How Human Single-Neuron Recordings Can Help Us Understand Cognition: Insights from Memory Studies
Abstract
1. Introduction
2. Human Single-Neuron Recordings
3. Grandmother, Gnostic and Concept Cells
4. Insights from Human Single-Neuron Recordings to Long-Term Memory (LTM)
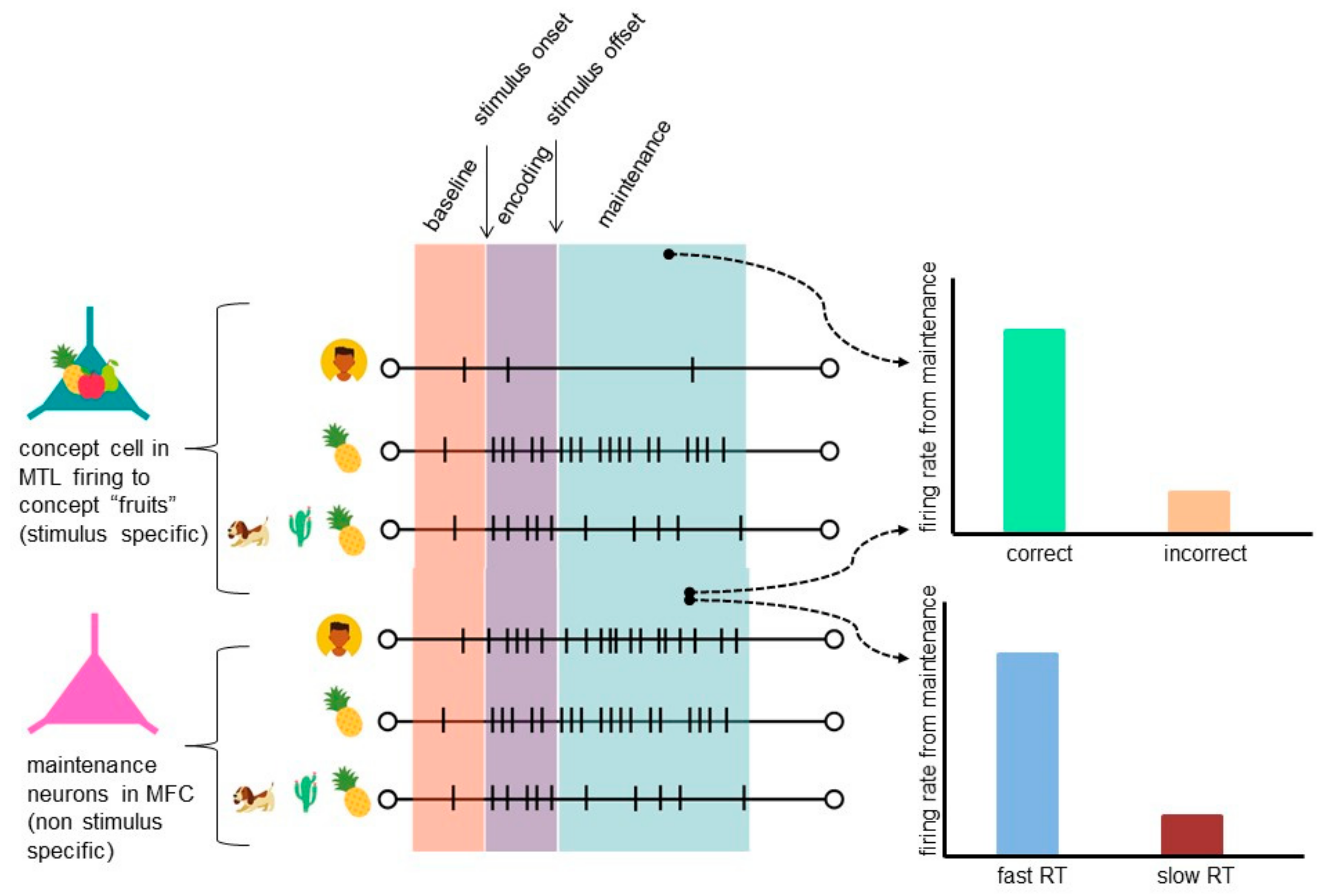
5. Insights from Human Single-Neuron Recordings to Working Memory (WM)
6. How Human Single-Neuron Recordings Can Help Us Understand Cognition
7. Human Single-Neuron Recordings of Pathological Brain
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baddeley, A.; Hitch, G. Working Memory. Psychol. Learn. Motiv. 1974, 8, 47–88. [Google Scholar] [CrossRef]
- Cowan, N.; Elliott, E.M.; Saults, S.J.; Morey, C.C.; Mattox, S.; Hismjatullina, A.; Conway, A.R.A. On the capacity of attention: Its estimation and its role in working memory and cognitive aptitudes. Cogn. Psychol. 2005, 51, 42–100. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Christ, S.E.; Cowan, N. Domain-general and domain-specific functional networks in working memory. Neuroimage 2014, 102, 646–656. [Google Scholar] [CrossRef]
- Oberauer, K. Access to Information in Working Memory: Exploring the Focus of Attention. J. Exp. Psychol. Learn. Mem. Cogn. 2002, 28, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Burle, B.; Spieser, L.; Roger, C.; Casini, L.; Hasbroucq, T.; Vidal, F. Spatial and temporal resolutions of EEG: Is it really black and white? A scalp current density view. Int. J. Psychophysiol. 2015, 97, 210–220. [Google Scholar] [CrossRef]
- Bandettini, P.A. Neuronal or hemodynamic? Grappling with the functional MRI signal. Brain Connect. 2014, 4, 487–498. [Google Scholar] [CrossRef]
- Kim, S.G.; Ogawa, S. Biophysical and physiological origins of blood oxygenation level-dependent fMRI signals. J. Cereb. Blood Flow Metab. 2012, 32, 1188–1206. [Google Scholar] [CrossRef]
- Logothetis, N.K. What we can do and what we cannot do with fMRI. Nature 2008, 453, 869–878. [Google Scholar] [CrossRef]
- Buzsáki, G. The Brain from Inside Out; Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Quian Quiroga, R. Plugging in to Human Memory: Advantages, Challenges, and Insights from Human Single-Neuron Recordings. Cell 2019, 179, 1015–1032. [Google Scholar] [CrossRef] [PubMed]
- Quian Quiroga, R.; Kraskov, A.; Koch, C.; Fried, I. Explicit Encoding of Multimodal Percepts by Single Neurons in the Human Brain. Curr. Biol. 2009, 19, 1308–1313. [Google Scholar] [CrossRef] [PubMed]
- Quian Quiroga, R.; Reddy, L.; Kreiman, G.; Koch, C.; Fried, I. Invariant visual representation by single neurons in the human brain. Nature 2005, 435, 1102–1107. [Google Scholar] [CrossRef]
- Fried, I.; Wilson, C.L.; Maidment, N.T.; Engel, J.; Behnke, E.; Fields, T.A.; Macdonald, K.A.; Morrow, J.W.; Ackerson, L. Cerebral microdialysis combined with single-neuron and electroencephalographic recording in neurosurgical patients: Technical note. J. Neurosurg. 1999, 91, 697–705. [Google Scholar] [CrossRef]
