MicroRNAs in Basolateral Amygdala Associated with Stress and Fear Memories Regulate Rapid Eye Movement Sleep in Rats
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Selection of Putative Res and Vul Rats for miRNA Profiling Studies
3.2. Differential Expression of BLA miRNAs in ST and CTX
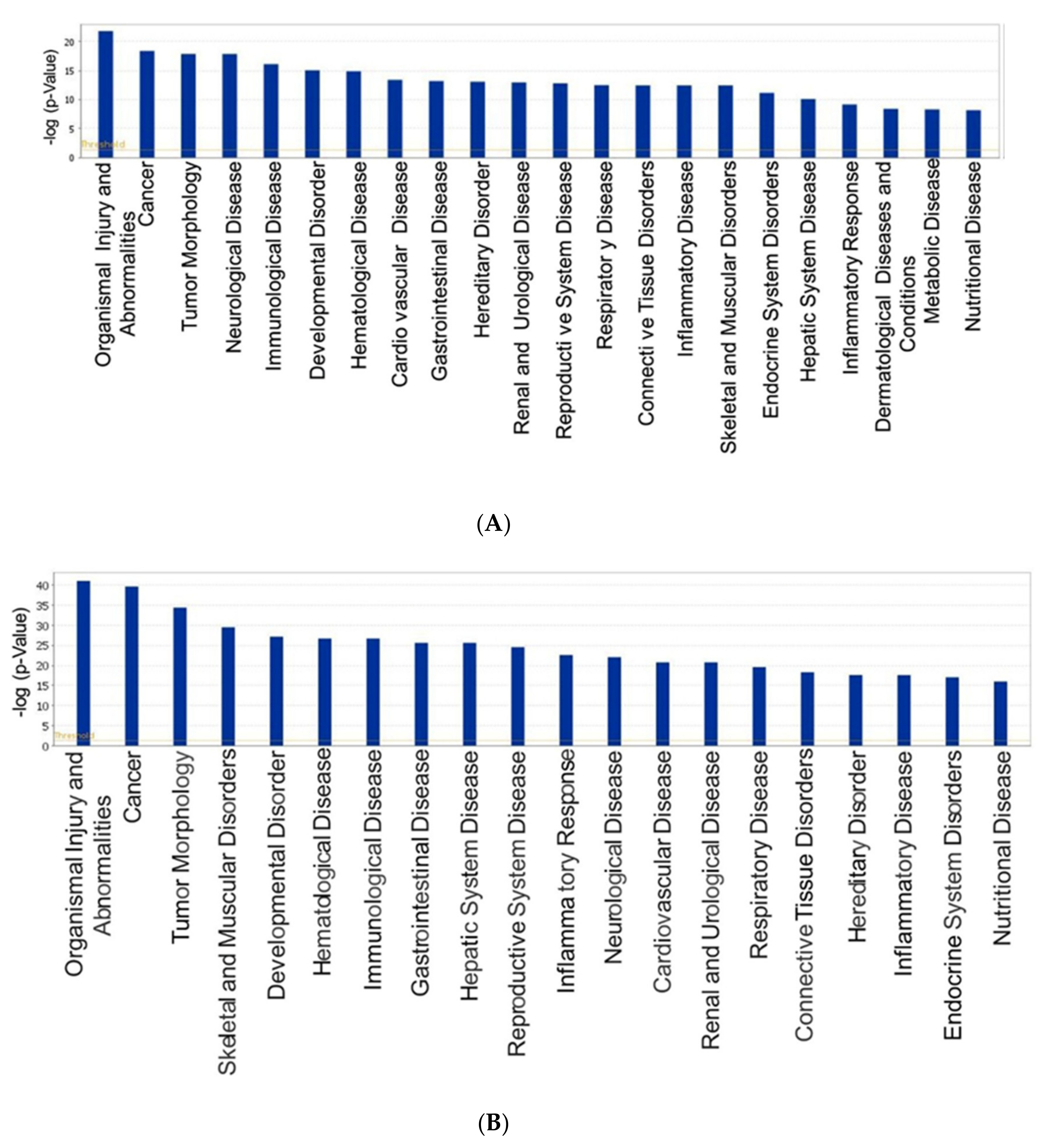
3.3. Biological Processes and Network Analysis of Altered miRNAs
3.4. Effects of AntagomiR-221 Microinjection on REM Sleep
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Gilbert, K.S.; Kark, S.M.; Gehrman, P.; Bogdanova, Y. Sleep disturbances, TBI and PTSD: Implications for treatment and recovery. Clin. Psychol. Rev. 2015, 40, 195–212. [Google Scholar] [CrossRef]
- Maher, M.J.; Rego, S.A.; Asnis, G.M. Sleep disturbances in patients with post-traumatic stress disorder: Epidemiology, impact and approaches to management. CNS Drugs 2006, 20, 567–590. [Google Scholar] [CrossRef]
- Moore, B.A.; Brock, M.S.; Brager, A.; Collen, J.; LoPresti, M.; Mysliwiec, V. Posttraumatic Stress Disorder, Traumatic Brain Injury, Sleep, and Performance in Military Personnel. Sleep Med. Clin. 2020, 15, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Koren, D.; Arnon, I.; Lavie, P.; Klein, E. Sleep Complaints as Early Predictors of Posttraumatic Stress Disorder: A 1-Year Prospective Study of Injured Survivors of Motor Vehicle Accidents. Am. J. Psychiatry 2002, 159, 855–857. [Google Scholar] [CrossRef] [PubMed]
- Lavie, P. Sleep Disturbances in the Wake of Traumatic Events. N. Engl. J. Med. 2001, 345, 1825–1832. [Google Scholar] [CrossRef]
- Bryant, R.A.; Creamer, M.; O’Donnell, M.; Silove, D.; McFarlane, A.C. Sleep Disturbance Immediately Prior to Trauma Predicts Subsequent Psychiatric Disorder. Sleep 2010, 33, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhao, N. Generalized anxiety disorder, depressive symptoms and sleep quality during COVID-19 outbreak in China: A web-based cross-sectional survey. Psychiatry Res. 2020, 288, 112954. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, F.; Wei, C.; Jia, Y.; Shang, Z.; Sun, L.; Wu, L.; Sun, Z.; Zhou, Y.; Wang, Y.; et al. Prevalence and predictors of PTSS during COVID-19 outbreak in China hardest-hit areas: Gender differences matter. Psychiatry Res. 2020, 287, 112921. [Google Scholar] [CrossRef]
- Yin, Q.; Sun, Z.; Liu, T.; Ni, X.; Deng, X.; Jia, Y.; Shang, Z.; Zhou, Y.; Liu, W. Posttraumatic stress symptoms of health care workers during the corona virus disease 2019. Clin. Psychol. Psychother. 2020, 27, 384–395. [Google Scholar] [CrossRef]
