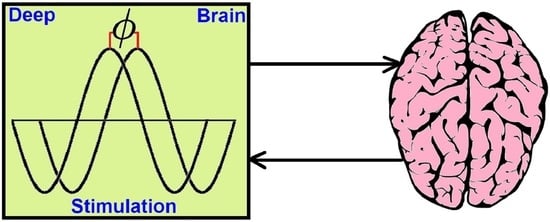
Phase-Dependent Deep Brain Stimulation: A Review
Abstract
1. Introduction
2. Neural Oscillations as Biomarkers
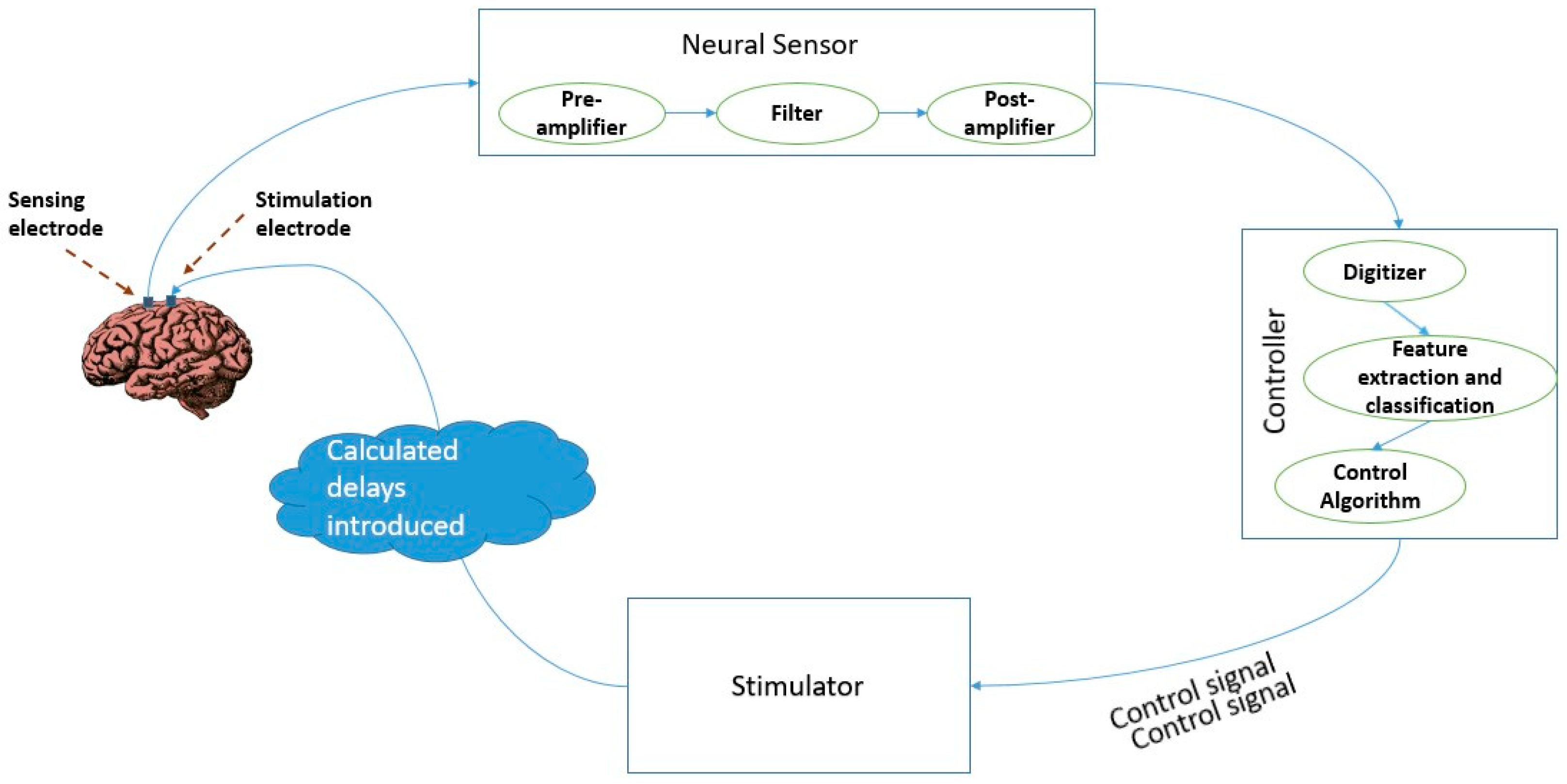
3. Closed Loop Brain Stimulation with Neural Oscillations as Biomarkers
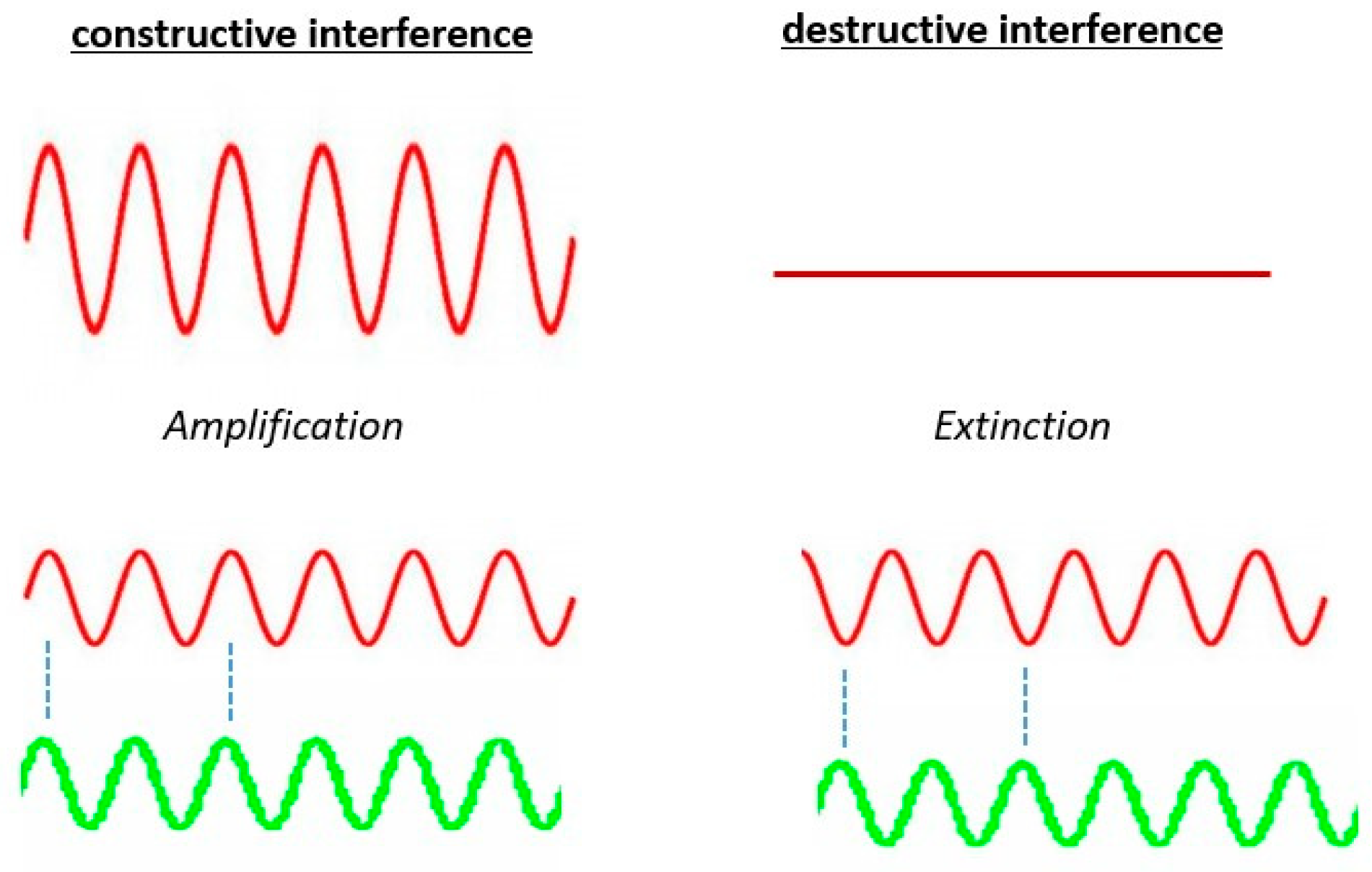
4. Phase Specific Stimulation
5. Outlook: Closed Loop Brain Stimulation with Phase Specific Stimulation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Budman, E.; Deeb, W.; Martinez-Ramirez, D.; Pilitsis, J.G.; Peng-Chen, Z.; Okun, M.S.; Ramirez-Zamora, A. Potential indications for deep brain stimulation in neurological disorders: An evolving field. Eur. J. Neurol. 2018, 25, 434-e30. [Google Scholar] [CrossRef]
- Lozano, A.M.; Lipsman, N.; Bergman, H.; Brown, P.; Chabardes, S.; Chang, J.W.; Matthews, K.; McIntyre, C.C.; Schlaepfer, T.E.; Schulder, M.; et al. Deep brain stimulation: Current challenges and future directions. Nat. Rev. Neurol. 2019, 15, 148–160. [Google Scholar] [CrossRef]
- Cagnan, H.; Denison, T.; McIntyre, C.; Brown, P. Emerging technologies for improved deep brain stimulation. Nat. Biotechnol. 2019, 37, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Bestmann, S.; Walsh, V. Transcranial electrical stimulation. Curr. Biol. 2017, 27, R1258–R1262. [Google Scholar] [CrossRef] [PubMed]
- Garcin, B.; Mesrati, F.; Hubsch, C.; Mauras, T.; Iliescu, I.; Naccache, L.; Vidailhet, M.; Roze, E.; Degos, B. Impact of transcranial magnetic stimulation on functional movement disorders: Cortical modulation or a behavioral effect? Front. Neurol. 2017, 8, 338. [Google Scholar] [CrossRef] [PubMed]
- Hänselmann, S.; Schneiders, M.; Weidner, N.; Rupp, R. Transcranial magnetic stimulation for individual identification of the best electrode position for a motor imagery-based brain-computer interface. J. Neuroeng. Rehabil. 2015, 12, 71. [Google Scholar] [CrossRef]
- Dayan, E.; Censor, N.; Buch, E.R.; Sandrini, M.; Cohen, L.G. Noninvasive brain stimulation: From physiology to network dynamics and back. Nat. Neurosci. 2013, 16, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, P.B.; Hoy, K.E.; Elliot, D.; McQueen, R.N.S.; Wambeek, L.E.; Daskalakis, Z.J. Accelerated repetitive transcranial magnetic stimulation in the treatment of depression. Neuropsychopharmacology 2018, 43, 1565–1572. [Google Scholar] [CrossRef]
- Lefaucheur, J.-P.; Aleman, A.; Baeken, C.; Benninger, D.H.; Brunelin, J.; Di Lazzaro, V.; Filipović, S.R.; Grefkes, C.; Hasan, A.; Hummel, F.C.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018). Clin. Neurophysiol. 2020, 131, 474–528. [Google Scholar] [CrossRef]