- Mian, M.K.; Sheth, S.A.; Patel, S.R.; Spiliopoulos, K.; Eskandar, E.N.; Williams, Z.M. Encoding of rules by neurons in the human dorsolateral prefrontal cortex. Cereb. Cortex 2014, 24, 807–816. [Google Scholar] [CrossRef]
- Jamali, M.; Grannan, B.; Haroush, K.; Moses, Z.B.; Eskandar, E.N.; Herrington, T.; Patel, S.; Williams, Z.M. Dorsolateral prefrontal neurons mediate subjective decisions and their variation in humans. Nat. Neurosci. 2019, 22, 1010–1020. [Google Scholar] [CrossRef]
- Kamiński, J.; Mamelak, A.N.; Birch, K.; Mosher, C.P.; Tagliati, M.; Rutishauser, U. Novelty-Sensitive Dopaminergic Neurons in the Human Substantia Nigra Predict Success of Declarative Memory Formation. Curr. Biol. 2018, 28, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Zaghloul, K.A.; Blanco, J.A.; Weidemann, C.T.; McGill, K.; Jaggi, J.L.; Baltuch, G.H.; Kahana, M.J. Human substantia nigra neurons encode unexpected financial rewards. Science 2009, 323, 1496–1499. [Google Scholar] [CrossRef]
- Rutishauser, U.; Aflalo, T.; Rosario, E.R.; Pouratian, N.; Andersen, R.A. Single-Neuron Representation of Memory Strength and Recognition Confidence in Left Human Posterior Parietal Cortex. Neuron 2018, 97, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Aflalo, T.; Zhang, C.Y.; Rosario, E.R.; Pouratian, N.; Orban, G.A.; Andersen, R.A. A shared neural substrate for action verbs and observed actions in human posterior parietal cortex. Sci. Adv. 2020, 6, eabb3984. [Google Scholar] [CrossRef] [PubMed]
- Rey, H.G.; Pedreira, C.; Quian Quiroga, R. Past, present and future of spike sorting techniques. Brain Res. Bull. 2015, 119, 106–117. [Google Scholar] [CrossRef]
- Gross, C.G. Genealogy of the “grandmother cell”. Neuroscientist 2002, 8, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Quian Quiroga, R.; Fried, I.; Koch, C. Brain cells for grandmother. Sci. Am. 2013, 308, 30–35. [Google Scholar] [CrossRef]
- Konorski, J. Integrative Activity of the Brain; University of Chicago Press: Chicago, IL, USA, 1967. [Google Scholar]
- Quian Quiroga, R. Concept cells: The building blocks of declarative memory functions. Nat. Rev. Neurosci. 2012, 13, 587–597. [Google Scholar] [CrossRef]
- De Falco, E.; Ison, M.J.; Fried, I.; Quian Quiroga, R. Long-term coding of personal and universal associations underlying the memory web in the human brain. Nat. Commun. 2016, 7, 1–11. [Google Scholar] [CrossRef]
- Quian Quiroga, R.; Kraskov, A.; Mormann, F.; Fried, I.; Koch, C. Single-Cell Responses to Face Adaptation in the Human Medial Temporal Lobe. Neuron 2014, 84, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Reber, T.P.; Faber, J.; Niediek, J.; Bostro, J.; Elger, C.E.; Mormann, F.; Elger, C.E.; Mormann, F. Single-Neuron Correlates of Conscious Perception in the Human Medial Temporal Lobe Report Single-Neuron Correlates of Conscious Perception in the Human Medial Temporal Lobe. Curr. Biol. 2017, 27, 1–8. [Google Scholar] [CrossRef]