- Rajkumar, R.P. COVID-19 and mental health: A review of the existing literature. Asian J. Psychiatry 2020, 52, 102066. [Google Scholar] [CrossRef]
- Mellman, T.A.; Kobayashi, I.; LaVela, J.; Wilson, B.; Brown, T.S.H. A Relationship between REM Sleep Measures and the Duration of Posttraumatic Stress Disorder in a Young Adult Urban Minority Population. Sleep 2014, 37, 1321–1326. [Google Scholar] [CrossRef]
- Ross, R.J. The Changing REM Sleep Signature of Posttraumatic Stress Disorder. Sleep 2014, 37, 1281–1282. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.J.; Ball, W.A.; Dinges, D.F.; Kribbs, N.B.; Morrison, A.R.; Silver, S.M.; Mulvaney, F.D. Rapid eye movement sleep disturbance in posttraumatic stress disorder. Biol. Psychiatry 1994, 35, 195–202. [Google Scholar] [CrossRef]
- Cousens, G.; Otto, T. Both pre- and posttraining excitotoxic lesions of the basolateral amygdala abolish the expression of olfactory and contextual fear conditioning. Behav. Neurosci. 1998, 112, 1092–1103. [Google Scholar] [CrossRef]
- Koo, J.W.; Han, J.-S.; Kim, J.J. Selective Neurotoxic Lesions of Basolateral and Central Nuclei of the Amygdala Produce Differential Effects on Fear Conditioning. J. Neurosci. 2004, 24, 7654–7662. [Google Scholar] [CrossRef] [PubMed]
- Maren, S.; Aharonov, G.; Fanselow, M.S. Retrograde Abolition of Conditional Fear after Excitotoxic Lesions in the Ba-solateral Amygdala of Rats: Absence of a Temporal Gradient. Behav. Neurosci. 1996, 110, 718–726. [Google Scholar] [CrossRef]
- Muller, J.; Corodimas, K.P.; Fridel, Z.; LeDoux, J.E. Functional Inactivation of the Lateral and Basal Nuclei of the Amygdala by Muscimol Infusion Prevents Fear Conditioning to an Explicit Conditioned Stimulus and to Contextual Stimuli. Behav. Neurosci. 1997, 111, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, B.; Lorenzini, C.A.; Baldi, E.; Tassoni, G.; Bucherelli, C. Auditory Thalamus, Dorsal Hippocampus, Basolateral Amygdala, and Perirhinal Cortex Role in the Consolidation of Conditioned Freezing to Context and to Acoustic Conditioned Stimulus in the Rat. J. Neurosci. 1999, 19, 9570–9578. [Google Scholar] [CrossRef] [PubMed]
- Wellman, L.L.; Fitzpatrick, M.E.; Hallum, O.Y.; Sutton, A.M.; Williams, B.L.; Sanford, L.D. Individual Differences in Animal Stress Models: Considering Resilience, Vulnerability, and the Amygdala in Mediating the Effects of Stress and Conditioned Fear on Sleep. Sleep 2016, 39, 1293–1303. [Google Scholar] [CrossRef]
- Wellman, L.L.; Fitzpatrick, M.E.; Hallum, O.Y.; Sutton, A.M.; Williams, B.L.; Sanford, L.D. The basolateral amygdala can mediate the effects of fear memory on sleep independently of fear behavior and the peripheral stress response. Neurobiol. Learn. Mem. 2017, 137, 27–35. [Google Scholar] [CrossRef]