- Maiti, R.; Mishra, B.R.; Hota, D. Effect of high-frequency transcranial magnetic stimulation on craving in substance use disorder: A meta-analysis. J. Neuropsychiatry Clin. Neurosci. 2017, 29, 160–171. [Google Scholar] [CrossRef]
- Ekhtiari, H.; Tavakoli, H.; Addolorato, G.; Baeken, C.; Bonci, A.; Campanella, S.; Castelo-Branco, L.; Challet-Bouju, G.; Clark, V.P.; Claus, E.; et al. Transcranial electrical and magnetic stimulation (tES and TMS) for addiction medicine: A consensus paper on the present state of the science and the road ahead. Neurosci. Biobehav. Rev. 2019, 104, 118–140. [Google Scholar] [CrossRef]
- Diana, M.; Raij, T.; Melis, M.; Nummenmaa, A.; Leggio, L.; Bonci, A. Rehabilitating the addicted brain with transcranial magnetic stimulation. Nat. Rev. Neurosci. 2017, 18, 685. [Google Scholar] [CrossRef]
- Fasano, A.; Aquino, C.C.; Krauss, J.K.; Honey, C.R.; Bloem, B.R. Axial disability and deep brain stimulation in patients with Parkinson disease. Nat. Rev. Neurol. 2015, 11, 98–110. [Google Scholar] [CrossRef]
- Krauss, J.K.; Lipsman, N.; Aziz, T.; Boutet, A.; Brown, P.; Chang, J.W.; Davidson, B.; Grill, W.M.; Hariz, M.I.; Horn, A.; et al. Technology of deep brain stimulation: Current status and future directions. Nat. Rev. Neurol. 2021, 17, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Bouthour, W.; Mégevand, P.; Donoghue, J.; Lüscher, C.; Birbaumer, N.; Krack, P. Biomarkers for closed-loop deep brain stimulation in Parkinson disease and beyond. Nat. Rev. Neurol. 2019, 15, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Hoang, K.B.; Cassar, I.R.; Grill, W.M.; Turner, D.A. Biomarkers and Stimulation Algorithms for Adaptive Brain Stimulation. Front. Neurosci. 2017, 11, 564. [Google Scholar] [CrossRef] [PubMed]
- Maetzler, W.; Liepelt, I.; Berg, D. Progression of Parkinson’s disease in the clinical phase: Potential markers. Lancet Neurol. 2009, 8, 1158–1171. [Google Scholar] [CrossRef]
- Carron, R.; Chaillet, A.; Filipchuk, A.; Pasillas-Lépine, W.; Hammond, C. Closing the loop of deep brain stimulation. Front. Syst. Neurosci. 2013, 7, 112. [Google Scholar] [CrossRef]
- Hosain, K.; Kouzani, A.; Tye, S. Closed loop deep brain stimulation: An evolving technology. Australas. Phys. Eng. Sci. Med. 2014, 37, 619–634. [Google Scholar] [CrossRef]
- Fleming, J.E.; Dunn, E.; Lowery, M.M. Simulation of Closed-Loop Deep Brain Stimulation Control Schemes for Suppression of Pathological Beta Oscillations in Parkinson’s Disease. Front. Neurosci. 2020, 14, 166. [Google Scholar] [CrossRef]