- Ison, M.J.; Quian Quiroga, R.; Fried, I. Rapid Encoding of New Memories by Individual Neurons in the Human Brain. Neuron 2015, 87, 220–230. [Google Scholar] [CrossRef]
- Kamiński, J.; Sullivan, S.; Chung, J.M.; Ross, I.B.; Mamelak, A.N.; Rutishauser, U. Persistently active neurons in human medial frontal and medial temporal lobe support working memory. Nat. Neurosci. 2017, 20, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Kornblith, S.; Quian Quiroga, R.; Koch, C.; Fried, I.; Mormann, F. Persistent Single-Neuron Activity during Working Memory in the Human Medial Temporal Lobe. Curr. Biol. 2017, 27, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Corkin, S. What’s new with the amnesic patient H.M.? Nat. Rev. Neurosci. 2002, 3, 153–160. [Google Scholar] [CrossRef]
- Squire, L.R.; Stark, C.E.L.; Clark, R.E. The medial temporal lobe. Annu. Rev. Neurosci. 2004, 27, 279–306. [Google Scholar] [CrossRef]
- Squire, L.R.; Zola-Morgan, S. The medial temporal lobe memory system. Science 1991, 253, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Tulving, E.; Markowitsch, H.J. Episodic and declarative memory: Role of the hippocampus. Hippocampus 1998, 8, 198–204. [Google Scholar] [CrossRef]
- Eichenbaum, H.; Yonelinas, A.P.; Ranganath, C. The medial temporal lobe and recognition memory. Annu. Rev. Neurosci. 2007, 30, 123–152. [Google Scholar] [CrossRef]
- Borders, A.A.; Aly, M.; Parks, C.M.; Yonelinas, A.P. The hippocampus is particularly important for building associations across stimulus domains. Neuropsychologia 2017, 99, 335–342. [Google Scholar] [CrossRef]
- Brasted, P.J.; Bussey, T.J.; Murray, E.A.; Wise, S.P. Role of the hippocampal system in associative learning beyond the spatial domain. Brain 2003, 126, 1202–1223. [Google Scholar] [CrossRef]
- Mayes, A.; Montaldi, D.; Migo, E. Associative memory and the medial temporal lobes. Trends Cogn. Sci. 2007, 11, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Scoville, W.B.; Milner, R.B. Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry 1957, 20, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Lisman, J.E.; Grace, A.A. The hippocampal-VTA loop: Controlling the entry of information into long-term memory. Neuron 2005, 46, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Lisman, J.; Grace, A.A.; Duzel, E. A neoHebbian framework for episodic memory; role of dopamine-dependent late LTP. Trends Neurosci. 2011, 34, 536–547. [Google Scholar] [CrossRef]
- Rutishauser, U.; Mamelak, A.N.; Schuman, E.M. Single-trial learning of novel stimuli by individual neurons of the human hippocampus-amygdala complex. Neuron 2006, 49, 805–813. [Google Scholar] [CrossRef]
- Rutishauser, U.; Schuman, E.M.; Mamelak, A.N. Activity of human hippocampal and amygdala neurons during retrieval of declarative memories. Proc. Natl. Acad. Sci. USA 2008, 105, 11032. [Google Scholar] [CrossRef] [PubMed]
