Use of Neuroanatomic Knowledge and Neuronavigation System for a Safe Anterior Petrosectomy
Abstract
1. Introduction
2. Materials and Methods
3. Results
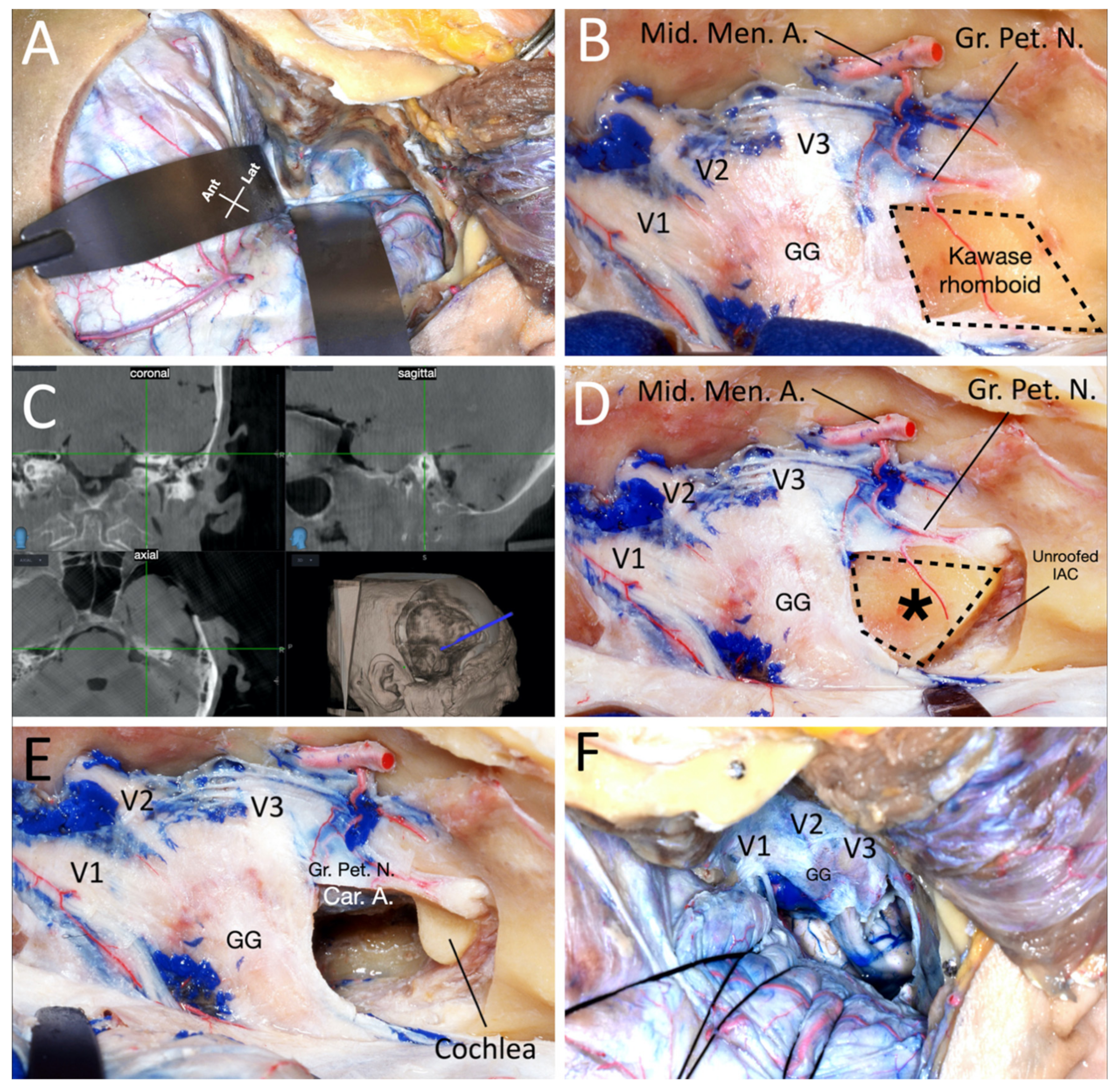
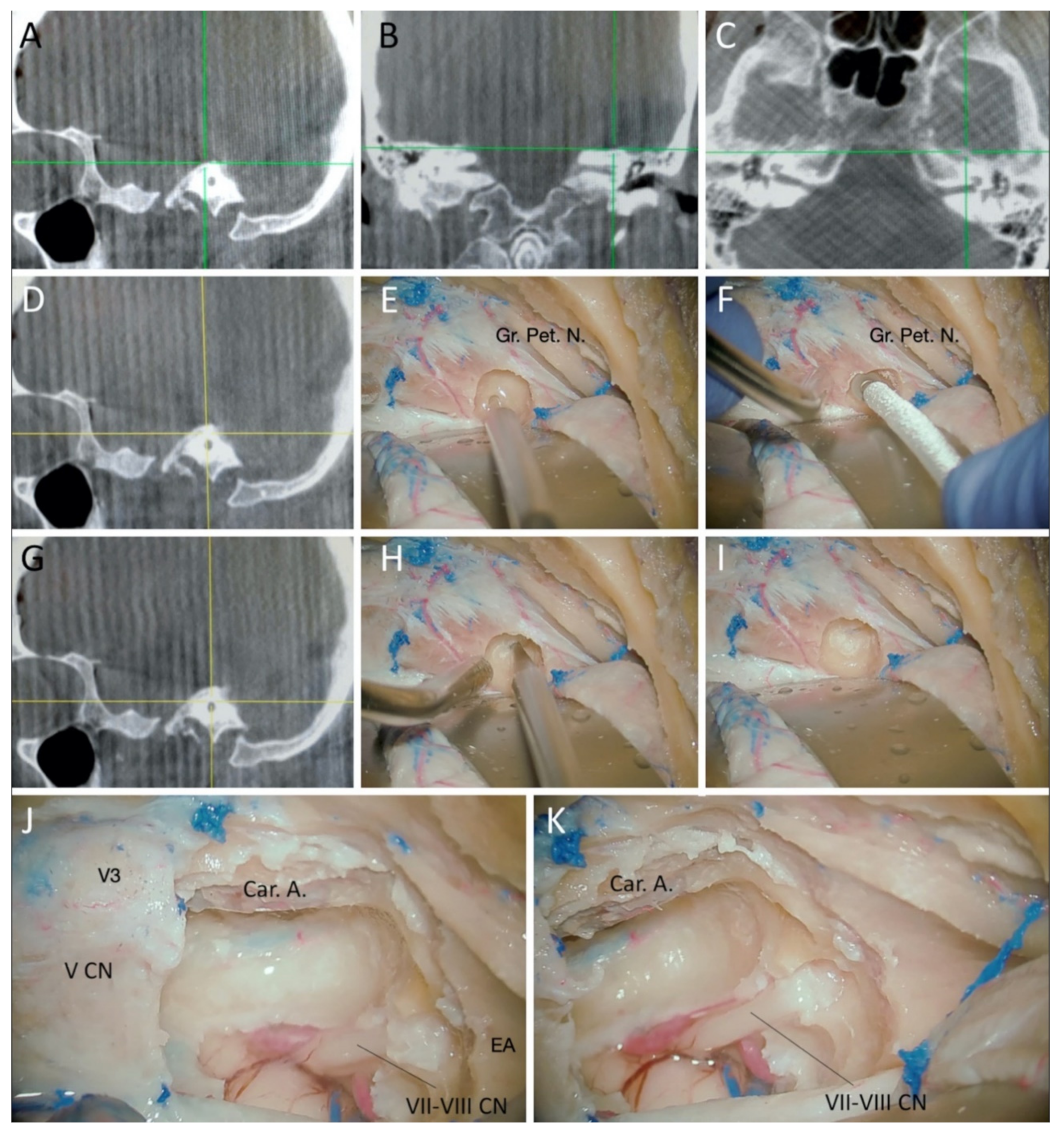
3.1. Cadaveric Dissection
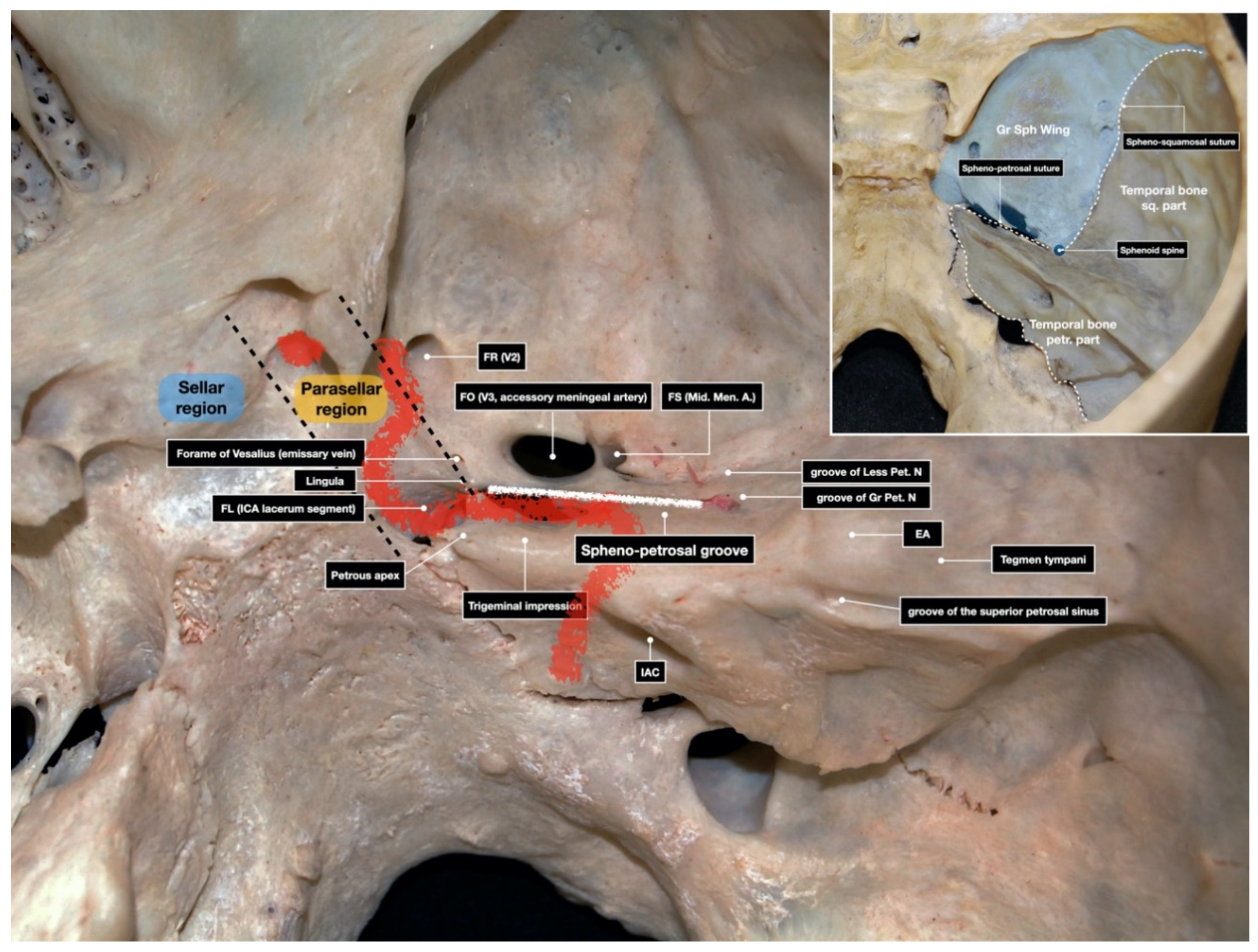
3.1.1. Surgical Position and Bony Exposure
3.1.2. Craniotomy and Middle Fossa Peeling
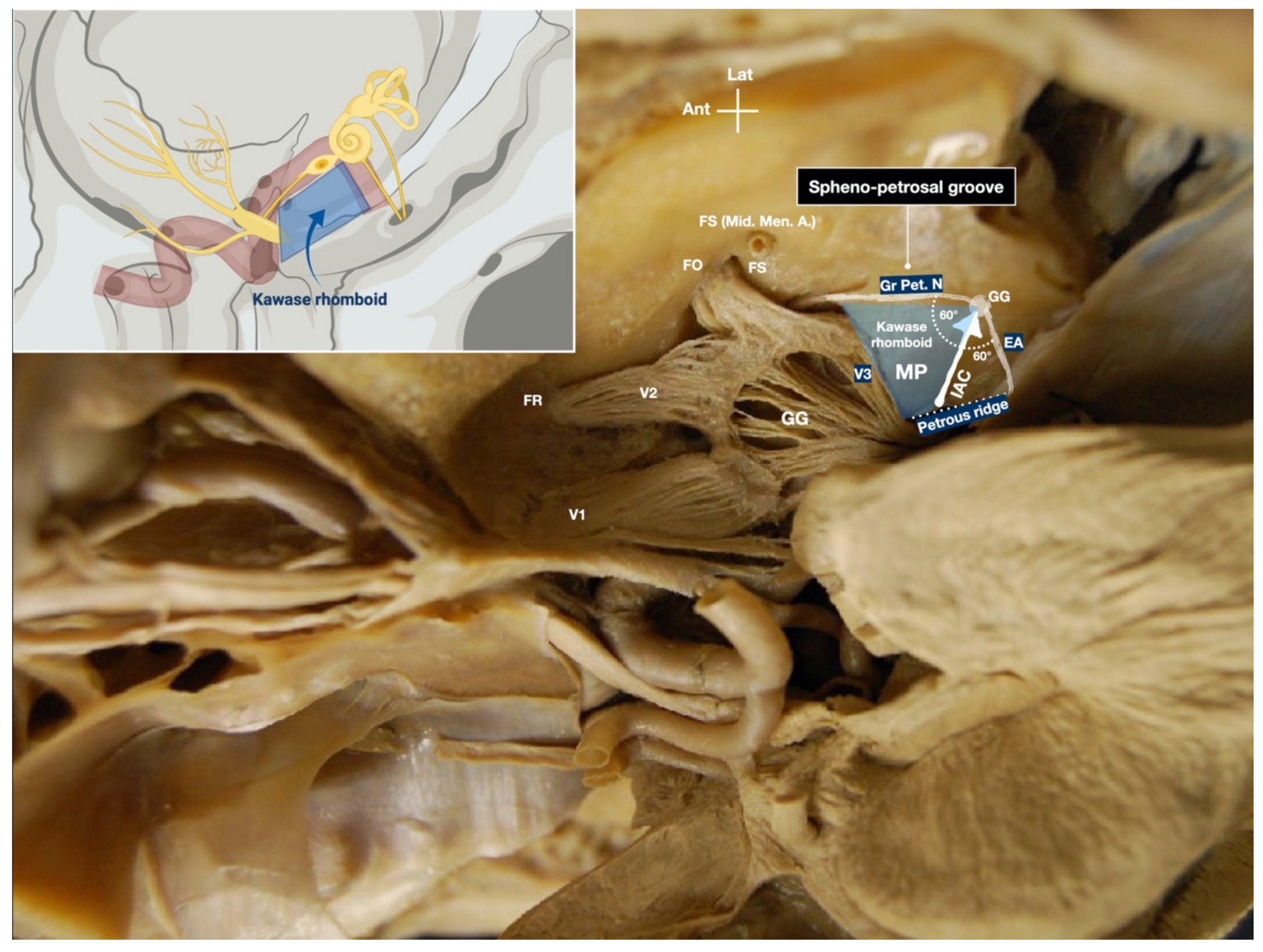
3.1.3. Drilling of the Petrous Bone under Neuronavigation
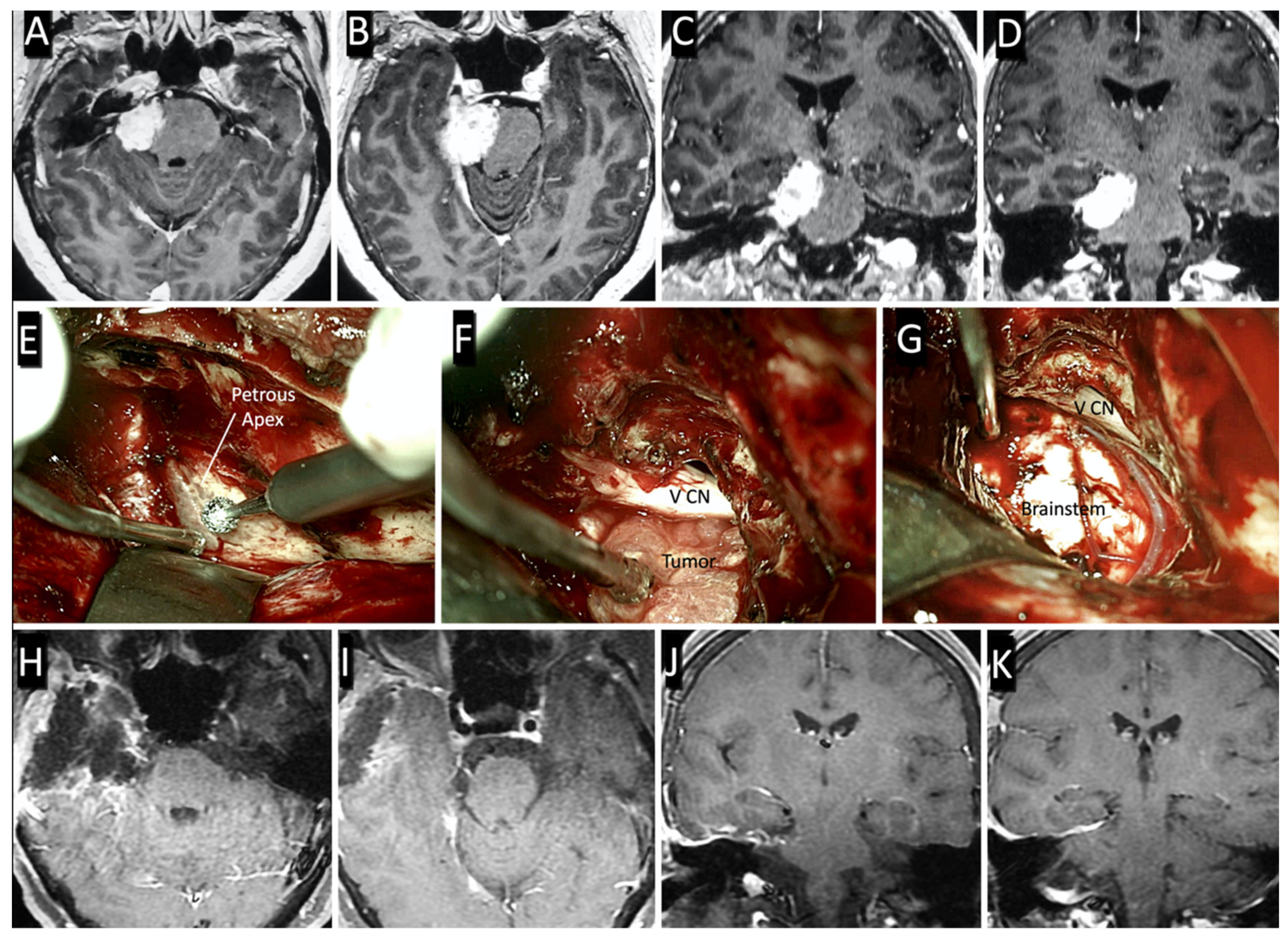
3.2. Surgical Case
4. Discussion
4.1. Previous Concepts
4.2. Anatomic Details and Neuronavigation
4.3. Advantages and Disadvantages of Anterior Petrosectomy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tripathi, M.; Deo, R.C.; Suri, A.; Srivastav, V.; Baby, B.; Kumar, S.; Kalra, P.; Banerjee, S.; Prasad, S.; Paul, K.; et al. Quantitative analysis of the Kawase versus the modified Dolenc-Kawase approach for middle cranial fossa lesions with variable anteroposterior extension. J. Neurosurg. 