- Arlotti, M.; Marceglia, S.; Foffani, G.; Volkmann, J.; Lozano, A.M.; Moro, E.; Cogiamanian, F.; Prenassi, M.; Bocci, T.; Cortese, F.; et al. Eight-hours adaptive deep brain stimulation in patients with Parkinson disease. Neuropsychopharmacology 2018, 90, e971–e976. [Google Scholar] [CrossRef]
- Swann, N.C.; De Hemptinne, C.; Thompson, M.C.; Miocinovic, S.; Miller, A.M.; Gilron, R.; Ostrem, J.L.; Chizeck, H.J.; Starr, P.A. Adaptive deep brain stimulation for Parkinson’s disease using motor cortex sensing. J. Neural Eng. 2018, 15, 046006. [Google Scholar] [CrossRef] [PubMed]
- Little, S.; Beudel, M.; Zrinzo, L.; Foltynie, T.; Limousin, P.; Hariz, M.; Neal, S.; Cheeran, B.; Cagnan, H.; Gratwicke, J.; et al. Bilateral adaptive deep brain stimulation is effective in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2016, 87, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Velisar, A.; Syrkin-Nikolau, J.; Blumenfeld, Z.; Trager, M.; Afzal, M.; Prabhakar, V.; Bronte-Stewart, H. Dual threshold neural closed loop deep brain stimulation in Parkinson disease patients. Brain Stimul. 2019, 12, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Little, S.; Tripoliti, E.; Beudel, M.; Pogosyan, A.; Cagnan, H.; Herz, D.; Bestmann, S.; Aziz, T.; Cheeran, B.; Zrinzo, L.; et al. Adaptive deep brain stimulation for Parkinson’s disease demonstrates reduced speech side effects compared to conventional stimulation in the acute setting. J. Neurol. Neurosurg. Psychiatry 2016, 87, 1388–1389. [Google Scholar] [CrossRef] [PubMed]
- Medtronic, P. Green light for deep brain stimulator incorporating neurofeedback. Nat. Biotechnol. 2020, 38, 1014–1015. [Google Scholar] [CrossRef]
- Biomarkers Definitions Working Group; Atkinson, A.J., Jr.; Colburn, W.A.; DeGruttola, V.G.; DeMets, D.L.; Downing, G.J.; Hoth, D.F.; Oates, J.A.; Peck, C.C.; Schooley, R.T.; et al. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar]
- Mirza, K.B.; Golden, C.T.; Nikolic, K.; Toumazou, C. Closed-Loop Implantable Therapeutic Neuromodulation Systems Based on Neurochemical Monitoring. Front. Neurosci. 2019, 13, 808. [Google Scholar] [CrossRef]
- Parastarfeizabadi, M.; Kouzani, A.Z. A Miniature Dual-Biomarker-Based Sensing and Conditioning Device for Closed-Loop DBS. IEEE J. Transl. Eng. Health Med. 2019, 7, 1–8. [Google Scholar] [CrossRef]
- David, S.V.; Malaval, N.; Shamma, S.A. Decoupling Action Potential Bias from Cortical Local Field Potentials. Comput. Intell. Neurosci. 2010, 2010, 1–12. [Google Scholar] [CrossRef]
- Waldert, S. Invasive vs. Non-Invasive Neuronal Signals for Brain-Machine Interfaces: Will One Prevail? Front. Neurosci. 2016, 10, 295. [Google Scholar] [CrossRef]
