrossRef]
- Bach, D.R.; Dayan, P. Algorithms for survival: A comparative perspective on emotions. Nat. Rev. Neurosci. 2017, 18, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Wadsworth, E.J.K.; Moss, S.C.; Simpson, S.A.; Smith, A.P. SSRIs and cognitive performance in a working sample. Hum. Psychopharmacol. Clin. Exp. 2005, 20, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Skandali, N.; Rowe, J.B.; Voon, V.; Deakin, J.B.; Cardinal, R.N.; Cormack, F.; Passamonti, L.; Bevan-Jones, W.R.; Regenthal, R.; Chamberlain, S.R.; et al. Dissociable effects of acute SSRI (Escitalopram) on executive, learning and emotional functions in healthy humans. Neuropsychopharmacology 2018, 43, 2645–2651. [Google Scholar] [CrossRef] [PubMed]
- McLean, C.P.; Asnaani, A.; Litz, B.T.; Hofmann, S.G. Gender differences in anxiety disorders: Prevalence, course of illness, comorbidity and burden of illness. J. Psychiatr. Res. 2011, 45, 1027–1035. [Google Scholar] [CrossRef]
- World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines, 6th ed.; World Health Organization: Geneva, Switzerland, 1992; ISBN 978-7-117-01957-6. [Google Scholar]
- Margraf, J. Mini-DIPS: Diagnostisches Kurz-Interview Bei Psychischen Störungen; Springer: Berlin/Heidelberg Germany, 2013. [Google Scholar]
- Beck, A.T.; Steer, R.A.; Brown, G.K. Beck depression inventory-II. In Beck Depression Inventory—II; Harcourt Assessment Inc.: San Antonio, TX, USA, 1996; Volume 78. [Google Scholar]
- Hautzinger, M.; Keller, F.; Kühner, C. Beck Depressions-Inventar (BDI-II); Harcourt Test Services: Frankfurt, Germany, 2006. [Google Scholar]
- Su, R.; Tay, L.; Diener, E. The development and validation of the comprehensive inventory of thriving (CIT) and the brief inventory of thriving (BIT). Appl. Psychol. Health Well Being 2014, 6, 251–279. [Google Scholar] [CrossRef] [PubMed]