- Wellman, L.L.; Fitzpatrick, M.E.; Sutton, A.M.; Williams, B.L.; Machida, M.; Sanford, L.D. Antagonism of corticotropin releasing factor in the basolateral amygdala of resilient and vulnerable rats: Effects on fear-conditioned sleep, temperature and freezing. Horm. Behav. 2018, 100, 20–28. [Google Scholar] [CrossRef]
- Wellman, L.L.; Fitzpatrick, M.E.; Machida, M.; Sanford, L.D. The basolateral amygdala determines the effects of fear memory on sleep in an animal model of PTSD. Exp. Brain Res. 2014, 232, 1555–1565. [Google Scholar] [CrossRef] [PubMed]
- Murkar, A.L.; De Koninck, J. Consolidative mechanisms of emotional processing in REM sleep and PTSD. Sleep Med. Rev. 2018, 41, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Balakathiresan, N.S.; Sharma, A.; Chandran, R.; Bhomia, M.; Zhang, Z.; Wang, K.K.W.; Maheshwari, R.K. Molecular Mech-anisms and Biomarker Perspective of Micrornas in Traumatic Brain Injury. In Biomarkers of Brain Injury and Neurological Disorders; Wang, K.K.W., Zhang, Z., Kobeissy, F.H., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 76–115. [Google Scholar]
- Brown, B.D.; Naldini, L. Exploiting and antagonizing microRNA regulation for therapeutic and experimental applications. Nat. Rev. Genet. 2009, 10, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Sayed, D.; Abdellatif, M. MicroRNAs in Development and Disease. Physiol. Rev. 2011, 91, 827–887. [Google Scholar] [CrossRef]
- Hrdlickova, B.; de Almeida, R.C.; Borek, Z.; Withoff, S. Genetic variation in the non-coding genome: Involvement of micro-RNAs and long non-coding RNAs in disease. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2014, 1842, 1910–1922. [Google Scholar] [CrossRef] [PubMed]
- Balakathiresan, N.S.; Bhomia, M.; Chandran, R.; Chavko, M.; McCarron, R.M.; Maheshwari, R.K. MicroRNA Let-7i Is a Promising Serum Biomarker for Blast-Induced Traumatic Brain Injury. J. Neurotrauma 2012, 29, 1379–1387. [Google Scholar] [CrossRef]
- Balakathiresan, N.S.; Chandran, R.; Bhomia, M.; Jia, M.; Li, H.; Maheshwari, R.K. Serum and amygdala microRNA signatures of posttraumatic stress: Fear correlation and biomarker potential. J. Psychiatr. Res. 2014, 57, 65–73. [Google Scholar] [CrossRef]
- Bhomia, M.; Balakathiresan, N.S.; Wang, K.K.; Papa, L.; Maheshwari, R.K. A Panel of Serum MiRNA Biomarkers for the Diagnosis of Severe to Mild Traumatic Brain Injury in Humans. Sci. Rep. 2016, 6, 28148. [Google Scholar] [CrossRef] [PubMed]