- Vosskuhl, J.; Strüber, D.; Herrmann, C.S. Non-invasive brain stimulation: A paradigm shift in understanding brain oscillations. Front. Hum. Neurosci. 2018, 12, 211. [Google Scholar] [CrossRef] [PubMed]
- Deisseroth, K. Optogenetics. Nat. Methods 2011, 8, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Piantadosi, S.C.; Ahmari, S.E. Using Optogenetics to Dissect the Neural Circuits Underlying OCD and Related Disorders. Curr. Treat. Options Psychiatry 2015, 2, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Başar, E. Brain oscillations in neuropsychiatric disease. Dialogues Clin. Neurosci. 2013, 15, 291–300. [Google Scholar] [PubMed]
- Başar, E.; Başar-Eroglu, C.; Karakaş, S.; Schürmann, M. Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int. J. Psychophysiol. 2001, 39, 241–248. [Google Scholar] [CrossRef]
- Düzel, E.; Penny, W.D.; Burgess, N. Brain oscillations and memory. Curr. Opin. Neurobiol. 2010, 20, 143–149. [Google Scholar] [CrossRef]
- Singh, A. Oscillatory activity in the cortico-basal ganglia-thalamic neural circuits in Parkinson’s disease. Eur. J. Neurosci. 2018, 48, 2869–2878. [Google Scholar] [CrossRef]
- Ahnaou, A.; Moechars, D.; Raeymaekers, L.; Biermans, R.; Manyakov, N.V.; Bottelbergs, A.; Wintmolders, C.; Van Kolen, K.; Van De Casteele, T.; Kemp, J.A.; et al. Emergence of early alterations in network oscillations and functional connectivity in a tau seeding mouse model of Alzheimer’s disease pathology. Sci. Rep. 2017, 7, 14189. [Google Scholar] [CrossRef]
- Kitchigina, V.F. Alterations of Coherent Theta and Gamma Network Oscillations as an Early Biomarker of Temporal Lobe Epilepsy and Alzheimer’s Disease. Front. Integr. Neurosci. 2018, 12, 36. [Google Scholar] [CrossRef]
- Cole, S.R.; Voytek, B. Brain Oscillations and the Importance of Waveform Shape. Trends Cogn. Sci. 2017, 21, 137–149. [Google Scholar] [CrossRef]
- Başar, E.; Başar-Eroğlu, C.; Güntekin, B.; Yener, G.G. Brain’s alpha, beta, gamma, delta, and theta oscillations in neuropsychiatric diseases: Proposal for biomarker strategies. In Supplements to Clinical Neurophysiology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 62, pp. 19–54. [Google Scholar]
- Little, S.; Brown, P. The functional role of beta oscillations in Parkinson’s disease. Park. Relat. Disord. 2014, 20, S44–S48. [Google Scholar] [CrossRef]
- Lofredi, R.; Tan, H.; Neumann, W.-J.; Yeh, C.-H.; Schneider, G.-H.; Kühn, A.A.; Brown, P. Beta bursts during continuous movements accompany the velocity decrement in Parkinson’s disease patients. Neurobiol. Dis. 2019, 127, 462–471. [Google Scholar] [CrossRef]
- Heinrichs-Graham, E.; Santamaria, P.M.; Gendelman, H.E.; Wilson, T.W. The cortical signature of symptom laterality in Parkinson’s disease. NeuroImage Clin. 2017, 14, 433–440. [Google Scholar] [CrossRef]