- Åkerstedt, T.; Gillberg, M. Subjective and objective sleepiness in the active individual. Int. J. Neurosci. 1990, 52, 29–37. [Google Scholar] [CrossRef]
- Clayson, P.E.; Larson, M.J. Psychometric properties of conflict monitoring and conflict adaptation indices: Response time and conflict N2 event-related potentials: Conflict adaptation psychometrics. Psychophysiology 2013, 50, 1209–1219. [Google Scholar] [CrossRef]
- Obrig, H.; Villringer, A. Beyond the visible—Imaging the human brain with light. J. Cereb. Blood Flow Metab. 2003, 23, 1–18. [Google Scholar] [CrossRef]
- Fehm, L.; Margraf, J. Thought suppression: Specificity in agoraphobia versus broad impairment in social phobia? Behav. Res. Ther. 2002, 40, 57–66. [Google Scholar] [CrossRef]
- Leutgeb, V.; Schäfer, A.; Schienle, A. An event-related potential study on exposure therapy for patients suffering from spider phobia. Biol. Psychol. 2009, 82, 293–300. [Google Scholar] [CrossRef]
- Berger, T.; Hämmerli, K.; Gubser, N.; Andersson, G.; Caspar, F. Internet-based treatment of depression: A randomized controlled trial comparing guided with unguided self-help. Cogn. Behav. Ther. 2011, 40, 251–266. [Google Scholar] [CrossRef]
- Garb, H.N. Clinical judgment, clinical training, and professional experience. Psychol. Bull. 1989, 105, 387–396. [Google Scholar] [CrossRef]
- Japer, H.H. The 10–20 electrode system of the international federation in electroencephalography and clinical neurophysiology. Electroencephalogr. Clin. Neurophysiol. 1958, 10, 371–375. [Google Scholar]
- Sharbrough, F.C.G.E. American electroencephalographic society guidelines for standard electrode position nomenclature. Clin. Neurophysiol. 1991, 8, 200–202. [Google Scholar]
