Inhibitory Control on a Stop Signal Task in Tourette Syndrome before and after Deep Brain Stimulation of the Internal Segment of the Globus Pallidus
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Design and Procedure
2.3. Stop Signal Task
2.4. Statistical Analysis
3. Results
3.1. Comparison of TS before DBS Surgery with Age-Matched Healthy Controls
3.2. Effect of DBS Surgery on Inhibitory Control on the Stop Signal Task—Within-Subjects Comparison
3.3. Effect of DBS Surgery—Comparison of Post-DBS Data of TS Patients with Healthy Controls
4. Discussion
4.1. Motor Inhibition in TS: Comparison of TS before DBS Surgery with Healthy Controls
4.2. Effect of GPi-DBS Surgery on Motor Inhibition in TS
4.3. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Jahanshahi, M.; Obeso, I.; Rothwell, J.C.; Obeso, J.A. A fronto-striato-subthalamic-pallidal network for goal-directed and habitual inhibition. Nat. Rev. Neurosci. 2015, 16, 719–732. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar] [CrossRef]
- Mink, J.W. Neurobiology of basal ganglia circuits in Tourette syndrome: Faulty inhibition of unwanted motor patterns? Adv. Inneurol. 2001, 85, 113–122. [Google Scholar]
- McNaught, K.S.P.; Mink, J.W. Advances in understanding and treatment of Tourette syndrome. Nat. Rev. Neurol. 2011, 7, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Kalanithi, P.S.A.; Zheng, W.; Kataoka, Y.; DiFiglia, M.; Grantz, H.; Saper, C.B.; Schwartz, M.L.; Leckman, J.F.; Vaccarino, F.M. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proc. Natl. Acad. Sci. USA 2005, 102, 13307–13312. [Google Scholar] [CrossRef]
- Worbe, Y.; Marrakchi-Kacem, L.; Lecomte, S.; Valabregue, R.; Poupon, F.; Guevara, P.; Tucholka, A.; Mangin, J.F.; Vidailhet, M.; Lehericy, S.; et al. Altered structural connectivity of cortico-striato-pallido-thalamic networks in Gilles de la Tourette syndrome. Brain 2015, 138 Pt 2, 472–482. [Google Scholar] [CrossRef]
- Jahanshahi, M.; Rothwell, J.C. Inhibitory dysfunction contributes to some of the motor and non-motor symptoms of movement disorders and psychiatric disorders. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2017, 372, 20160198. [Google Scholar] [CrossRef]
- Kefalopoulou, Z.; Zrinzo, L.; Jahanshahi, M.; Candelario, J.; Milabo, C.; Beigi, M.; Akram, H.; Hyam, J.; Clayton, J.; Kass-Iliyya, L.; et al. Bilateral globus pallidus stimulation for severe tourette’s syndrome: A double-blind, randomised crossover trial. Lancet Neurol. 2015, 14, 595–605. [Google Scholar] [CrossRef]
- Logan, G.D.; Cowan, W.B. On the ability to inhibit thought and action: A theory of an act of control. Psychol. Rev. 1984, 91, 295–327. [Google Scholar] [CrossRef]
- Obeso, I.; Wilkinson, L.; Rodríguez-Oroz, M.C.; Obeso, J.A.; Jahanshahi, M. Bilateral stimulation of the subthalamic nucleus has differential effects on reactive and proactive inhibition and conflict-induced slowing in Parkinson’s disease. Exp. Brain Res. 2013, 226, 451–462. [Google Scholar] [CrossRef]
- Roessner, V.; Albrecht, B.; Dechent, P.; Baudewig, J.; Rothenberger, A. Normal response inhibition in boys with Tourette syndrome. Behav. Brain Funct. 2008, 4. [Google Scholar] [CrossRef]
- Kantini, E.; Cassaday, H.J.; Hollis, C.; Jackson, G.M. The normal inhibition of associations is impaired by clonidine in Tourette syndrome. J. Can. Acad. Child Adolesc. Psychiatry 2011, 20, 96–106. Available online: https://www.ncbi.nlm.nih.gov/pubmed/21541098 (accessed on 28 June 2014).