- Chung, J.W.; Burciu, R.G.; Ofori, E.; Coombes, S.A.; Christou, E.A.; Okun, M.S.; Hess, C.W.; Vaillancourt, D.E. Beta-band oscillations in the supplementary motor cortex are modulated by levodopa and associated with functional activity in the basal ganglia. NeuroImage Clin. 2018, 19, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Woerd, E.S.T.; Oostenveld, R.; De Lange, F.P.; Praamstra, P. A shift from prospective to reactive modulation of beta-band oscillations in Parkinson’s disease. NeuroImage 2014, 100, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Rowland, N.C.; De Hemptinne, C.; Swann, N.C.; Qasim, S.; Miocinovic, S.; Ostrem, J.L.; Knight, R.T.; Starr, P.A. Task-related activity in sensorimotor cortex in Parkinson’s disease and essential tremor: Changes in beta and gamma bands. Front. Hum. Neurosci. 2015, 9, 512. [Google Scholar] [CrossRef]
- Wang, J.; Hirschmann, J.; Elben, S.; Hartmann, C.J.; Vesper, J.; Wojtecki, L.; Schnitzler, A. High-frequency oscillations in Parkinson’s disease: Spatial distribution and clinical relevance. Mov. Disord. 2014, 29, 1265–1272. [Google Scholar] [CrossRef]
- Swann, N.C.; De Hemptinne, C.; Miocinovic, S.; Qasim, S.; Wang, S.S.; Ziman, N.; Ostrem, J.L.; Luciano, M.S.; Galifianakis, N.B.; Starr, P.A. Gamma Oscillations in the Hyperkinetic State Detected with Chronic Human Brain Recordings in Parkinson’s Disease. J. Neurosci. 2016, 36, 6445–6458. [Google Scholar] [CrossRef] [PubMed]
- De Hemptinne, C.; Swann, N.C.; Ostrem, J.L.; Ryapolova-Webb, E.S.; Luciano, M.S.; Galifianakis, N.B.; Starr, P.A. Therapeutic deep brain stimulation reduces cortical phase-amplitude coupling in Parkinson’s disease. Nat. Neurosci. 2015, 18, 779–786. [Google Scholar] [CrossRef]
- Özkurt, T.E.; Butz, M.; Homburger, M.; Elben, S.; Vesper, J.; Wojtecki, L.; Schnitzler, A. High frequency oscillations in the subthalamic nucleus: A neurophysiological marker of the motor state in Parkinson’s disease. Exp. Neurol. 2011, 229, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Asch, N.; Herschman, Y.; Maoz, R.; Auerbach-Asch, C.R.; Valsky, D.; Abu-Snineh, M.; Arkadir, D.; Linetsky, E.; Eitan, R.; Marmor, O.; et al. Independently together: Subthalamic theta and beta opposite roles in predicting Parkinson’s tremor. Brain Commun. 2020, 2, fcaa074. [Google Scholar] [CrossRef]
- Chaudhuri, K.R.; Tolosa, E.; Schapira, A.H.; Poewe, W. Non-Motor Symptoms of Parkinson’s Disease; OUP: Oxford, UK, 2014. [Google Scholar]