- Uludağ, K.; Dubowitz, D.J.; Yoder, E.J.; Restom, K.; Liu, T.T.; Buxton, R.B. Coupling of cerebral blood flow and oxygen consumption during physiological activation and deactivation measured with FMRI. NeuroImage 2004, 23, 148–155. [Google Scholar] [CrossRef]
- Logothetis, N.K.; Wandell, B.A. Interpreting the BOLD signal. Annu. Rev. Physiol. 2004, 66, 735–769. [Google Scholar] [CrossRef]
- Fox, P.T.; Raichle, M.E. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc. Natl. Acad. Sci. USA 1986, 83, 1140–1144. [Google Scholar] [CrossRef] [PubMed]
- Steinbrink, J.; Villringer, A.; Kempf, F.; Haux, D.; Boden, S.; Obrig, H. Illuminating the BOLD signal: Combined FMRI–FNIRS studies. Magn. Reson. Imaging 2006, 24, 495–505. [Google Scholar] [CrossRef]
- Cope, M.; Delpy, D.T.; Wray, S.; Wyatt, J.S.; Reynolds, E.O.R. A CCD Spectrophotometer to quantitate the concentration of chromophores in living tissue utilising the absorption peak of water at 975 nm. In Oxygen Transport to Tissue XI; Rakusan, K., Biro, G.P., Goldstick, T.K., Turek, Z., Eds.; Advances in Experimental Medicine and Biology; Springer US: Boston, MA, USA, 1989; pp. 33–40. ISBN 978-1-4684-5643-1. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Gratton, G.; Coles, M.G.H.; Donchin, E. A new method for off-line removal of artifact. Electroencephalogr. Clin. Neurophysiol. 1983, 55, 468–484. [Google Scholar] [CrossRef]
- Greenhouse, S.W.; Geisser, S. On methods in the analysis of profile data. Psychometrika 1959, 24, 95–112. [Google Scholar] [CrossRef]
- Huettel, S.A.; Song, A.W.; McCarthy, G. Functional Magnetic Resonance Imaging, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2004; Volume 1. [Google Scholar]
