Effects of Open-Label Placebos on State Anxiety and Glucocorticoid Stress Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
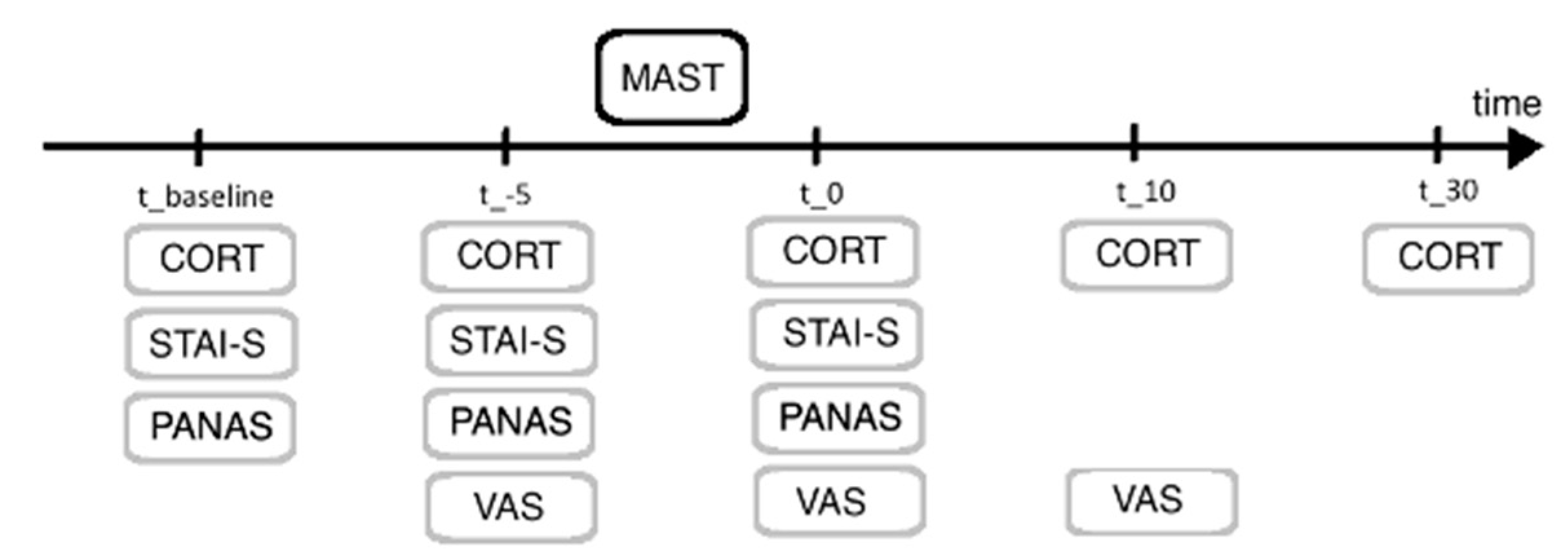
2.2. Maastricht Acute Stress Test
2.3. Neuroendocrine Stress Responses
2.4. Procedure
2.5. Statistical Analysis
3. Results
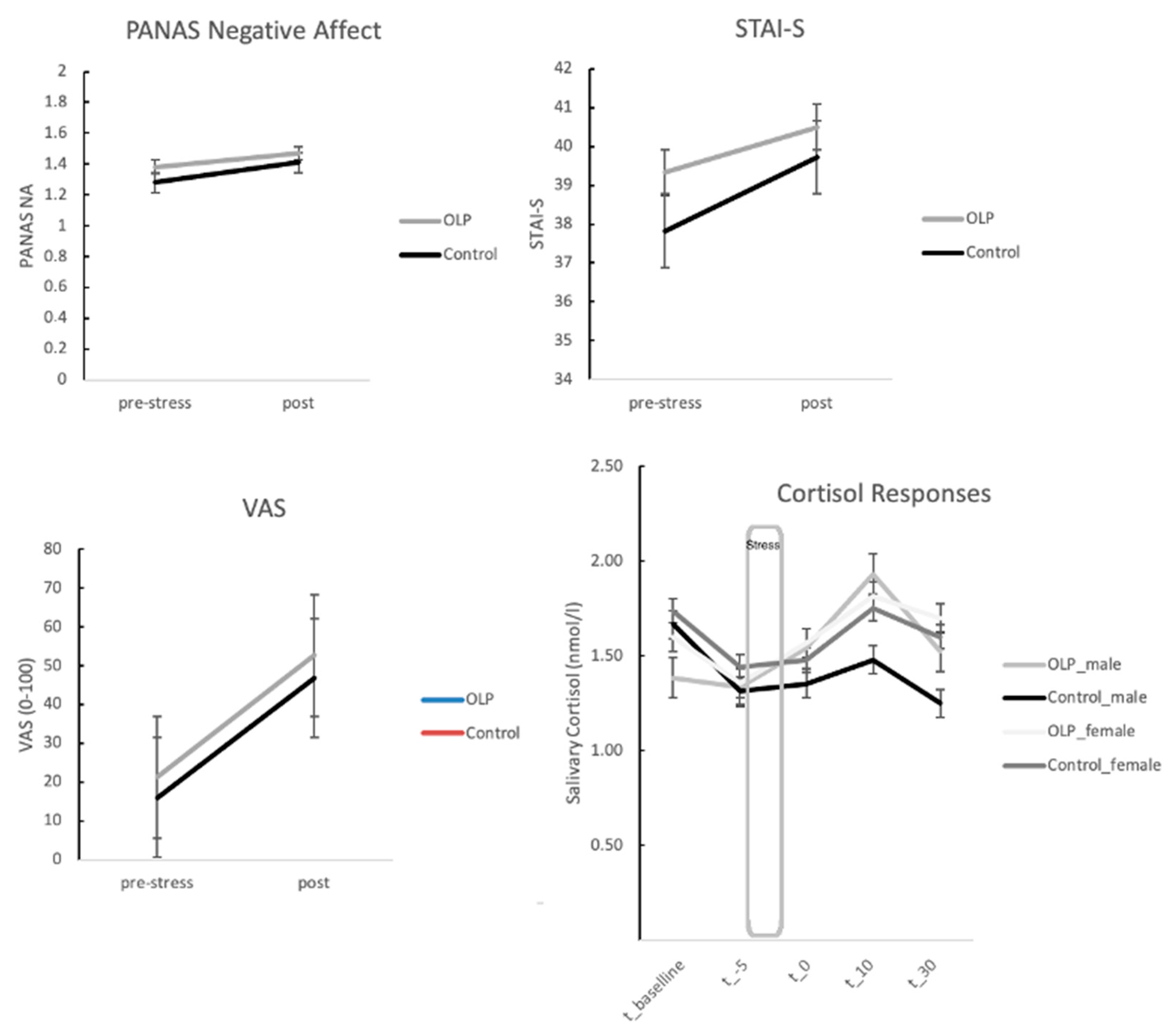
3.1. Stress Responses: Self-Report Measures between OLP and Control Group
3.2. Stress Responses: Neuroendocrine Responses between OLP and Control Group
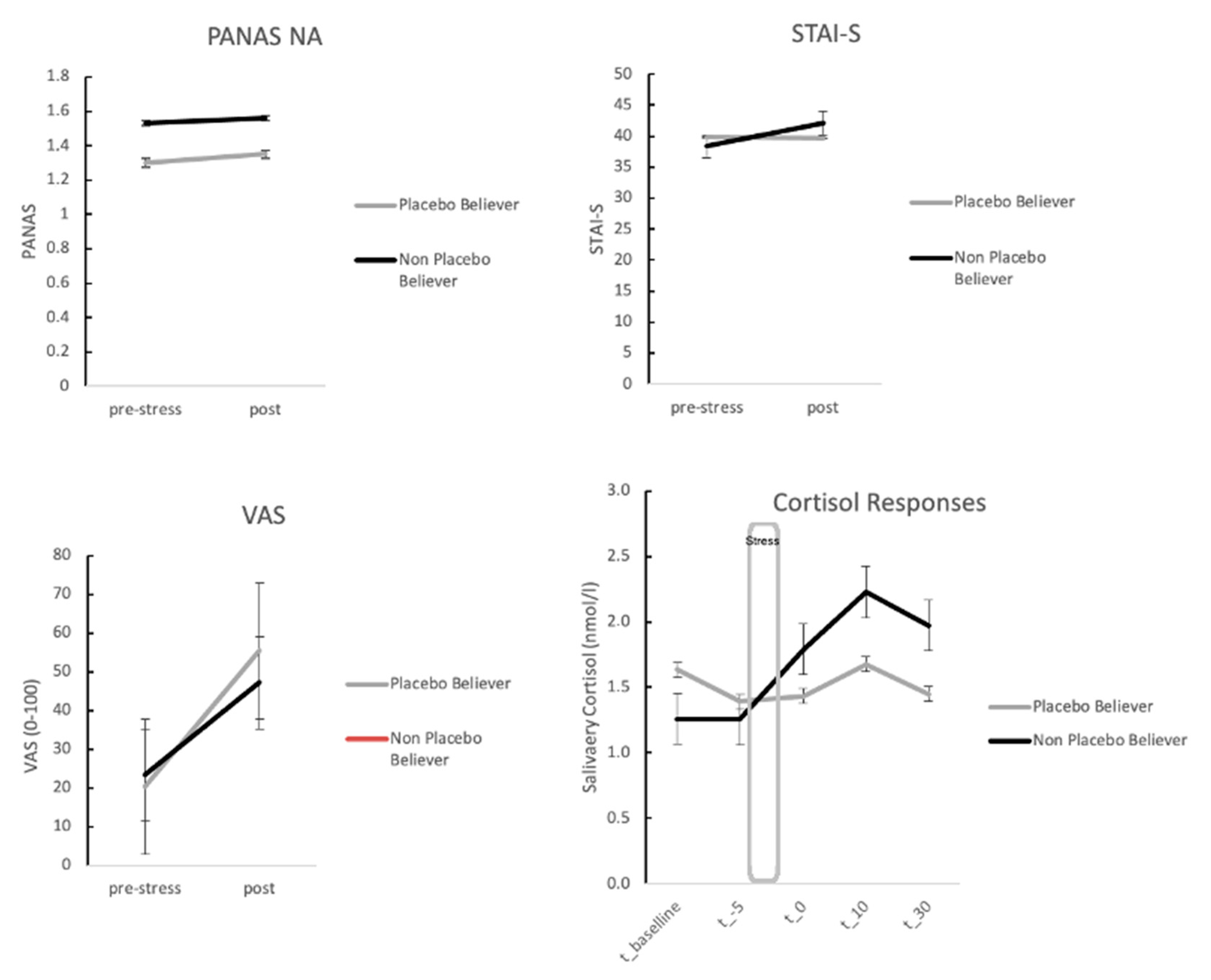
3.3. Stress Responses for OLP Group Only: Self-Report Measures for Stress Depending on High vs. Low Belief
3.4. Stress Responses for OLP Group Only: Neuroendocrine Measures Depending on High vs. Low Belief in Placebos
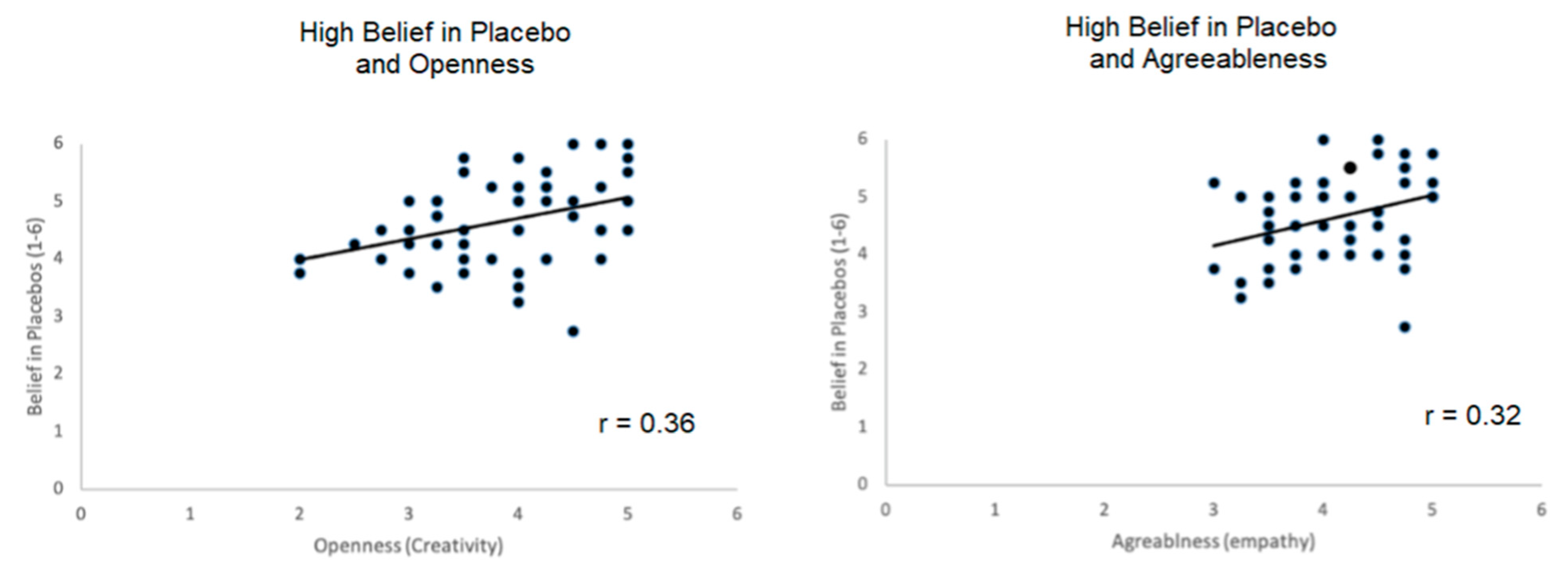
3.5. Correlations of Personality Measures with Belief in Placebos
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hellhammer, D.H.; Wüst, S.; Kudielka, B.M. Salivary Cortisol as a Biomarker in Stress Research. Psychoneuroendocrinology 2009, 34, 163–171. [Google Scholar] [CrossRef]
- Ulrich-Lai, Y.M.; Herman, J.P. Neural Regulation of Endocrine and Autonomic Stress Responses. Nat. Rev. Neurosci. 2009, 10, 397–409. [Google Scholar] [CrossRef]
- Greely, H.; Sahakian, B.; Harris, J.; Kessler, R.C.; Gazzaniga, M.; Campbell, P.; Farah, M.J. Towards Responsible Use of Cognitive-Enhancing Drugs by the Healthy. Nature 2008, 456, 702–705. [Google Scholar] [CrossRef]
- Shiralkar, M.T.; Harris, T.B.; Eddins-Folensbee, F.F.; Coverdale, J.H. A Systematic Review of Stress-Management Programs for Medical Students. Acad. Psychiatry 2013, 37, 158–164. [Google Scholar] [CrossRef]