- Jung, J.; Jackson, S.R.; Parkinson, A.; Jackson, G.M. Cognitive control over motor output in Tourette syndrome. Neurosci. Biobehav. Rev. 2013, 37, 1016–1025. [Google Scholar] [CrossRef]
- Johannes, S.; Wieringa, B.M.; Mantey, M.; Nager, W.; Rada, D.; Müller-Vahl, K.R.; Emrich, H.M.; Dengler, R.; Münte, T.F.; Dietrich, D. Altered inhibition of motor responses in Tourette Syndrome and Obsessive-Compulsive Disorder. Acta Neurol. Scand. 2001, 104, 36–43. [Google Scholar] [CrossRef]
- Jackson, G.M.; Mueller, S.C.; Hambleton, K.; Hollis, C.P. Enhanced cognitive control in Tourette Syndrome during task uncertainty. Exp. Brain Res. 2007, 182, 357–364. [Google Scholar] [CrossRef]
- Mueller, S.C.; Jackson, G.M.; Dhalla, R.; Datsopoulos, S.; Hollis, C.P. Enhanced cognitive control in young people with Tourette’s syndrome. Curr. Biol. 2006, 16, 570–573. [Google Scholar] [CrossRef]
- Eichele, H.; Eichele, T.; Hammar, Å.; Freyberger, H.J.; Hugdahl, K.; Plessen, K.J. Go/NoGo performance in boys with Tourette syndrome. Child Neuropsychol. 2010, 16, 162–168. [Google Scholar] [CrossRef]
- Li, C.S.R.; Chang, H.L.; Hsu, Y.P.; Wang, H.S.; Ko, N.C. Motor response inhibition in children with Tourette’s disorder. J. Neuropsychiatry Clin. Neurosci. Off. J. Am. Neuropsychiatr. Assoc. 2006, 18, 417–419. [Google Scholar] [CrossRef]
- Mancini, C.; Cardona, F.; Baglioni, V.; Panunzi, S.; Pantano, P.; Suppa, A.; Mirabella, G. Inhibition is impaired in children with obsessive-compulsive symptoms but not in those with tics. Mov. Disord. 2018, 33, 950–959. [Google Scholar] [CrossRef]
- Rawji, V.; Modi, S.; Latorre, A.; Rocchi, L.; Hockey, L.; Bhatia, K.; Joyce, E.; Rothwell, J.C.; Jahanshahi, M. Impaired automatic but intact volitional inhibition in primary tic disorders. Brain 2020, 143, 906–919. [Google Scholar] [CrossRef]
- Mirabella, G.; Upadhyay, N.; Mancini, C.; Giannì, C.; Panunzi, S.; Petsas, N.; Suppa, A.; Cordona, F.; Pantano, P. Corrigendum to “loss in grey matter in a small network of brain areas underpins poor reactive inhibition in obsessive-compulsive disorder patients.”. Psychiatry Res. Neuroimaging Sect. 2020, 305. [Google Scholar] [CrossRef]
- Jackson, S.R.; Parkinson, A.; Jung, J.; Ryan, S.E.; Morgan, P.S.; Hollis, C.; Jackson, G.M. Compensatory neural reorganization in Tourette syndrome. Curr. Biol. 2011, 21, 580–585. [Google Scholar] [CrossRef] [PubMed]