- Boynton, G.M.; Engel, S.A.; Glover, G.H.; Heeger, D.J. Linear systems analysis of functional magnetic resonance imaging in human V1. J. Neurosci. 1996, 16, 4207–4221. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Fox, S.; Blasi, A.; Elwell, C.E. Illuminating the developing brain: The past, present and future of functional near infrared spectroscopy. Neurosci. Biobehav. Rev. 2010, 34, 269–284. [Google Scholar] [CrossRef]
- Pires, L.; Leitão, J.; Guerrini, C.; Simões, M.R. Event-related brain potentials in the study of inhibition: Cognitive control, source localization and age-related modulations. Neuropsychol. Rev. 2014, 24, 461–490. [Google Scholar] [CrossRef] [PubMed]
- Clark, V.P.; Hillyard, S.A. Spatial Selective attention affects early extrastriate but not striate components of the visual evoked potential. J. Cogn. Neurosci. 1996, 8, 387–402. [Google Scholar] [CrossRef]
- Luck, S.J. Multiple mechanisms of visual-spatial attention: Recent evidence from human electrophysiology. Behav. Brain Res. 1995, 71, 113–123. [Google Scholar] [CrossRef]
- Mangun, G.R.; Hillyard, S.A. Modulations of sensory-evoked brain potentials indicate changes in perceptual processing during visual-spatial priming. J. Exp. Psychol. Hum. Percept. Perform. 1991, 17, 1057. [Google Scholar] [CrossRef]
- Anllo-vento, L. Shifting attention in visual space: The effects of peripheral cueing on brain cortical potentials. Int. J. Neurosci. 1995, 80, 353–370. [Google Scholar] [CrossRef]
- Debruille, J.B.; Touzel, M.; Segal, J.; Snidal, C.; Renoult, L. A central component of the N1 event-related brain potential could index the early and automatic inhibition of the actions systematically activated by objects. Front. Behav. Neurosci. 2019, 13. [Google Scholar] [CrossRef]