2015, 123, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Muto, J.; Prevedello, D.M.; Ditzel Filho, L.F.; Tang, I.P.; Oyama, K.; Kerr, E.E.; Otto, B.A.; Kawase, T.; Yoshida, K.; Carrau, R.L. Comparative analysis of the anterior transpetrosal approach with the endoscopic endonasal approach to the petroclival region. J. Neurosurg. 2016, 125, 1171–1186. [Google Scholar] [CrossRef] [PubMed]
- Fournier, H.D.; Mercier, P.; Roche, P.H. Surgical anatomy of the petrous apex and petroclival region. Adv. Tech. Stand. Neurosurg. 2007, 32, 91–146. [Google Scholar] [CrossRef] [PubMed]
- Altieri, R.; Sameshima, T.; Pacca, P.; Crobeddu, E.; Garbossa, D.; Ducati, A.; Zenga, F. Detailed anatomy knowledge: First step to approach petroclival meningiomas through the petrous apex. Anatomy lab experience and surgical series. Neurosurg. Rev. 2017, 40, 231–239. [Google Scholar] [CrossRef]
- Peris-Celda, M.; Perry, A.; Carlstrom, L.P.; Graffeo, C.S.; Driscoll, C.L.W.; Link, M.J. Key anatomical landmarks for middle fossa surgery: A surgical anatomy study. J. Neurosurg. 2018, 1–10. [Google Scholar] [CrossRef]
- Samii, M.; Tatagiba, M.; Carvalho, G.A. Retrosigmoid intradural suprameatal approach to Meckel’s cave and the middle fossa: Surgical technique and outcome. J. Neurosurg. 2000, 92, 235–241. [Google Scholar] [CrossRef]