- Rutishauser, U.; Ross, I.B.; Mamelak, A.N.; Schuman, E.M. Human memory strength is predicted by theta-frequency phase-locking of single neurons. Nature 2010, 464, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Nieoullon, A. Dopamine and the regulation of cognition and attention. Prog. Neurobiol. 2002, 67, 53–83. [Google Scholar] [CrossRef]
- Standing, L.; Conezio, J.; Haber, R.N. Perception and memory for pictures: Single-trial learning of 2500 visual stimuli. Psychon. Sci. 1970, 19, 73–74. [Google Scholar] [CrossRef]
- Atkinson, R.C.; Shiffrin, R.M. Human Memory: A proposed system and its control processes BT—The Psychology of Learning and Motivation. Psychol. Learn. Motiv. 1968, 2, 89–195. [Google Scholar]
- Oberauer, K. Working Memory and Attention—A Conceptual Analysis and Review. J. Cogn. 2019, 2, 1–23. [Google Scholar] [CrossRef]
- Baddeley, A. Working memory: Theories, models, and controversies. Annu. Rev. Psychol. 2012, 63, 1–29. [Google Scholar] [CrossRef]
- Fuster, J.M.; Alexander, G.E. Neuron Activity related to short-term memory. Science 1971, 173, 652–654. [Google Scholar] [CrossRef]
- Constantinidis, C.; Funahashi, S.; Lee, D.; Murray, J.D.; Qi, X.L.; Wang, M.; Arnsten, A.F.T. Persistent spiking activity underlies working memory. J. Neurosci. 2018, 38, 7020–7028. [Google Scholar] [CrossRef]
- Olshausen, B.A.; Field, D.J. Sparse coding of sensory inputs. Curr. Opin. Neurobiol. 2004, 14, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Compte, A.; Brunel, N.; Goldman-Rakic, P.S.; Wang, X.J. Synaptic mechanisms and network dynamics underlying spatial working memory in a cortical network model. Cereb. Cortex 2000, 10, 910–923. [Google Scholar] [CrossRef]
- Kamiński, J.; Rutishauser, U. Between persistently active and activity-silent frameworks: Novel vistas on the cellular basis of working memory. Ann. N. Y. Acad. Sci. 2020, 1464, 64–75. [Google Scholar] [CrossRef]
- Jeneson, A.; Squire, L.R. Working memory, long-term memory, and medial temporal lobe function. Learn. Mem. 2012, 19, 15–25. [Google Scholar] [CrossRef]
- Christophel, T.B.; Klink, P.C.; Spitzer, B.; Roelfsema, P.R.; Haynes, J.D. The Distributed Nature of Working Memory. Trends Cogn. Sci. 2017, 21, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, J.; Brzezicka, A.; Mamelak, A.N.; Rutishauser, U. Combined Phase-Rate Coding by Persistently Active Neurons as a Mechanism for Maintaining Multiple Items in Working Memory in Humans. Neuron 2020, 106, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Boran, E.; Fedele, T.; Klaver, P.; Hilfiker, P.; Stieglitz, L.; Grunwald, T.; Sarnthein, J. Persistent hippocampal neural firing and hippocampal-cortical coupling predict verbal working memory load. Sci. Adv. 2019, 5, eaav3687. [Google Scholar] [CrossRef]