- Tillman, C.M.; Wiens, S. Behavioral and ERP indices of response conflict in stroop and flanker tasks. Psychophysiology 2011, 48, 1405–1411. [Google Scholar] [CrossRef]
- Grützmann, R.; Riesel, A.; Klawohn, J.; Kathmann, N.; Endrass, T. Complementary modulation of N2 and CRN by conflict frequency. Psychophysiology 2014, 51, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.H.; Azzam, P.N. Characterization of N200 and P300: Selected studies of the event-related potential. Int. J. Med. Sci. 2005, 8. [Google Scholar] [CrossRef]
- Eimer, M. The N2pc component as an indicator of attentional selectivity. Electroencephalogr. Clin. Neurophysiol. 1996, 99, 225–234. [Google Scholar] [CrossRef]
- Luck, S.J.; Hillyard, S.A. Electrophysiological correlates of feature analysis during visual search. Psychophysiology 1994, 31, 291–308. [Google Scholar] [CrossRef]
- Luck, S.J.; Hillyard, S.A. Spatial filtering during visual search: Evidence from human electrophysiology. J. Exp. Psychol. Hum. Percept. Perform. 1994, 20, 1000–1014. [Google Scholar] [CrossRef]
- Zhou, S.; Xiong, S.; Cheng, W.; Wang, Y. Flanker paradigm contains conflict and distraction factors with distinct neural mechanisms: An erp analysis in a 2-1 mapping task. Cogn. Neurodyn. 2019, 13, 341–356. [Google Scholar] [CrossRef] [PubMed]
- Shulman, G.L.; McAvoy, M.P.; Cowan, M.C.; Astafiev, S.V.; Tansy, A.P.; d’Avossa, G.; Corbetta, M. Quantitative analysis of attention and detection signals during visual search. J. Neurophysiol. 2003, 90, 3384–3397. [Google Scholar] [CrossRef]
- Shulman, G.L.; Astafiev, S.V.; McAvoy, M.P.; d’Avossa, G.; Corbetta, M. Right TPJ deactivation during visual search: Functional significance and support for a filter hypothesis. Cereb. Cortex 2007, 17, 2625–2633. [Google Scholar] [CrossRef]