- Bam, M.; Yang, X.; Zumbrun, E.E.; Zhong, Y.; Zhou, J.; Ginsberg, J.P.; Leyden, Q.; Zhang, J.; Nagarkatti, P.S.; Nagarkatti, M. Dysregulated immune system networks in war veterans with PTSD is an outcome of altered miRNA expression and DNA methylation. Sci. Rep. 2016, 6, 31209. [Google Scholar] [CrossRef] [PubMed]
- Bam, M.; Yang, X.; Zumbrun, E.E.; Ginsberg, J.P.; Leyden, Q.; Zhang, J.; Nagarkatti, P.S.; Nagarkatti, M. Decreased AGO2 and DCR1 in PBMCs from War Veterans with PTSD leads to diminished miRNA resulting in elevated inflammation. Transl. Psychiatry 2017, 7, e1222. [Google Scholar] [CrossRef] [PubMed]
- Hunsberger, J.G.; Fessler, E.B.; Chibane, F.L.; Leng, Y.; Maric, A.; Elkahloun, A.G.; Chuang, D.-M. Mood stabilizer-regulated miRNAs in neuropsychiatric and neurodegenerative diseases: Identifying associations and functions. Am. J. Transl. Res. 2013, 5, 450–464. [Google Scholar]
- Jung, S.H.; Wang, Y.; Kim, T.; Tarr, A.; Reader, B.; Powell, N.; Sheridan, J.F. Molecular mechanisms of repeated social defeat-induced glucocorticoid resistance: Role of microRNA. Brain, Behav. Immun. 2015, 44, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Xu, A.; Cui, S.; Sun, M.-R.; Xue, Y.-C.; Wang, J.-H. Impaired GABA synthesis, uptake and release are associated with depression-like behaviors induced by chronic mild stress. Transl. Psychiatry 2016, 6, e910. [Google Scholar] [CrossRef]
- Li, C.; Liu, Y.; Liu, D.; Jiang, H.; Pan, F. Dynamic Alterations of Mir-34c Expression in the Hypo-thalamus of Male Rats after Early Adolescent Traumatic Stress. Neural Plast. 2016, 2016, 8. [Google Scholar] [CrossRef]
- Fanselow, M.S.; LeDoux, J.E. Why We Think Plasticity Underlying Pavlovian Fear Conditioning Occurs in the Basolateral Amygdala. Neuron 1999, 23, 229–232. [Google Scholar] [CrossRef]
- Nader, K.; Schafe, G.E.; Le Doux, J.E. Fear Memories Require Protein Synthesis in the Amygdala for Re-consolidation after Retrieval. Nature 2000, 406, 722–726. [Google Scholar] [CrossRef]
- Gallo Cantafio, M.E.; Nielsen, B.S.; Mignogna, C.; Arbitrio, M.; Botta, C.; Frandsen, N.M.; Rolfo, C.; Tagliaferri, P.; Tassone, P.; di Martino, M.T. Pharmacokinetics and Pharmacodynamics of a 13-Mer Lna-Inhibitor-Mir-221 in Mice and Non-Human Primates. Mol. Ther. Nucleic Acids 2016, 5, 6. [Google Scholar] [CrossRef]
- Shen, M.; Song, Z.; Wang, J.H. Microrna and Mrna Profiles in the Amygdala Are Associated with Stress-Induced De-pression and Resilience in Juvenile Mice. Psychopharmacology 2019, 236, 2119–2142. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, W.; Du, K.; Wang, J.-H. microRNA and mRNA profiles in the amygdala are relevant to fear memory induced by physical or psychological stress. J. Neurophysiol. 2019, 122, 1002–1022. [Google Scholar] [CrossRef] [PubMed]