- Poewe, W. Non-motor symptoms in Parkinson’s disease. Eur. J. Neurol. 2008, 15, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Bin Yoo, H.; De La Concha, E.O.; De Ridder, D.; Pickut, B.A.; Vanneste, S. The Functional Alterations in Top-Down Attention Streams of Parkinson’s disease Measured by EEG. Sci. Rep. 2018, 8, 10609. [Google Scholar] [CrossRef] [PubMed]
- Tarsy, D.; Simon, D.K. Dystonia. N. Engl. J. Med. 2006, 355, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.; Di Giovanni, M.; Lalli, S. Dystonia: Diagnosis and management. Eur. J. Neurol. 2018, 26, 5–17. [Google Scholar] [CrossRef]
- Ostrem, J.L.; Starr, P.A. Treatment of dystonia with deep brain stimulation. Neurotherapeutics 2008, 5, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Moll, C.K.E.; Galindo-Leon, E.; Sharott, A.; Gulberti, A.; Buhmann, C.; Koeppen, J.A.; Biermann, M.; Bäumer, T.; Zittel, S.; Westphal, M.; et al. Asymmetric pallidal neuronal activity in patients with cervical dystonia. Front. Syst. Neurosci. 2014, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Neumann, W.; Horn, A.; Ewert, S.; Huebl, J.; Brücke, C.; Slentz, C.; Schneider, G.; Kühn, A.A. A localized pallidal physiomarker in cervical dystonia. Ann. Neurol. 2017, 82, 912–924. [Google Scholar] [CrossRef]
- Neumann, W.-J.; Jha, A.; Bock, A.; Huebl, J.; Horn, A.; Schneider, G.-H.; Sander, T.H.; Litvak, V.; Kühn, A.A. Cortico-pallidal oscillatory connectivity in patients with dystonia. Brain 2015, 138, 1894–1906. [Google Scholar] [CrossRef]
- Velickovic, M.; Benabou, R.; Brin, M.F. Cervical Dystonia. Drugs 2001, 61, 1921–1943. [Google Scholar] [CrossRef]
- Uhlhaas, P.J.; Singer, W. Oscillations and neuronal dynamics in schizophrenia: The search for basic symptoms and translational opportunities. Biol. Psychiatry 2015, 77, 1001–1009. [Google Scholar] [CrossRef]
- Uhlhaas, P.J.; Haenschel, C.; Nikolić, D.; Singer, W. The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr. Bull. 2008, 34, 927–943. [Google Scholar] [CrossRef] [PubMed]
- Symond, M.B.; Harris, A.W.; Gordon, E.; Williams, L.M. “Gamma Synchrony” in First-Episode Schizophrenia: A Disorder of Temporal Connectivity? Am. J. Psychiatry 2005, 162, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Gandal, M.J.; Edgar, J.C.; Klook, K.; Siegel, S.J. Gamma synchrony: Towards a translational biomarker for the treatment-resistant symptoms of schizophrenia. Neuropharmacology 2012, 62, 1504–1518. [Google Scholar] [CrossRef]
- Jaworska, N.; Blier, P.; Fusee, W.; Knott, V. Alpha power, alpha asymmetry and anterior cingulate cortex activity in depressed males and females. J. Psychiatr. Res. 2012, 46, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- Shim, M.; Im, C.-H.; Kim, Y.-W.; Lee, S.-H. Altered cortical functional network in major depressive disorder: A resting-state electroencephalogram study. NeuroImage Clin. 2018, 19, 1000–1007. [Google Scholar] [CrossRef]