- Carlson, L.E.; Toivonen, K.; Subnis, U. Integrative Approaches to Stress Management. Cancer J. 2019, 25, 329–336. [Google Scholar] [CrossRef]
- Alkhawaldeh, J.M.A.; Soh, K.L.; Mukhtar, F.B.M.; Peng, O.C.; Anshasi, H.A. Stress Management Interventions for Intensive and Critical Care Nurses: A Systematic Review. Nurs. Crit. Care 2020, 25, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Garakani, A.; Murrough, J.W.; Freire, R.C.; Thom, R.P.; Larkin, K.; Buono, F.D.; Iosifescu, D.V. Pharmacotherapy of Anxiety Disorders: Current and Emerging Treatment Options. Front. Psychiatry 2020, 11, 595584. [Google Scholar] [CrossRef]
- Darragh, M.; Yow, B.; Kieser, A.; Booth, R.J.; Kydd, R.R.; Consedine, N.S. A Take-Home Placebo Treatment Can Reduce Stress, Anxiety and Symptoms of Depression in a Non-Patient Population. Aust. N. Z. J. Psychiatry 2016, 50, 858–865. [Google Scholar] [CrossRef]
- Furmark, T.; Appel, L.; Henningsson, S.; Ahs, F.; Faria, V.; Linnman, C.; Fredrikson, M. A Link Between Serotonin-Related Gene Polymorphisms, Amygdala Activity, and Placebo-Induced Relief from Social Anxiety. J. Neurosci. 2008, 28, 13066–13074. [Google Scholar] [CrossRef] [PubMed]
- Balodis, I.M.; Wynne-Edwards, K.E.; Olmstead, M.C. The Stress-Response-Dampening Effects of Placebo. Horm. Behav. 2011, 59, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, P.; Dietrich, T.; Fransson, P.; Andersson, J.; Carlsson, K.; Ingvar, M. Placebo in Emotional Processing-Induced Expectations of Anxiety Relief Activate a Generalized Modulatory Network. Neuron 2005, 46, 957–969. [Google Scholar] [CrossRef]
- Wendt, L.; Albring, A.; Schedlowski, M. Learned Placebo Responses in Neuroendocrine and Immune Functions. Handb. Exp. Pharmacol. 2014, 225, 159–181. [Google Scholar] [CrossRef]