- Kawase, T.; Shiobara, R.; Toya, S. Anterior transpetrosal-transtentorial approach for sphenopetroclival meningiomas: Surgical method and results in 10 patients. Neurosurgery 1991, 28, 869–875, discussion 875-866. [Google Scholar] [CrossRef]
- Al-Mefty, O.; Fox, J.L.; Smith, R.R. Petrosal approach for petroclival meningiomas. Neurosurgery 1988, 22, 510–517. [Google Scholar] [CrossRef]
- Van Gompel, J.J.; Alikhani, P.; Youssef, A.S.; Loveren, H.R.; Boyev, K.P.; Agazzi, S. Anterior Petrosectomy: Consecutive Series of 46 Patients with Attention to Approach-Related Complications. J. Neurol. Surg. B Skull Base 2015, 76, 379–384. [Google Scholar] [CrossRef]
- Megerian, C.A.; Chiocca, E.A.; McKenna, M.J.; Harsh, G.F.T.; Ojemann, R.G. The subtemporal-transpetrous approach for excision of petroclival tumors. Am. J. Otol. 1996, 17, 773–779. [Google Scholar]
- Kawase, T.; Toya, S.; Shiobara, R.; Mine, T. Transpetrosal approach for aneurysms of the lower basilar artery. J. Neurosurg. 1985, 63, 857–861. [Google Scholar] [CrossRef]
- Dew, L.A.; Shelton, C.; Harnsberger, H.R.; Thompson, B.G., Jr. Surgical exposure of the petrous internal carotid artery: Practical application for skull base surgery. Laryngoscope 1997, 107, 967–976. [Google Scholar] [CrossRef]