| P | Sex | Age at Onset | Age at Surgery | Comorbidities | Stimulation Location | YGTSS Pre | YGTSS Post | GTS-QoL Pre | GTS-QoL Post |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 8 | 24 | Anxiety | Posteroventral pallidum | 87 | 63 | 82.4 | 74 |
| 2 | M | 11 | 22 | Anxiety | Anteromedial pallidum | 81 | 66 | 79.6 | 47.2 |
| 3 | M | 6 | 26 | Obsessive Compulsive Behaviours (OCB) | Anteromedial pallidum | 93 | 48 | 67.6 | 39.8 |
| 4 | F | 12 | 60 | Depression | Anteromedial pallidum | 93 | 49 | 62 | 14.8 |
| 5 | M | 7 | 25 | OCB | Anteromedial pallidum | 80 | 62 | 40.7 | 43.5 |
| 6 | M | 3 | 34 | ADHD, OCD | GPi | 93 | 58 | N/A | 18.5 |
| 7 | M | 6 | 21 | Mild learning difficulties, traumatic myelopathy secondary to tics | GPi | 94 | 83 | 78.7 | 47.2 |
| 8 | F | 10 | 25 | Nil | GPi | 94 | 37 | 63.9 | 28.7 |
| 9 | M | 6 | 39 | Traumatic myelopathy secondary to tics | GPi | 63 | 29 | N/A | 16.7 |
| 10 | M | 9 | 60 | Segmental dystonia, probably secondary to neuroleptic use | GPi | 60 | 22 | 42.6 | 41.7 |
| 11 | M | 6 | 18 | OCD | GPi | 94 | 51 | 80.6 | 47.2 |
| 12 | M | 7 | 32 | OCD, Anxiety | GPi | 74 | 74 | 81.5 | 82.4 |
| 13 | F | 9 | 55 | Nil | GPi | 93 | 4 | 47.2 | 1.9 |
| 14 | M | - | 38 | OCD, Anxiety, Depression | GPi | 82 | 47 | 52.8 | 48.2 |
| Mean (SD) | 7.69 (2.46) | 34.21 (14.5) | 85.38 (11.69) | 58.67 (21.16) | 65.5 (15.94) | 47.7 (22.2) |
| Measure | TS Patients (N = 9) Mean (SD) | Controls (N = 8) Mean (SD) | p-Value (One-Tailed) | Effect Sizes (Cohen’s d) |
|---|---|---|---|---|
| GoRT ms | 505.35 (55.41) | 403.50 (70.89) | 0.001 | 1.601 |
| % StopInhibit | 54.51 (3.08) | 49.48 (3.52) | 0.001 | 1.528 |
| StopRespond RT ms | 414.27 (30.45) | 351.25 (55.76) | 0.002 | 1.403 |
| SSD ms | 280.81 (69.37) | 165.63 (83.16) | 0.001 | 1.504 |
| SSRT ms | 224.54 (67.77) | 237.87 (19.08) | 0.071 | 0.268 |
| Omission Errors | 8.44 (23.48) | 1.00 (1.41) | 0.069 | 0.447 |
| Discrimination Errors | 6.67 (5.55) | 5.25 (6.41) | 0.012 | 0.237 |
| Measure | Pre DBS-GPi (N = 6) Mean (SD) | Post DBS-GPi (N = 6) Mean (SD) | p-Value (One-Tailed) | Effect Sizes (Cohen’s d) |
|---|---|---|---|---|
| GoRT ms | 516.06 (61.00) | 496.02 (56.13) | 0.3 | 0.342 |
| % StopInhibit | 54.69 (2.61) | 53.13 (2.55) | 0.147 | 0.605 |