- Hadamitzky, M.; Sondermann, W.; Benson, S.; Schedlowski, M. Placebo Effects in the Immune System. Int. Rev. Neurobiol. 2018, 138, 39–59. [Google Scholar] [CrossRef]
- Goebel, M.U.; Trebst, A.E.; Steiner, J.; Xie, Y.F.; Exton, M.S.; Frede, S.; Schedlowski, M. Behavioral Conditioning of Immunosuppression is Possible in Humans. Faseb. J. 2002, 16, 1869–1873. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.E.; Cohen, S. Psychological Interventions and the Immune System: A Meta-Analytic Review and Critique. Health Psychol. 2001, 20, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Black, S. Inhibition of Immediate-Type Hypersensitivity Response by Direct Suggestion Under Hypnosis. Br. Med. J. 1963, 1, 925–929. [Google Scholar] [CrossRef]
- Heinrichs, M.; Baumgartner, T.; Kirschbaum, C.; Ehlert, U. Social Support and Oxytocin Interact to Suppress Cortisol and Subjective Responses to Psychosocial Stress. Biol. Psychiatry 2003, 54, 1389–1398. [Google Scholar] [CrossRef]
- Pulopulos, M.M.; Baeken, C.; De Raedt, R. Cortisol Response to Stress: The Role of Expectancy and Anticipatory Stress Regulation. Horm. Behav. 2020, 117, 104587. [Google Scholar] [CrossRef]
- Shields, G.S.; Spahr, C.M.; Slavich, G.M. Psychosocial Interventions and Immune System Function: A Systematic Review and Meta-analysis of Randomized Clinical Trials. JAMA Psychiatry 2020, 77, 1031–1043. [Google Scholar] [CrossRef]
- Urizar, G.G.; Jr Miller, K.; Saldaña, K.S.; Garovoy, N.; Sweet, C.M.C.; King, A.C. Effects of Health Behavior Interventions on Psychosocial Outcomes and Cortisol Regulation Among Chronically Stressed Midlife and Older Adults. Int. J. Behav. Med. 2021. [Google Scholar] [CrossRef]