- Dolenc, V.V. Frontotemporal epidural approach to trigeminal neurinomas. Acta Neurochir. 1994, 130, 55–65. [Google Scholar] [CrossRef]
- Diaz Day, J. The middle fossa approach and extended middle fossa approach: Technique and operative nuances. Neurosurgery 2012, 70, 192–201. [Google Scholar] [CrossRef]
- Kartush, J.M.; Kemink, J.L.; Graham, M.D. The arcuate eminence. Topographic orientation in middle cranial fossa surgery. Ann. Otol. Rhinol. Laryngol. 1985, 94, 25–28. [Google Scholar] [CrossRef]
- Bochenek, Z.; Kukwa, A. An extended approach through the middle cranial fossa to the internal auditory meatus and the cerebello-pontine angle. Acta Otolaryngol. 1975, 80, 410–414. [Google Scholar] [CrossRef]
- Kawase, T.; Shiobara, R.; Toya, S. Middle fossa transpetrosal-transtentorial approaches for petroclival meningiomas. Selective pyramid resection and radicality. Acta Neurochir. 1994, 129, 113–120. [Google Scholar] [CrossRef]
- Gross, B.A.; Dunn, I.F.; Du, R.; Al-Mefty, O. Petrosal approaches to brainstem cavernous malformations. Neurosurg. Focus 2012, 33, E10. [Google Scholar] [CrossRef]