- Miller, G.A. The Magical number 7 plus or minus two: Some limits on our capacity for processing information. Psychol. Rev. 1956, 63, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Cowan, N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behav. Brain Sci. 2001, 24, 87–114. [Google Scholar] [CrossRef]
- Gläscher, J.; Adolphs, R.; Damasio, H.; Bechara, A.; Rudrauf, D.; Calamia, M.; Paul, L.K.; Tranel, D. Lesion mapping of cognitive control and value-based decision making in the prefrontal cortex. Proc. Natl. Acad. Sci. USA 2012, 109, 14681–14686. [Google Scholar] [CrossRef]
- Lisman, J.E.; Idiart, M.A.P. Storage of 7 ± 2 short-term memories in oscillatory subcycles. Science 1995, 267, 1512–1515. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, S. In defence of high-speed memory scanning. Q. J. Exp. Psychol. 2016, 69, 2020–2075. [Google Scholar] [CrossRef] [PubMed]
- Dosher, B.A.; Sperling, G. A Century of Human Information-Processing Theory: Vision, Attention, and Memory; Woodhead Publishing Limited: Cambridge, UK, 1998. [Google Scholar]
- Henson, R.; Hartley, T.; Burgess, N.; Hitch, G.; Flude, B. Selective interference with verbal short-term memory for serial order information: A new paradigm and tests of a timing-signal hypothesis. Q. J. Exp. Psychol. Sect. A Hum. Exp. Psychol. 2003, 56, 1307–1334. [Google Scholar] [CrossRef] [PubMed]
- Jonides, J.; Lewis, R.L.; Nee, D.E.; Lustig, C.A.; Berman, M.G.; Moore, K.S. The mind and brain of short-term memory. Annu. Rev. Psychol. 2008, 59, 193–224. [Google Scholar] [CrossRef]
- Kawasaki, H.; Adolphs, R.; Oya, H.; Kovach, C.; Damasio, H.; Kaufman, O.; Howard, M. Analysis of single-unit responses to emotional scenes in human ventromedial prefrontal cortex. J. Cogn. Neurosci. 2005, 17, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, D.B.; Zaghloul, K.A. The role of the subthalamic nucleus in cognition. Rev. Neurosci. 2013, 24, 125–138. [Google Scholar] [CrossRef]
- Simpson, E.H.; Kellendonk, C.; Kandel, E. A Possible Role for the Striatum in the Pathogenesis of the Cognitive Symptoms of Schizophrenia. Neuron 2010, 65, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Hanganu, A.; Provost, J.S.; Monchi, O. Neuroimaging studies of striatum in cognition part II: Parkinson’s disease. Front. Syst. Neurosci. 2015, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Aum, D.J.; Tierney, T.S. Deep brain stimulation: Foundations and future trends. Front. Biosci. 2018, 23, 162–182. [Google Scholar] [CrossRef]
- Graat, I.; Figee, M.; Denys, D. The application of deep brain stimulation in the treatment of psychiatric disorders. Int. Rev. Psychiatry 2017, 29, 178–190. [Google Scholar] [CrossRef]
- Khodagholy, D.; Gelinas, J.N.; Thesen, T.; Doyle, W.; Devinsky, O.; Malliaras, G.G.; Buzsáki, G. NeuroGrid: Recording action potentials from the surface of the brain. Nat. Neurosci. 2015, 18, 310–315. [Google Scholar] [CrossRef]