- Carrico, A.W.; Antoni, M.H. Effects of Psychological Interventions on Neuroendocrine Hormone Regulation and Immune Status in HIV-Positive Persons: A Review of Randomized Controlled Trials. Psychosom. Med. 2008, 7, 575–584. [Google Scholar] [CrossRef]
- Moraes, L.J.; Miranda, M.B.; Loures, L.F.; Mainieri, A.G.; Mármora, C.H.C. A Systematic Review of Psychoneuroimmunology-Based Interventions. Psychol. Health Med. 2018, 23, 635–652. [Google Scholar] [CrossRef]
- Park, L.C.; Covi, L. Nonblind Placebo Trial: An Exploration of Neurotic Patients’ Responses to Placebo when its Inert Content is Disclosed. Arch. Gen. Psychiatry 1965, 12, 336–345. [Google Scholar] [CrossRef]
- Sandler, A.D.; Bodfish, J.W. Open-Label Use of Placebos in the Treatment of ADHD: A Pilot Study. Child. Care Health Dev. 2008, 34, 104–110. [Google Scholar] [CrossRef]
- Kaptchuk, T.J.; Friedlander, E.; Kelley, J.M.; Sanchez, M.N.; Kokkotou, E.; Singer, J.P.; Lembo, A.J. Placebos Without Deception: A Randomized Controlled Trial in Irritable Bowel Syndrome. PLoS ONE 2010, 5, e15591. [Google Scholar] [CrossRef]
- Kelley, J.M.; Kaptchuk, T.J.; Cusin, C.; Lipkin, S.; Fava, M. Open-Label Placebo for Major Depressive Disorder: A Pilot Randomized Controlled Trial. Psychother. Psychosom. 2012, 81, 312–314. [Google Scholar] [CrossRef] [PubMed]
- Kam-Hansen, S.; Jakubowski, M.; Kelley, J.M.; Kirsch, I.; Hoaglin, D.C.; Kaptchuk, T.J.; Burstein, R. Altered Placebo and Drug Labeling Changes the Outcome of Episodic Migraine Attacks. Sci. Transl. Med. 2014, 6, 218ra215. [Google Scholar] [CrossRef]
- Carvalho, C.; Caetano, J.M.; Cunha, L.; Rebouta, P.; Kaptchuk, T.J.; Kirsch, I. Open-Label Placebo Treatment in Chronic Low Back Pain: A Randomized Controlled Trial. Pain 2016, 157, 2766–2772. [Google Scholar] [CrossRef]