- Komune, N.; Matsushima, K.; Matsuo, S.; Safavi-Abbasi, S.; Matsumoto, N.; Rhoton, A.L., Jr. The accuracy of an electromagnetic navigation system in lateral skull base approaches. Laryngoscope 2017, 127, 450–459. [Google Scholar] [CrossRef]
- Luzzi, S.; Giotta Lucifero, A.; Del Maestro, M.; Marfia, G.; Navone, S.E.; Baldoncini, M.; Nuñez, M.; Campero, A.; Elbabaa, S.K.; Galzio, R. Anterolateral Approach for Retrostyloid Superior Parapharyngeal Space Schwannomas Involving the Jugular Foramen Area: A 20-Year Experience. World Neurosurg. 2019, 132, e40–e52. [Google Scholar] [CrossRef]
- Anand, V.K.; Kacker, A. Value of radiologic imaging and computer assisted surgery in surgical decisions of the anterior skull base lesions. Rhinology 2000, 38, 17–22. [Google Scholar]
- Day, J.D.; Fukushima, T.; Giannotta, S.L. Microanatomical study of the extradural middle fossa approach to the petroclival and posterior cavernous sinus region: Description of the rhomboid construct. Neurosurgery 1994, 34, 1009–1016. [Google Scholar] [CrossRef]
- Luzzi, S.; Maestro, M.D.; Elia, A.; Vincitorio, F.; Perna, G.D.; Zenga, F.; Garbossa, D.; Elbabaa, S.K.; Galzio, R. Morphometric and Radiomorphometric Study of the Correlation Between the Foramen Magnum Region and the Anterior and Posterolateral Approaches to Ventral Intradural Lesions. Turk. Neurosurg. 2019, 29, 875–886. [Google Scholar] [CrossRef]