| StopRespond RT ms | 417.65 (33.28) | 399.06 (36.11) | 0.173 | 0.535 |
| SSD ms | 295.86 (65.73) | 260.67 (70.73) | 0.173 | 0.515 |
| SSRT ms | 220.19 (77.80) | 235.35 (50.95) | 0.231 | 0.231 |
| Omission Errors | 12.50 (28.68) | 1.00 (1.55) | 0.297 | 0.567 |
| Discrimination Errors | 6.5 (6.75) | 5.50 (6.06) | 0.342 | 0.156 |
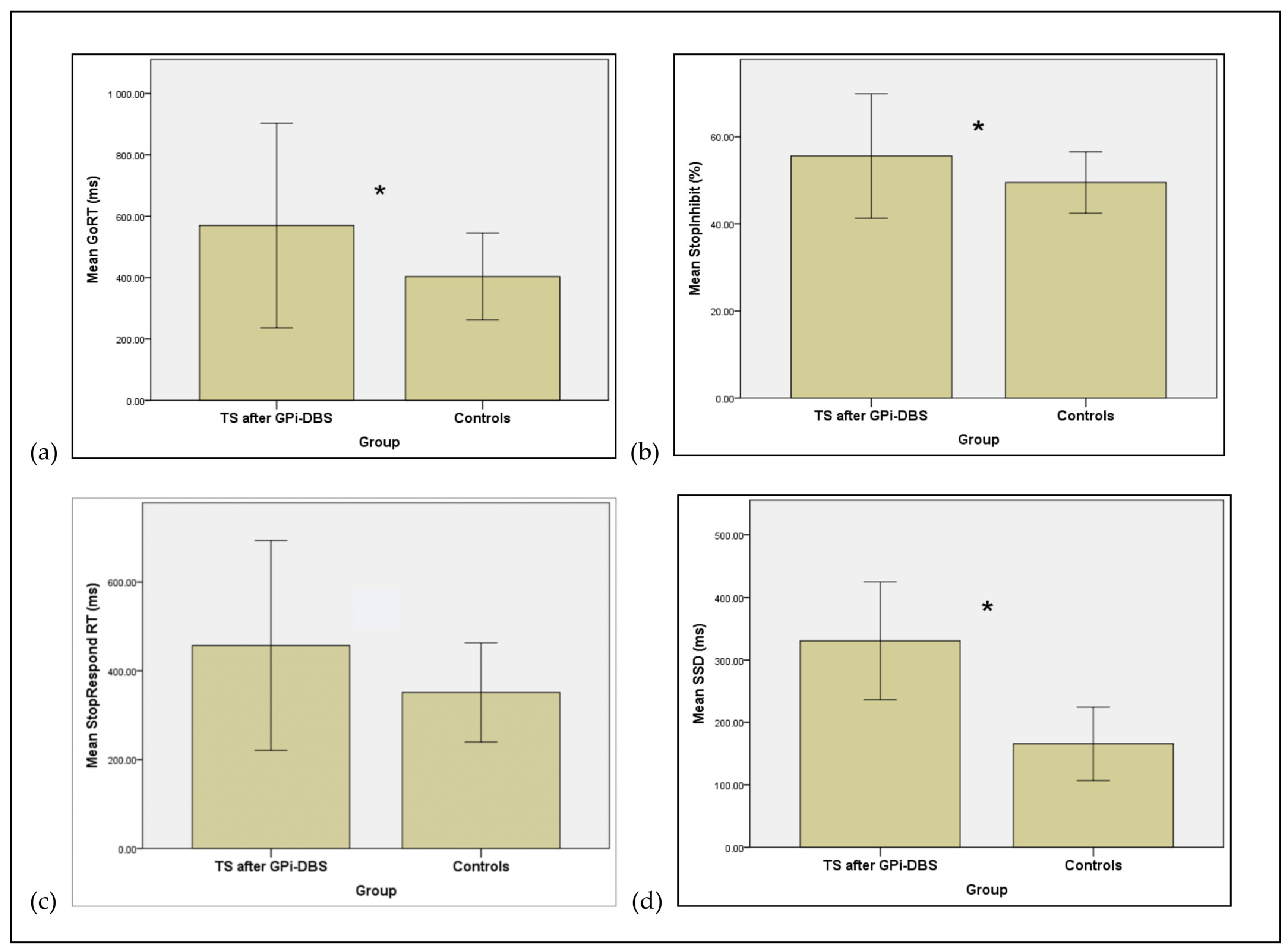
| Measure | TS Post-Surgery (N = 11) Mean (SD) | Controls (N = 8) Mean (SD) | p-Value (One-Tailed) | Effect Sizes (Cohen’s d) |
|---|---|---|---|---|
| Go RT ms | 569.60 (166.77) | 403.50 (70.90) | 0.001 | 1.296 |
| % StopInhibit | 55.59 (7.14) | 49.48 (3.52) | 0.001 | 1.09 |
| StopRespond RT ms | 456.79 (117.90) | 351.25 (55.76) | 0.004 | 1.144 |
| SSD ms | 330.89 (156.41) | 165.63 (83.16) | 0.001 | 1.319 |
| SSRT ms | 238.72 (51.25) | 237.87 (19.08) | 0.06 | 0.022 |
| Omission Errors | 0.91 (1.38) | 1.00 (1.41) | 0.06 | 0.065 |
| Discrimination Errors | 3.73 (4.82) | 5.25 (6.41) | 0.065 | 0.268 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morreale, F.; Kefalopoulou, Z.; Zrinzo, L.; Limousin, P.; Joyce, E.; Foltynie, T.; Jahanshahi, M. Inhibitory Control on a Stop Signal Task in Tourette Syndrome before and after Deep Brain Stimulation of the Internal Segment of the Globus Pallidus. Brain Sci. 2021, 11, 461. https://doi.org/10.3390/brainsci11040461
Morreale F, Kefalopoulou Z, Zrinzo L, Limousin P, Joyce E, Foltynie T, Jahanshahi M. Inhibitory Control on a Stop Signal Task in Tourette Syndrome before and after Deep Brain Stimulation of the Internal Segment of the Globus Pallidus. Brain Sciences. 2021; 11(4):461. https://doi.org/10.3390/brainsci11040461
Chicago/Turabian StyleMorreale, Francesca, Zinovia Kefalopoulou, Ludvic Zrinzo, Patricia Limousin, Eileen Joyce, Tom Foltynie, and Marjan Jahanshahi. 2021. "Inhibitory Control on a Stop Signal Task in Tourette Syndrome before and after Deep Brain Stimulation of the Internal Segment of the Globus Pallidus" Brain Sciences 11, no. 4: 461. https://doi.org/10.3390/brainsci11040461
APA StyleMorreale, F., Kefalopoulou, Z., Zrinzo, L., Limousin, P., Joyce, E., Foltynie, T., & Jahanshahi, M. (2021). Inhibitory Control on a Stop Signal Task in Tourette Syndrome before and after Deep Brain Stimulation of the Internal Segment of the Globus Pallidus. Brain Sciences, 11(4), 461. https://doi.org/10.3390/brainsci11040461