- Schaefer, M.; Harke, R.; Denke, C. Open-Label Placebos Improve Symptoms in Allergic Rhinitis. Psychother. Psychosom. 2016, 85, 373–374. [Google Scholar] [CrossRef] [PubMed]
- Hoenemeyer, T.W.; Kaptchuk, T.J.; Mehta, T.S.; Fontaine, K.R. Open-Label Placebo Treatment for Cancer-Related Fatigue: A Randomized-Controlled Clinical Trial. Sci. Rep. 2018, 8, 2784. [Google Scholar] [CrossRef]
- Schaefer, M.; Sahin, T.; Berstecher, B. Why do Open-Label Placebos Work? A Randomized Controlled Trial of an Open-Label Placebo Induction with and Without Extended Information about the Placebo Effect in Allergic Rhinitis. PLoS ONE 2018, 13, e0192758. [Google Scholar] [CrossRef]
- Blease, C.R.; Bishop, F.L.; Kaptchuk, T.J. Informed Consent and Clinical Trials: Where is the Placebo Effect? BMJ 2017, 356, j463. [Google Scholar] [CrossRef]
- Kaptchuk, T.J. Open-Label Placebo: Reflections on a Research Agenda. Perspect Biol. Med. 2018, 61, 311–334. [Google Scholar] [CrossRef] [PubMed]
- Saunders, B.; Saito, T.; Klosterhoff, R.; de Oliveira, L.F.; Barreto, G.; Perim, P.; Gualano, B. I Put it in My Head that the Supplement Would Help Me: Open-Placebo Improves Exercise Performance in Female Cyclists. PLoS ONE 2019, 14, e0222982. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, M.; Denke, C.; Harke, R.; Olk, N.; Erkovan, M.; Enge, S. Open-Label Placebos Reduce Test Anxiety and Improve Self-Management Skills: A Randomized-Controlled Trial. Sci. Rep. 2019, 9, 13317. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.; Jarrett, P.; Broadbent, E.; Petrie, K.J. Open-Label Placebos for Wound Healing: A Randomized Controlled Trial. Ann. Behav. Med. 2018, 52, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Smeets, T.; Cornelisse, S.; Quaedflieg, C.W.; Meyer, T.; Jelicic, M.; Merckelbach, H. Introducing