- Ogiwara, T.; Goto, T.; Hara, Y.; Hongo, K. Real-Time Navigation-Guided Drilling Technique for Skull Base Surgery in the Middle and Posterior Fossae. J. Neurol. Surg. B Skull Base 2018, 79, S334–S339. [Google Scholar] [CrossRef]
- Canzi, P.; Avato, I.; Marconi, S.; Del Maestro, M.; Lucifero, A.G.; Magnetto, M.; Carlotto, E.; Auricchio, F.; Luzzi, S.; Benazzo, M. A 3D printed custom-made mask model for frameless neuronavigation during retrosigmoid craniotomy. A preclinical cadaveric feasibility study. Ann. Ital. Chir. 2020, 91, 526–533. [Google Scholar]
- Dolenc, V.V. Anatomy and Surgery of the Cavernous Sinus; Springer: Vienna, Austria, 1989; pp. 68–87. [Google Scholar] [CrossRef]
- Tedeschi, H.; Rhoton, A.L., Jr. Lateral approaches to the petroclival region. Surg. Neurol. 1994, 41, 180–216. [Google Scholar] [CrossRef]
- Miller, R.S.; Pensak, M.L. The superior petrosal triangle as a constant anatomical landmark for subtemporal middle fossa orientation. Laryngoscope 2003, 113, 1327–1331. [Google Scholar] [CrossRef]