- Khodagholy, D.; Gelinas, J.N.; Zhao, Z.; Yeh, M.; Long, M.; Greenlee, J.D.; Doyle, W.; Devinsky, O.; Buzsáki, G. Organic electronics for high-resolution electrocorticography of the human brain. Sci. Adv. 2016, 2, e1601027. [Google Scholar] [CrossRef]
- Csercsa, R.; Dombovári, B.; Fabó, D.; Wittner, L.; Erss, L.; Entz, L.; Sólyom, A.; Rásonyi, G.; Szcs, A.; Kelemen, A.; et al. Laminar analysis of slow wave activity in humans. Brain 2010, 133, 2814–2829. [Google Scholar] [CrossRef]
- Ulbert, I.; Halgren, E.; Heit, G.; Karmos, G. Multiple microelectrode-recording system for human intracortical applications. J. Neurosci. Methods 2001, 106, 69–79. [Google Scholar] [CrossRef]
- Nadasdy, Z.; Nguyen, T.P.; Török, Á.; Shen, J.Y.; Briggs, D.E.; Modur, P.N.; Buchanan, R.J. Context-dependent spatially periodic activity in the human entorhinal cortex. Proc. Natl. Acad. Sci. USA 2017, 114, E3516–E3525. [Google Scholar] [CrossRef]
- Despouy, E.; Curot, J.; Reddy, L.; Nowak, L.G.; Deudon, M.; Sol, J.C.; Lotterie, J.A.; Denuelle, M.; Maziz, A.; Bergaud, C.; et al. Recording local field potential and neuronal activity with tetrodes in epileptic patients. J. Neurosci. Methods 2020, 341, 108759. [Google Scholar] [CrossRef] [PubMed]
- Buzsáki, G. Large-scale recording of neuronal ensembles. Nat. Neurosci. 2004, 7, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, J.; Rutishauser, U. Insights on vision derived from studying human single neurons. Cogn. Sci. Technol. 2017, 25–39. [Google Scholar] [CrossRef]
- Steinlein, O.K. Genetics and epilepsy. Dialogues Clin. Neurosci. 2008, 10, 29–38. [Google Scholar]
- Dayal, V.; Limousin, P.; Foltynie, T. Subthalamic nucleus deep brain stimulation in Parkinson’s disease: The effect of varying stimulation parameters. J. Parkinsons Dis. 2017, 7, 235–245. [Google Scholar] [CrossRef]
- Weiss, D.; Breit, S.; Wächter, T.; Plewnia, C.; Gharabaghi, A.; Krüger, R. Combined stimulation of the substantia nigra pars reticulata and the subthalamic nucleus is effective in hypokinetic gait disturbance in Parkinson’s disease. J. Neurol. 2011, 258, 1183–1185. [Google Scholar] [CrossRef]
- Mikell, C.B.; Sheehy, J.P.; Youngerman, B.E.; McGovern, R.A.; Wojtasiewicz, T.J.; Chan, A.K.; Pullman, S.L.; Yu, Q.; Goodman, R.R.; Schevon, C.A.; et al. Features and timing of the response of single neurons to novelty in the substantia nigra. Brain Res. 2014, 1542, 79–84. [Google Scholar] [CrossRef][Green Version]
- Damier, P.; Hirsch, E.C.; Agid, Y.; Graybiel, A.M. The substantia nigra of the human brain: II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain 1999, 122, 1437–1448. [Google Scholar] [CrossRef]
- Ungless, M.A.; Grace, A.A. Are you or aren’t you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci. 2012, 35, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Stauffer, W.R.; Lak, A.; Yang, A.; Borel, M.; Paulsen, O.; Boyden, E.S.; Schultz, W. Dopamine Neuron-Specific Optogenetic Stimulation in Rhesus Macaques. Cell 2016, 166, 1564–1571. [Google Scholar] [CrossRef]