the Maastricht Acute Stress Test (MAST): A Quick and Non-Invasive Approach to Elicit Robust Autonomic and Glucocorticoid Stress Responses. Psychoneuroendocrinology 2012, 37, 1998–2008. [Google Scholar] [CrossRef] [PubMed]
- Charlesworth, J.E.G.; Petkovic, G.; Kelley, J.M.; Hunter, M.; Onakpoya, I.; Roberts, N.; Miller, F.G.; Howick, J. Effects of Placebos without Deception Compared with no Treatment: A Systematic Review and Meta-Analysis. J. Evid. Based Med. 2017, 10, 97–107. [Google Scholar] [CrossRef]
- Rombold, F.; Wingenfeld, K.; Renneberg, B.; Schwarzkopf, F.; Hellmann-Regen, J.; Otte, C.; Roepke, S. Impact of Exogenous Cortisol on the Formation of Intrusive Memories in Healthy Women. J. Psychiatr. Res. 2016, 83, 71–78. [Google Scholar] [CrossRef]
- Schultebraucks, K.; Deuter, C.E.; Duesenberg, M.; Schulze, L.; Hellmann-Regen, J.; Domke, A.; Wingenfeld, K. Selective Attention to Emotional Cues and Emotion Recognition in Healthy Subjects: The Role of Mineralocorticoid Receptor Stimulation. Psychopharmacology 2016, 233, 3405–3415. [Google Scholar] [CrossRef]
- Spitzer, C.; Otte, C.; Kuehl, L.K.; May, A.; Schultebraucks, K.; Hellmann-Regen, J.; Wingenfeld, K. The Dexamethasone Corticotropin Releasing Hormone Test in Healthy and Depressed Women with and Without Childhood Adversity. Psychoneuroendocrinology 2018, 87, 147–151. [Google Scholar] [CrossRef]
- Schulz, P.; Schlotz, W.; Becker, P. Trierer Inventar zum Chronischen Stress (TICS); Hogrefe: Göttingen, Germany, 2008. [Google Scholar]
- Danner, D.; Rammstedt, B.; Bluemke, M.; Treiber, L.; Berres, S.; Soto, C.; John, O. Die Deutsche Version des Big Five Inventory 2 (BFI-2). Diagnostica 2019, 65, 1–12. [Google Scholar] [CrossRef]