- Felmingham, K.L.; Dobson-Stone, C.; Schofield, P.R.; Quirk, G.J.; Bryant, R.A. The Brain-Derived Neurotrophic Factor Val66Met Polymorphism Predicts Response to Exposure Therapy in Posttraumatic Stress Disorder. Biol. Psychiatry 2013, 73, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Shumyatsky, G.P.; Malleret, G.; Shin, R.-M.; Takizawa, S.; Tully, K.; Tsvetkov, E.; Zakharenko, S.S.; Joseph, J.; Vronskaya, S.; Yin, D.; et al. stathmin, a Gene Enriched in the Amygdala, Controls Both Learned and Innate Fear. Cell 2005, 123, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tang, X.; Sanford, L.D. Fear-conditioned suppression of REM sleep: Relationship to Fos expression patterns in limbic and brainstem regions in BALB/cJ mice. Brain Res. 2003, 991, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Snijders, C.; Krauskopf, J.; Pishva, E.; Eijssen, L.; Machiels, B.; Kleinjans, J.; Kenis, G.; Hove, D.V.D.; Kim, M.O.; Boks, M.P.M.; et al. Circulating Serum MicroRNAs as Potential Diagnostic Biomarkers of Posttraumatic Stress Disorder: A Pilot Study. Front. Genet. 2019, 10, 1042. [Google Scholar] [CrossRef]
- Sweeten, B.L.W.; Sutton, A.M.; Wellman, L.L.; Sanford, L.D. Predicting Stress Resilience and Vulnerability: Brain-Derived Neurotrophic Factor and Rapid Eye Movement Sleep as Potential Biomarkers of Individual Stress Responses. Sleep 2019, 43. [Google Scholar] [CrossRef] [PubMed]




| S# | Detector | miRBase Accession | Fold Change | p Value |
|---|---|---|---|---|
| Stress Vulnerable (ST-Vul) | ||||
| 1 | miR-503 | MIMAT0004790 | 160.43 | 0.003 |
| 2 | miR-330 | MIMAT0000568 | 120.59 | 0.0005 |
| 3 | miR-331 | MIMAT0004643 | 43.96 | 0.02 |
| 4 | miR-431 | MIMAT0001626 | 28.87 | 0.04 |
| 5 | miR-136 | MIMAT0004532 | 9.93 | 0.02 |
| 6 | miR-9 | MIMAT0000781 | 9.69 | 0.01 |
| 7 | miR-455 | MIMAT0003742 | −7.54 | 0.03 |
| 8 | miR-203 | MIMAT0000876 | −8.24 | 0.05 |
| 9 | miR-381 | MIMAT0003199 | −10.36 | 0.03 |
| 10 | miR-185 | MIMAT0000862 | −13.72 | 0.05 |
| 11 | miR-126 | MIMAT0000831 | −23.55 | 0.02 |
| 12 | miR-181a | MIMAT0000858 | −27.80 | 0.04 |
| Stress Resilient (ST-Res) | ||||
| 1 | miR-126 | MIMAT0000831 | −18.49 | 0.03 |
| 2 | miR-185 | MIMAT0000862 | −23.94 | 0.01 |
| 3 | miR-344 | MIMAT0000592 | −119.02 | 0.008 |
| S# | Detector | miRBase Accession | Fold Change | p Value |
|---|---|---|---|---|
| Context Re-exposure Vulnerable (CTX-Vul) | ||||