- Todd, J.J.; Fougnie, D.; Marois, R. Visual short-term memory load suppresses temporo-parietal junction activity and induces inattentional blindness. Psychol. Sci. 2005, 16, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhong, F. Interpreting experience enhances early attentional processing, conflict monitoring and interference suppression along the time course of processing. Neuropsychologia 2017, 95, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Polich, J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef] [PubMed]
- Kałamała, P.; Szewczyk, J.; Senderecka, M.; Wodniecka, Z. Flanker Task with equiprobable congruent and incongruent conditions does not elicit the conflict N2. Psychophysiology 2018, 55, e12980. [Google Scholar] [CrossRef]
- Johnson, R.; Donchin, E. P300 and stimulus categorization: Two plus one is not so different from one plus one. Psychophysiology 1980, 17, 167–178. [Google Scholar] [CrossRef]
- Doucet, C.; Stelmack, R.M. The effect of response execution on P3 latency, reaction time, and movement time. Psychophysiology 1999, 36, 351–363. [Google Scholar] [CrossRef]
- Galvao-Carmona, A.; González-Rosa, J.J.; Hidalgo-Muñoz, A.R.; Páramo, D.; Benítez, M.L.; Izquierdo, G.; Vázquez-Marrufo, M. Disentangling the attention network test: Behavioral, event related potentials, and neural source analyses. Front. Hum. Neurosci. 2014, 8. [Google Scholar] [CrossRef]
- Luijten, M.; Machielsen, M.W.J.; Veltman, D.J.; Hester, R.; de Haan, L.; Franken, I.H.A. Systematic review of ERP and FMRI studies investigating inhibitory control and error processing in people with substance dependence and behavioural addictions. J. Psychiatry Neurosci. 