- Mahato, S.; Paul, S. Electroencephalogram (EEG) Signal Analysis for Diagnosis of Major Depressive Disorder (MDD): A Review. In Nanoelectronics, Circuits and Communication Systems; Nath, V., Mandal, J., Eds.; Lecture Notes in Electrical Engineering; Springer: Singapore, 2019; Volume 511, pp. 323–335. [Google Scholar] [CrossRef]
- Gheza, D.; Bakic, J.; Baeken, C.; De Raedt, R.; Pourtois, G. Abnormal approach-related motivation but spared reinforcement learning in MDD: Evidence from fronto-midline Theta oscillations and frontal Alpha asymmetry. Cogn. Affect. Behav. Neurosci. 2019, 19, 759–777. [Google Scholar] [CrossRef]
- Fitzgerald, P.J.; Watson, B.O. Gamma oscillations as a biomarker for major depression: An emerging topic. Transl. Psychiatry 2018, 8, 1–7. [Google Scholar] [CrossRef]
- Jafari, Z.; Kolb, B.E.; Mohajerani, M.H. Neural oscillations and brain stimulation in Alzheimer’s disease. Prog. Neurobiol. 2020, 194, 101878. [Google Scholar] [CrossRef] [PubMed]
- Etter, G.; van der Veldt, S.; Manseau, F.; Zarrinkoub, I.; Trillaud-Doppia, E.; Williams, S. Optogenetic gamma stimulation rescues memory impairments in an Alzheimer’s disease mouse model. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kilias, A.; Canales, A.; Froriep, U.P.; Park, S.; Egert, U.; Anikeeva, P. Optogenetic entrainment of neural oscillations with hybrid fiber probes. J. Neural Eng. 2018, 15, 056006. [Google Scholar] [CrossRef]
- Widge, A.S.; Boggess, M.; Rockhill, A.P.; Mullen, A.; Sheopory, S.; Loonis, R.; Freeman, D.K.; Miller, E.K. Altering alpha-frequency brain oscillations with rapid analog feedback-driven neurostimulation. PLoS ONE 2018, 13, e0207781. [Google Scholar] [CrossRef] [PubMed]
- Cagnan, H.; Pedrosa, D.; Little, S.; Pogosyan, A.; Cheeran, B.; Aziz, T.; Green, A.; Fitzgerald, J.; Foltynie, T.; Limousin, P.; et al. Stimulating at the right time: Phase-specific deep brain stimulation. Brain 2017, 140, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Siegle, J.H.; Wilson, M.A. Enhancement of encoding and retrieval functions through theta phase-specific manipulation of hippocampus. eLife 2014, 3, e03061. [Google Scholar] [CrossRef]
- Weerasinghe, G.; Duchet, B.; Cagnan, H.; Brown, P.; Bick, C.; Bogacz, R. Predicting the effects of deep brain stimulation using a reduced coupled oscillator model. PLoS Comput. Biol. 2019, 15, e1006575. [Google Scholar] [CrossRef]