- Kim, S.M.; Lee, H.Y.; Kim, H.K.; Zabramski, J.M. Cochlear line: A novel landmark for hearing preservation using the anterior petrosal approach. J. Neurosurg. 2015, 123, 9–13. [Google Scholar] [CrossRef]
- Steiger, H.J.; Hänggi, D.; Stummer, W.; Winkler, P.A. Custom-tailored transdural anterior transpetrosal approach to ventral pons and retroclival regions. J. Neurosurg. 2006, 104, 38–46. [Google Scholar] [CrossRef]
- Wang, J.; Yoshioka, F.; Joo, W.; Komune, N.; Quilis-Quesada, V.; Rhoton, A.L. The cochlea in skull base surgery: An anatomy study. J. Neurosurg. 2016, 125, 1–11. [Google Scholar] [CrossRef]
- Van Havenbergh, T.; Koekelkoren, E.; De Ridder, D.; Van De Heyning, P.; Verlooy, J. Image guided surgery for petrous apex lesions. Acta Neurochir. 2003, 145, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Sure, U.; Alberti, O.; Petermeyer, M.; Becker, R.; Bertalanffy, H. Advanced image-guided skull base surgery. Surg. Neurol. 2000, 53, 563–572. [Google Scholar] [CrossRef]
- Danner, C.; Cueva, R.A. Extended middle fossa approach to the petroclival junction and anterior cerebellopontine angle. Otol. Neurotol. 2004, 25, 762–768. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Justa, A.; Luzzi, S.; Giotta Lucifero, A.; Villalonga, J.F.; Saenz, A.; Santin-Amo, J.M.; Baldoncini, M.; Campero, A. Use of Neuroanatomic Knowledge and Neuronavigation System for a Safe Anterior Petrosectomy. Brain Sci. 2021, 11, 488. https://doi.org/10.3390/brainsci11040488
Flores-Justa A, Luzzi S, Giotta Lucifero A, Villalonga JF, Saenz A, Santin-Amo JM, Baldoncini M, Campero A. Use of Neuroanatomic Knowledge and Neuronavigation System for a Safe Anterior Petrosectomy. Brain Sciences. 2021; 11(4):488. https://doi.org/10.3390/brainsci11040488
Chicago/Turabian StyleFlores-Justa, Ana, Sabino Luzzi, Alice Giotta Lucifero, Juan F. Villalonga, Amparo Saenz, José María Santin-Amo, Matias Baldoncini, and Alvaro Campero. 2021. "Use of Neuroanatomic Knowledge and Neuronavigation System for a Safe Anterior Petrosectomy" Brain Sciences 11, no. 4: 488. https://doi.org/10.3390/brainsci11040488
APA StyleFlores-Justa, A., Luzzi, S., Giotta Lucifero, A., Villalonga, J. F., Saenz, A., Santin-Amo, J. M., Baldoncini, M., & Campero, A. (2021). Use of Neuroanatomic Knowledge and Neuronavigation System for a Safe Anterior Petrosectomy. Brain Sciences, 11(4), 488. https://doi.org/10.3390/brainsci11040488