2014, 39, 149–169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; De Beuckelaer, A.; Chen, L.; Zhou, R. ERP evidence for inhibitory control deficits in test-anxious individuals. Front. Psychiatry 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Moser, J.S.; Hajcak, G.; Simons, R.F. The effects of fear on performance monitoring and attentional allocation. Psychophysiology 2005, 42, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Metzger, L.J.; Orr, S.P.; Lasko, N.B.; Pitman, R.K. Auditory event-related potentials to tone stimuli in combat-related posttraumatic stress disorder. Biol. Psychiatry 1997, 42, 1006–1015. [Google Scholar] [CrossRef]
- Tumati, S.; Paulus, M.P.; Northoff, G. Out-of-step: Brain-heart desynchronization in anxiety disorders. Mol. Psychiatry 2021. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Schwerdtfeger, A.; Fink, D.; Brunner, C.; Aigner, C.S.; Brito, J.; Andrade, A. MRI-related anxiety in healthy individuals, intrinsic BOLD oscillations at 0.1 Hz in precentral gyrus and insula, and heart rate variability in low frequency bands. PLoS ONE 2018, 13, e0206675. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Schwerdtfeger, A.; Seither-Preisler, A.; Brunner, C.; Aigner, C.S.; Calisto, J.; Gens, J.; Andrade, A. Synchronization of intrinsic 0.1-Hz blood-oxygen-level-dependent oscillations in amygdala and prefrontal cortex in subjects with increased state anxiety. Eur. J. Neurosci. 2018, 47, 417–426. [Google Scholar] [CrossRef] [PubMed]
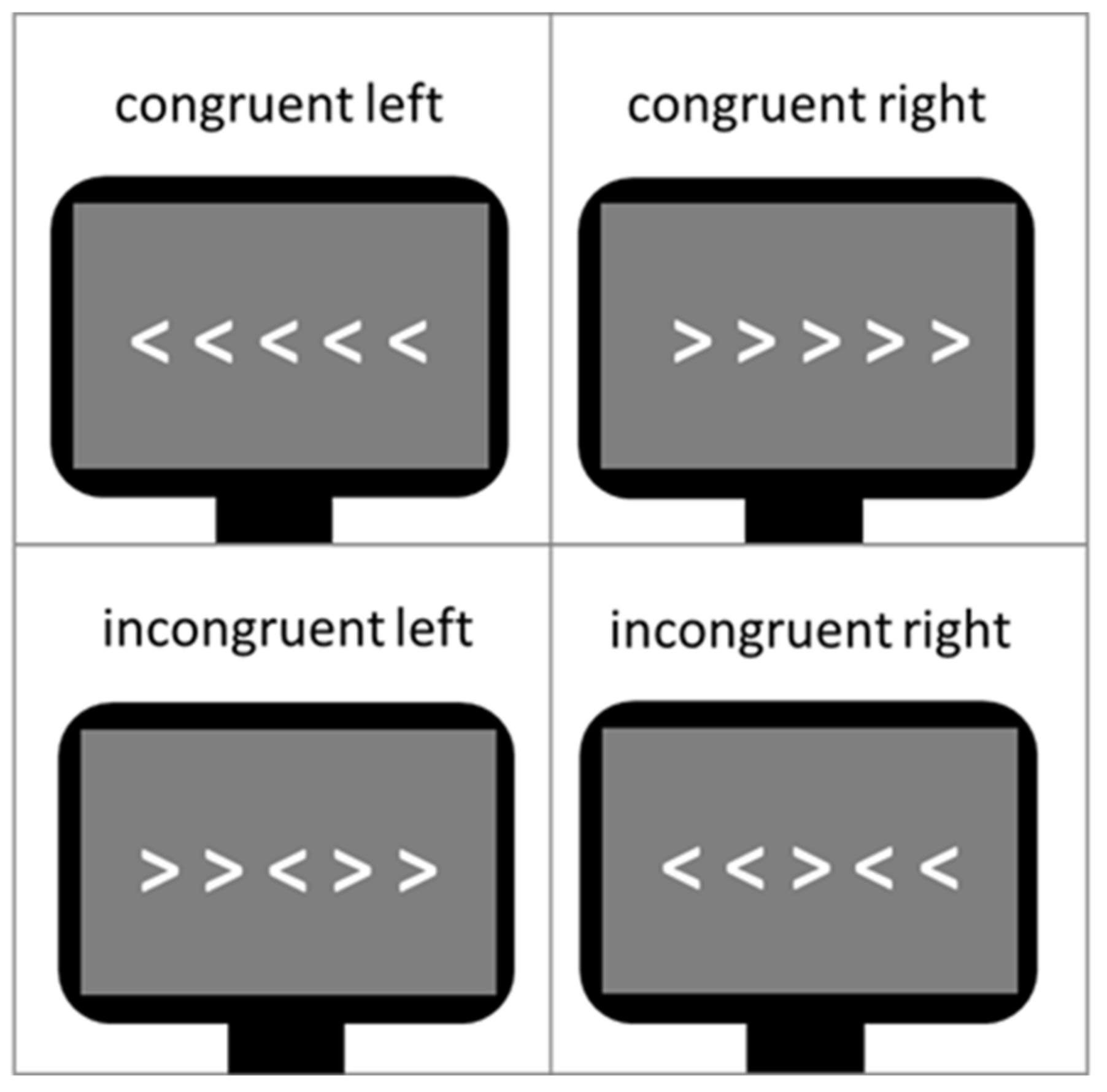
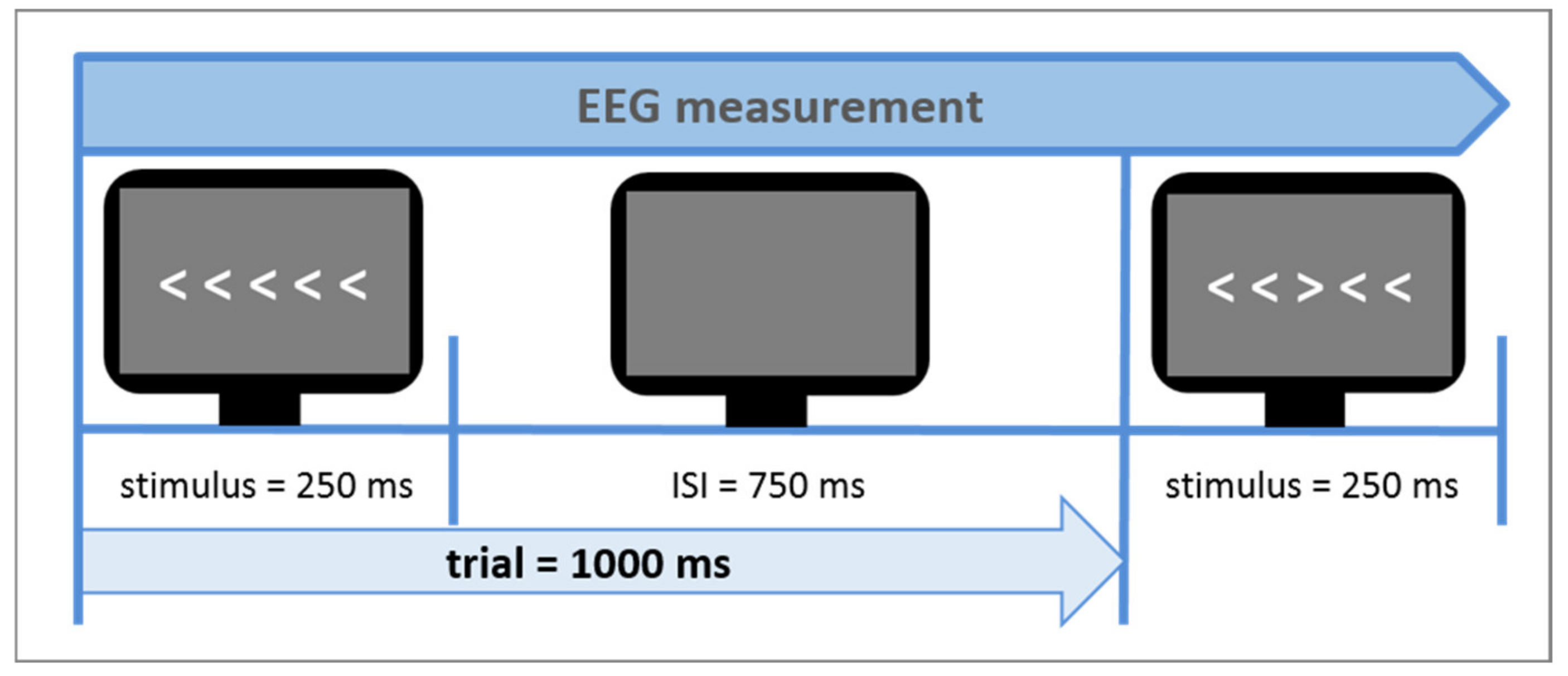
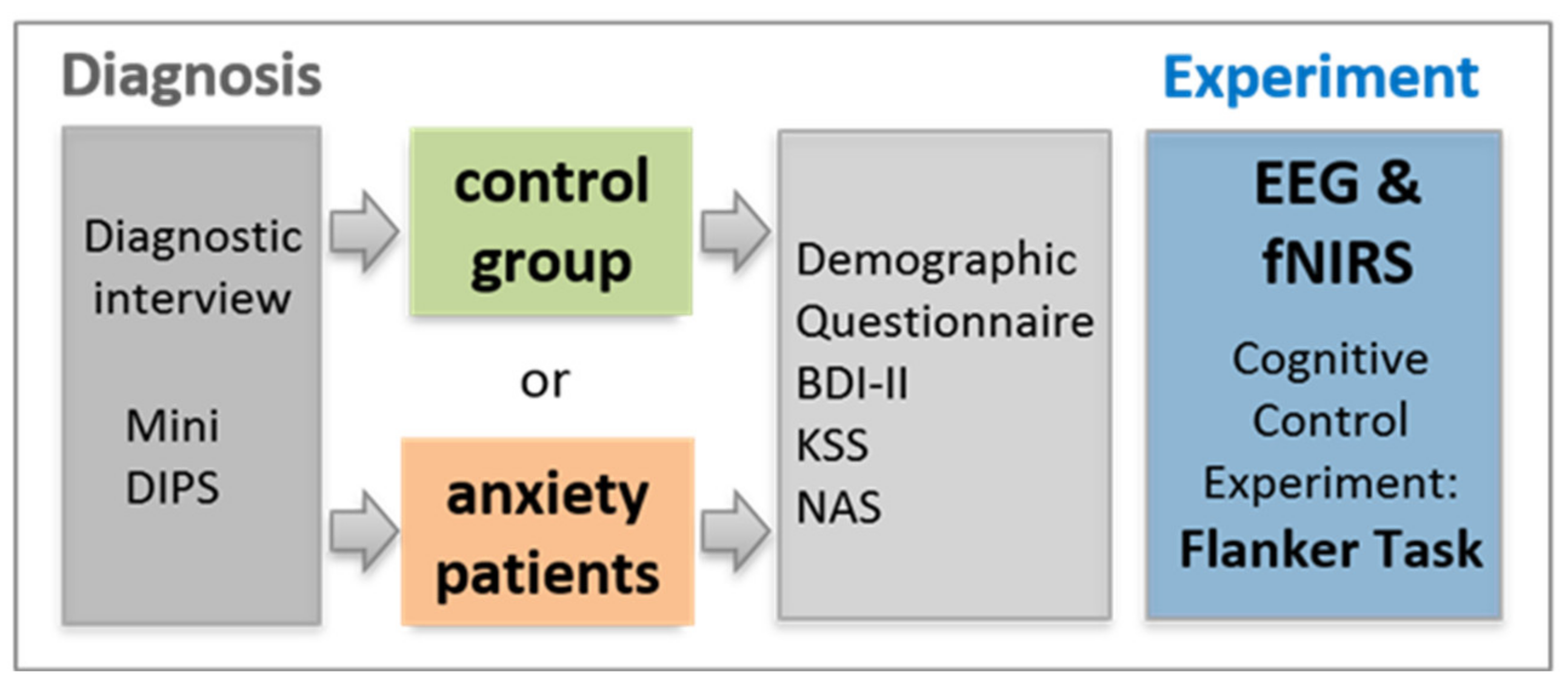
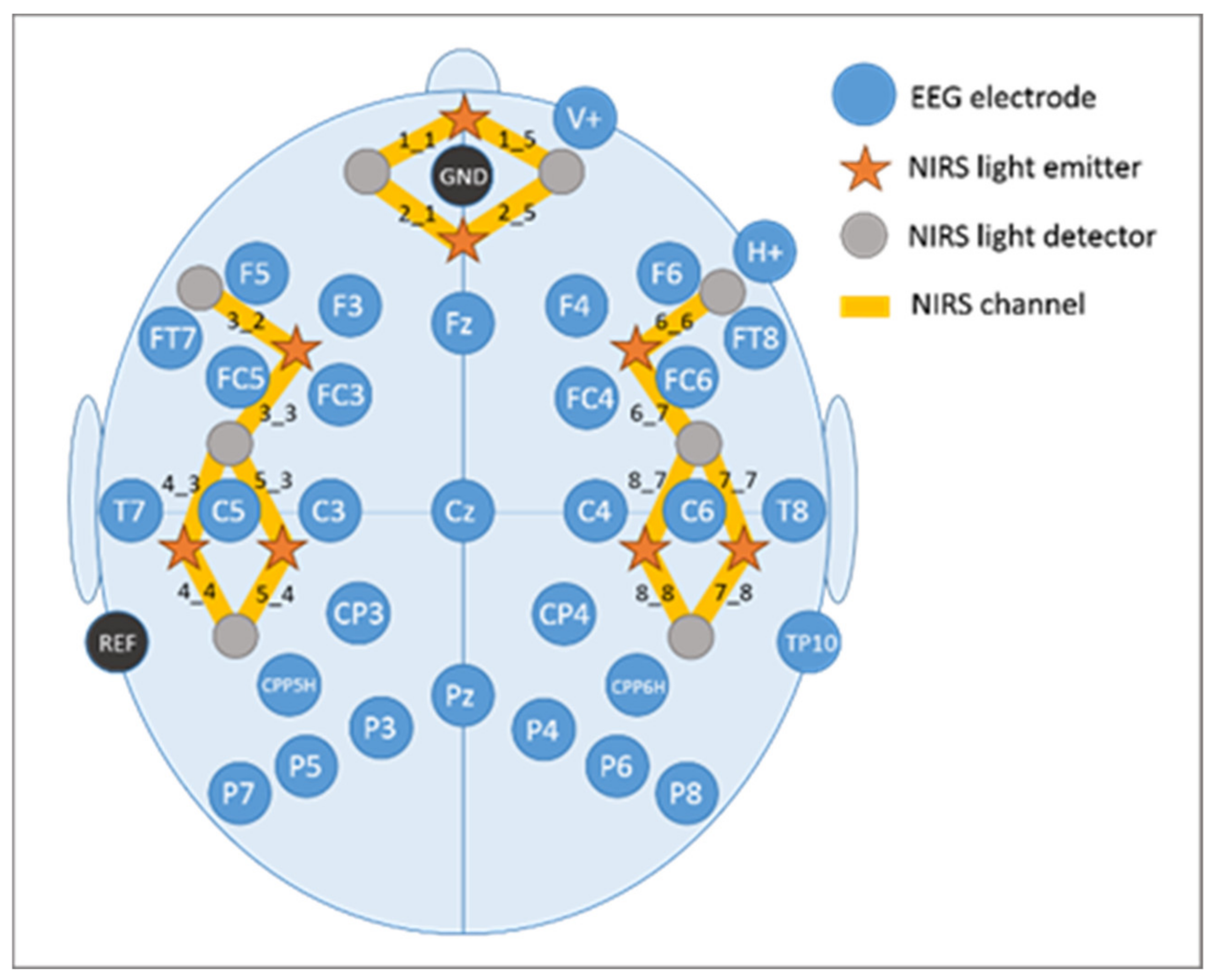
| Controls (n = 29) | Patients (n = 28) | df | t | p | |
|---|---|---|---|---|---|
| Age (Mean) | 24.79 (Range 20–38) | 25.7 (Range 19–40) | 54 | −0.687 | 0.495 |
| BDI-II score | 4.46 | 13.11 | 53 | −4.888 | <0.0001 |