- Mansouri, F.; Fettes, P.; Schulze, L.; Giacobbe, P.; Zariffa, J.; Downar, J. A Real-Time Phase-Locking System for Non-invasive Brain Stimulation. Front. Neurosci. 2018, 12, 877. [Google Scholar] [CrossRef]
- Cagnan, H.; Brittain, J.-S.; Little, S.; Foltynie, T.; Limousin, P.; Zrinzo, L.; Hariz, M.; Joint, C.; Fitzgerald, J.; Green, A.L.; et al. Phase dependent modulation of tremor amplitude in essential tremor through thalamic stimulation. Brain 2013, 136, 3062–3075. [Google Scholar] [CrossRef] [PubMed]
- Zarubin, G.; Gundlach, C.; Nikulin, V.; Villringer, A.; Bogdan, M. Transient Amplitude Modulation of Alpha-Band Oscillations by Short-Time Intermittent Closed-Loop tACS. Front. Hum. Neurosci. 2020, 14. [Google Scholar] [CrossRef]
- Heuvel, M.P.V.D.; Pol, H.E.H. Exploring the brain network: A review on resting-state fMRI functional connectivity. Eur. Neuropsychopharmacol. 2010, 20, 519–534. [Google Scholar] [CrossRef]
- Shafi, M.M.; Westover, M.B.; Fox, M.D.; Pascual-Leone, A. Exploration and modulation of brain network interactions with noninvasive brain stimulation in combination with neuroimaging. Eur. J. Neurosci. 2012, 35, 805–825. [Google Scholar] [CrossRef]
- Horn, A.; Li, N.; Dembek, T.A.; Kappel, A.; Boulay, C.; Ewert, S.; Tietze, A.; Husch, A.; Perera, T.; Neumann, W.-J.; et al. Lead-DBS v2: Towards a comprehensive pipeline for deep brain stimulation imaging. NeuroImage 2019, 184, 293–316. [Google Scholar] [CrossRef] [PubMed]
- Horn, A.; Reich, M.; Vorwerk, J.; Li, N.; Wenzel, G.; Fang, Q.; Schmitz-Hübsch, T.; Nickl, R.; Kupsch, A.; Volkmann, J.; et al. Connectivity predicts deep brain stimulation outcome in Parkinson disease. Ann. Neurol. 2017, 82, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.D.; Buckner, R.L.; White, M.P.; Greicius, M.D.; Pascual-Leone, A. Efficacy of Transcranial Magnetic Stimulation Targets for Depression Is Related to Intrinsic Functional Connectivity with the Subgenual Cingulate. Biol. Psychiatry 2012, 72, 595–603. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumari, L.S.; Kouzani, A.Z. Phase-Dependent Deep Brain Stimulation: A Review. Brain Sci. 2021, 11, 414. https://doi.org/10.3390/brainsci11040414
Kumari LS, Kouzani AZ. Phase-Dependent Deep Brain Stimulation: A Review. Brain Sciences. 2021; 11(4):414. https://doi.org/10.3390/brainsci11040414
Chicago/Turabian StyleKumari, Lekshmy Sudha, and Abbas Z. Kouzani. 2021. "Phase-Dependent Deep Brain Stimulation: A Review" Brain Sciences 11, no. 4: 414. https://doi.org/10.3390/brainsci11040414
APA StyleKumari, L. S., & Kouzani, A. Z. (2021). Phase-Dependent Deep Brain Stimulation: A Review. Brain Sciences, 11(4), 414. https://doi.org/10.3390/brainsci11040414