| 1 | miR-345 | MIMAT0000595 | 105.86 | 0.003 |
| 2 | miR-24 | MIMAT0005441 | 103.29 | 0.0004 |
| 3 | miR-431 | MIMAT0001626 | 98.90 | 0.01 |
| 4 | miR-93 | MIMAT0000817 | 83.27 | 0.004 |
| 5 | miR-490 | MIMAT0012823 | 55.29 | 0.01 |
| 6 | miR-382 | MIMAT0003201 | 49.48 | 0.01 |
| 7 | miR-15b | MIMAT0000784 | 46.86 | 0.003 |
| 8 | miR-17 | MIMAT0000786 | 44.21 | 0.02 |
| 9 | miR-339 | MIMAT0004648 | 43.93 | 0.02 |
| 10 | miR-187 | MIMAT0000864 | 35.86 | 0.02 |
| 11 | miR-339 | MIMAT0000583 | 34.64 | 0.01 |
| 12 | miR-20b | MIMAT0003211 | 26.60 | 0.03 |
| 13 | miR-331 | MIMAT0004643 | 22.05 | 0.03 |
| 14 | miR-351 | MIMAT0000609 | 17.87 | 0.04 |
| 15 | miR-330 | MIMAT0004641 | 16.18 | 0.05 |
| 16 | let-7a | MIMAT0005439 | 14.00 | 0.03 |
| 17 | miR-221 | MIMAT0000890 | 10.11 | 0.03 |
| 18 | miR-24 | MIMAT0000218 | −12.57 | 0.05 |
| 19 | miR-28 | MIMAT0004661 | −193.43 | 0.002 |
| Context Re-exposure Resilient (CTX-Res) | ||||
| 1 | miR-345 | MIMAT0000595 | 36.07 | 0.02 |
| 2 | miR-431 | MIMAT0001626 | 23.70 | 0.05 |
| 3 | miR-296 | MIMAT0000898 | −14.47 | 0.02 |
| 4 | miR-761 | MIMAT0012853 | −19.23 | 0.04 |
| 5 | miR-24 | MIMAT0000218 | −40.31 | 0.01 |
| 6 | miR-181a | MIMAT0000858 | −45.72 | 0.02 |
| 7 | miR-28 | MIMAT0004661 | −15351.58 | 0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balakathiresan, N.S.; Bhomia, M.; Zhai, M.; Sweeten, B.L.W.; Wellman, L.L.; Sanford, L.D.; Knollmann-Ritschel, B. MicroRNAs in Basolateral Amygdala Associated with Stress and Fear Memories Regulate Rapid Eye Movement Sleep in Rats. Brain Sci. 2021, 11, 489. https://doi.org/10.3390/brainsci11040489
Balakathiresan NS, Bhomia M, Zhai M, Sweeten BLW, Wellman LL, Sanford LD, Knollmann-Ritschel B. MicroRNAs in Basolateral Amygdala Associated with Stress and Fear Memories Regulate Rapid Eye Movement Sleep in Rats. Brain Sciences. 2021; 11(4):489. https://doi.org/10.3390/brainsci11040489
Chicago/Turabian StyleBalakathiresan, Nagaraja S., Manish Bhomia, Min Zhai, Brook L. W. Sweeten, Laurie L. Wellman, Larry D. Sanford, and Barbara Knollmann-Ritschel. 2021. "MicroRNAs in Basolateral Amygdala Associated with Stress and Fear Memories Regulate Rapid Eye Movement Sleep in Rats" Brain Sciences 11, no. 4: 489. https://doi.org/10.3390/brainsci11040489
APA StyleBalakathiresan, N. S., Bhomia, M., Zhai, M., Sweeten, B. L. W., Wellman, L. L., Sanford, L. D., & Knollmann-Ritschel, B. (2021). MicroRNAs in Basolateral Amygdala Associated with Stress and Fear Memories Regulate Rapid Eye Movement Sleep in Rats. Brain Sciences, 11(4), 489. https://doi.org/10.3390/brainsci11040489