- Aarsland, D.; Creese, B.; Politis, M.; Chaudhuri, K.R.; Ffytche, D.H.; Weintraub, D.; Ballard, C. Cognitive decline in Parkinson disease. Nat. Rev. Neurol. 2017, 13, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Barone, P.; Aarsland, D.; Burn, D.; Emre, M.; Kulisevsky, J.; Weintraub, D. Cognitive impairment in nondemented Parkinson’s disease. Mov. Disord. 2011, 26, 2483–2495. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Horta, S.; Kulisevsky, J. Mild cognitive impairment in Parkinson’s disease. J. Neural Transm. 2019, 126, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Shah, A.; Bhalsing, K.S.; Kumar, K.J.; Saini, J.; Ingalhalikar, M.; Pal, P.K. Abnormal hippocampal subfields are associated with cognitive impairment in Essential Tremor. J. Neural Transm. 2019, 126, 597–606. [Google Scholar] [CrossRef]
- Louis, E.D.; Joyce, J.L.; Cosentino, S. Mind the gaps: What we don’t know about cognitive impairment in essential tremor. Park. Relat. Disord. 2019, 63, 10–19. [Google Scholar] [CrossRef]
- Weil, R.S.; Costantini, A.A.; Schrag, A.E. Mild Cognitive Impairment in Parkinson’s Disease—What Is It? Curr. Neurol. Neurosci. Rep. 2018, 18. [Google Scholar] [CrossRef]
- Williams-Gray, C.H.; Mason, S.L.; Evans, J.R.; Foltynie, T.; Brayne, C.; Robbins, T.W.; Barker, R.A. The CamPaIGN study of Parkinson’s disease: 10-year outlook in an incident population-based cohort. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.; Rohl, B.; Morgan, S.; Huey, E.D.; Louis, E.D.; Cosentino, S. Mild Cognitive Impairment Subtypes in a Cohort of Elderly Essential Tremor Cases. J. Int. Neuropsychol. Soc. 2017, 23, 390–399. [Google Scholar] [CrossRef]
- Smith, C.R.; Cullen, B.; Sheridan, M.P.; Cavanagh, J.; Grosset, K.A.; Grosset, D.G. Cognitive impairment in Parkinson’s disease is multifactorial: A neuropsychological study. Acta Neurol. Scand. 2020, 141, 500–508. [Google Scholar] [CrossRef]
- Bowles, B.; Crupi, C.; Pigott, S.; Parrent, A.; Wiebe, S.; Janzen, L.; Köhler, S. Double dissociation of selective recollection and familiarity impairments following two different surgical treatments for temporal-lobe epilepsy. Neuropsychologia 2010, 48, 2640–2647. [Google Scholar] [CrossRef]
- Illman, N.A.; Kemp, S.; Souchay, C.; Morris, R.G.; Moulin, C.J.A. Assessing a Metacognitive Account of Associative Memory Impairments in Temporal Lobe Epilepsy. Epilepsy Res. Treat. 2016, 2016, 6746938. [Google Scholar] [CrossRef] [PubMed]
- Moscovitch, D.A.; McAndrews, M.P. Material-specific deficits in “remembering” in patients with unilateral temporal lobe epilepsy and excisions. Neuropsychologia 2002, 40, 1335–1342. [Google Scholar] [CrossRef]