- Leibowitz, K.A.; Hardebeck, E.J.; Goyer, J.P.; Crum, A.J. The Role of Patient Beliefs in Open-Label Placebo Effects. Health Psychol. 2019, 38, 613–622. [Google Scholar] [CrossRef]
- Hoeger Bement, M.; Weyer, A.; Keller, M.; Harkins, A.L.; Hunter, S.K. Anxiety and Stress Can Predict Pain Perception Following a Cognitive Stress. Physiol. Behav. 2010, 101, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.; Vagg, P.R.; Jacobs, G.A. Manual for the State-Trait Anxiety Inventory; Consulting Psychologists Press: Palo Alto, CA, USA, 1983. [Google Scholar]
- Laux, L.; Hock, M.; Bergner-Köhler, R.; Hodapp, V.; Renner, K.-H. Das State-Trait-Angst-Depressions-Inventar; Hogrefe: Göttingen, Germany, 2013. [Google Scholar]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and Validation of Brief Measures of Positive and Negative Affect: The PANAS Scales. J. Personal. Soc. Psychol. 1988, 54, 1063–1070. [Google Scholar] [CrossRef]
- Krohne, H.W.; Egloff, B.; Kohlmann, C.; Tausch, A. Investigations with a German Version of the Positive and Negative Affect Schedule (PANAS). Diagnostica 1996, 42, 139–156. [Google Scholar]
- Vachon-Presseau, E.; Berger, S.E.; Abdullah, T.B.; Huang, L.; Cecchi, G.A.; Griffith, J.W.; Apkarian, A.V. Brain and Psychological Determinants of Placebo Pill Response in Chronic Pain Patients. Nat. Commun. 2018, 9, 3397. [Google Scholar] [CrossRef]
- Danner, D.; Rammstedt, B.; Bluemke, M.; Lechner, C.; Berres, S.; Knopf, T.; John, O.P. Die Deutsche Version des Big Five Inventory 2 (BFI-2). Zusammenstellung Sozialwissenschaftlicher Items und Skalen (ZIS). Available online: https://www.ssoar.info/ssoar/handle/document/65715 (accessed on 7 April 2021).