| BIT | 41.14 | 35.33 | 54 | 4.026 | <0.0001 |
| KSS score | 3.93 | 4.41 | 53 | −1.021 | 0.312 |
| NAS | 1.25 | 2.56 | 33.349 | −3.794 | <0.001 |
| Effect | df | F | p |
|---|---|---|---|
| 260–300 ms | |||
| congruency | 1, 43 | 7.901 | 0.007 |
| Congruency * electrode | 28, 1204 | 5.724 | <0.0001 |
| 300–400 ms | |||
| Congruency | 1, 43 | 43.213 | <0.0001 |
| Congruency * electrode | 28, 1204 | 10.491 | <0.0001 |
| 420–660 ms | |||
| Congruency | 1, 43 | 39.875 | <0.0001 |
| Congruency * electrode | 28, 1204 | 8.278 | <0.0001 |
| Electrode | df | t | p |
|---|---|---|---|
| C4 | 56 | 3.648 | 0.001 |
| CP4 | 56 | 3.523 | 0.001 |
| CPP6H | 56 | 3.767 | <0.0001 |
| P4 | 56 | 3.828 | <0.0001 |
| P8 | 55 | 4.729 | <0.0001 |
| Pz | 56 | 3.742 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
König, N.; Steber, S.; Borowski, A.; Bliem, H.R.; Rossi, S. Neural Processing of Cognitive Control in an Emotionally Neutral Context in Anxiety Patients. Brain Sci. 2021, 11, 543. https://doi.org/10.3390/brainsci11050543
König N, Steber S, Borowski A, Bliem HR, Rossi S. Neural Processing of Cognitive Control in an Emotionally Neutral Context in Anxiety Patients. Brain Sciences. 2021; 11(5):543. https://doi.org/10.3390/brainsci11050543
Chicago/Turabian StyleKönig, Nicola, Sarah Steber, Anna Borowski, Harald R. Bliem, and Sonja Rossi. 2021. "Neural Processing of Cognitive Control in an Emotionally Neutral Context in Anxiety Patients" Brain Sciences 11, no. 5: 543. https://doi.org/10.3390/brainsci11050543
APA StyleKönig, N., Steber, S., Borowski, A., Bliem, H. R., & Rossi, S. (2021). Neural Processing of Cognitive Control in an Emotionally Neutral Context in Anxiety Patients. Brain Sciences, 11(5), 543. https://doi.org/10.3390/brainsci11050543