- Kleen, J.K.; Scott, R.C.; Holmes, G.L.; Roberts, D.W.; Rundle, M.M.; Testorf, M.; Lenck-Santini, P.P.; Jobst, B.C. Hippocampal interictal epileptiform activity disrupts cognition in humans. Neurology 2013, 81, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Horak, P.C.; Meisenhelter, S.; Song, Y.; Testorf, M.E.; Kahana, M.J.; Viles, W.D.; Bujarski, K.A.; Connolly, A.C.; Robbins, A.A.; Sperling, M.R.; et al. Interictal epileptiform discharges impair word recall in multiple brain areas. Epilepsia 2017, 58, 373–380. [Google Scholar] [CrossRef]
- Reed, C.M.; Mosher, C.P.; Chandravadia, N.; Chung, J.M.; Mamelak, A.N.; Rutishauser, U. Extent of single-neuron activity modulation by hippocampal interictal discharges predicts declarative memory disruption in humans. J. Neurosci. 2020, 40, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Hwang, J.J.; Poston, K.L. Episodic Recognition Memory and the Hippocampus in Parkinson’s disease: A Review. Cortex 2019, 113, 191–209. [Google Scholar] [CrossRef]
- Carlson, A.A.; Rutishauser, U.; Mamelak, A.N. Safety and utility of hybrid depth electrodes for seizure localization and single-unit neuronal recording. Stereotact. Funct. Neurosurg. 2018, 96, 311–319. [Google Scholar] [CrossRef]
- Hefft, S.; Brandt, A.; Zwick, S.; Von Elverfeldt, D.; Mader, I.; Cordeiro, J.; Trippel, M.; Blumberg, J.; Schulze-Bonhage, A. Safety of hybrid electrodes for single-neuron recordings in humans. Neurosurgery 2013, 73, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Panov, F.; Levin, E.; De Hemptinne, C.; Swann, N.C.; Qasim, S.; Miocinovic, S.; Ostrem, J.L.; Starr, P.A. Intraoperative electrocorticography for physiological research in movement disorders: Principles and experience in 200 cases. J. Neurosurg. 2017, 126, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Sisterson, N.D.; Carlson, A.A.; Rutishauser, U.; Mamelak, A.N.; Flagg, M.; Pouratian, N.; Salimpour, Y.; Anderson, W.S.; Richardson, R.M. Electrocorticography During Deep Brain Stimulation Surgery: Safety Experience From 4 Centers Within the National Institute of Neurological Disorders and Stroke Research Opportunities in Human Consortium. Neurosurgery 2021. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubska, Z.R.; Kamiński, J. How Human Single-Neuron Recordings Can Help Us Understand Cognition: Insights from Memory Studies. Brain Sci. 2021, 11, 443. https://doi.org/10.3390/brainsci11040443
Kubska ZR, Kamiński J. How Human Single-Neuron Recordings Can Help Us Understand Cognition: Insights from Memory Studies. Brain Sciences. 2021; 11(4):443. https://doi.org/10.3390/brainsci11040443
Chicago/Turabian StyleKubska, Zuzanna Roma, and Jan Kamiński. 2021. "How Human Single-Neuron Recordings Can Help Us Understand Cognition: Insights from Memory Studies" Brain Sciences 11, no. 4: 443. https://doi.org/10.3390/brainsci11040443
APA StyleKubska, Z. R., & Kamiński, J. (2021). How Human Single-Neuron Recordings Can Help Us Understand Cognition: Insights from Memory Studies. Brain Sciences, 11(4), 443. https://doi.org/10.3390/brainsci11040443