- Cohen, F.; Kearney, K.A.; Zegans, L.S.; Kemeny, M.E.; Neuhaus, J.M.; Stites, D.P. Differential Immune System Changes with Acute and Persistent Stress for Optimists vs. Pessimists. Brain Behav. Immun. 1999, 13, 155–174. [Google Scholar] [CrossRef]
- Morton, D.L.; Watson, A.; El-Deredy, W.; Jones, A.K. Reproducibility of Placebo Analgesia: Effect of Dispositional Optimism. Pain 2009, 146, 194–198. [Google Scholar] [CrossRef]
- Kern, A.; Kramm, C.; Witt, C.M.; Barth, J. The Influence of Personality Traits on the Placebo/Nocebo Response: A Systematic Review. J. Psychosom. Res. 2020, 128, 109866. [Google Scholar] [CrossRef] [PubMed]
- Lidstone, S.C.; Schulzer, M.; Dinelle, K.; Mak, E.; Sossi, V.; Ruth, T.J.; Stoessl, A.J. Effects of Expectation on Placebo-Induced Dopamine Release in Parkinson Disease. Arch. Gen. Psychiatry 2010, 67, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Enck, P.; Bingel, U.; Schedlowski, M.; Rief, W. The Placebo Response in Medicine: Minimize, Maximize or Personalize? Nat. Rev. Drug Discov. 2013, 12, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Bargh, J.A. The Modern Unconscious. World Psychiatry 2019, 18, 225–226. [Google Scholar] [CrossRef]
- Ongaro, G.; Ward, D. An Enactive Account of Placebo Effects. Biol. Philos. 2017, 32, 507–533. [Google Scholar] [CrossRef]
- Guevarra, D.A.; Moser, J.S.; Wager, T.D.; Kross, E. Placebos without Deception Reduce Self-Report and Neural Measures of Emotional Distress. Nat. Commun. 2020, 11, 3785. [Google Scholar] [CrossRef] [PubMed]
- Moser, J.S.; Dougherty, A.; Mattson, W.I.; Katz, B.; Moran, T.P.; Guevarra, D.; Kross, E. Third-Person Self-Talk Facilitates Emotion Regulation Without Engaging Cognitive Control: Converging Evidence from ERP and fMRI. Sci. Rep. 2017, 7, 4519. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Open Label Placebo | Control |
|---|---|---|
| n | 24 | 29 |
| age (in years) | 25.25 ± 7.28 | 27.41 ± 10.25 |
| Females/males | 14/10 | 14/15 |
| SSCI | 31.13 ± 8.96 | 29.97 ± 8.26 |
| STAI-T | 39.75 ± 8.44 | 37.31 ± 7.87 |
| STAI-S (baseline) | 41.54 ± 7.82 | 39.31 ± 6.60 |
| PANAS NA (baseline) | 1.47 ± 0.65 | 1.33 ± 0.23 |
| VAS (baseline) | 21.33 ± 22.82 | 16.00 ± 17.69 |
| Self-Report Measures | Open Label Placebo | Control |
|---|---|---|
| STAI-S | 1.17 ± 5.21 | 1.90 ± 7.34 |
| PANAS | 0.05 ± 0.45 | 0.12 ± 0.46 |
| VAS | 31.29 ± 35.07 | 30.76 ± 34.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schaefer, M.; Hellmann-Regen, J.; Enge, S. Effects of Open-Label Placebos on State Anxiety and Glucocorticoid Stress Responses. Brain Sci. 2021, 11, 508. https://doi.org/10.3390/brainsci11040508
Schaefer M, Hellmann-Regen J, Enge S. Effects of Open-Label Placebos on State Anxiety and Glucocorticoid Stress Responses. Brain Sciences. 2021; 11(4):508. https://doi.org/10.3390/brainsci11040508
Chicago/Turabian StyleSchaefer, Michael, Julian Hellmann-Regen, and Sören Enge. 2021. "Effects of Open-Label Placebos on State Anxiety and Glucocorticoid Stress Responses" Brain Sciences 11, no. 4: 508. https://doi.org/10.3390/brainsci11040508
APA StyleSchaefer, M., Hellmann-Regen, J., & Enge, S. (2021). Effects of Open-Label Placebos on State Anxiety and Glucocorticoid Stress Responses. Brain Sciences, 11(4), 508. https://doi.org/10.3390/brainsci11040508






