Influence of Brain-Derived Neurotrophic Factor Genotype on Short-Latency Afferent Inhibition and Motor Cortex Metabolites
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Genetic Analysis
2.3. Electromyography (EMG)
2.4. TMS Settings
2.5. Inhibitory TMS Conditions
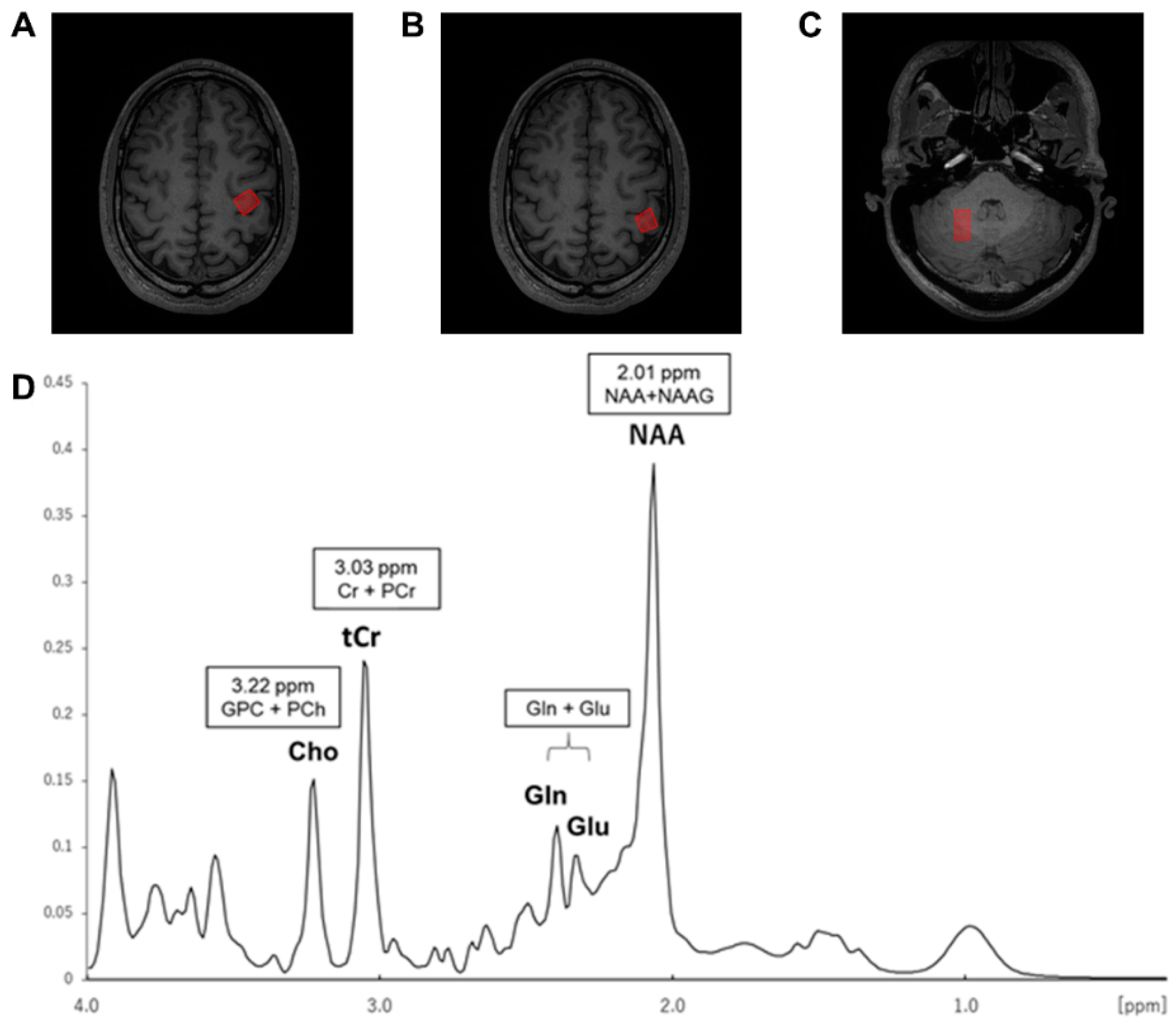
2.6. MR Data Acquisition
2.7. Experimental Protocol
2.8. Analysis of TMS Data
2.9. Analysis of MRS Data
2.10. Statistics
3. Results
3.1. Participants and Measurements
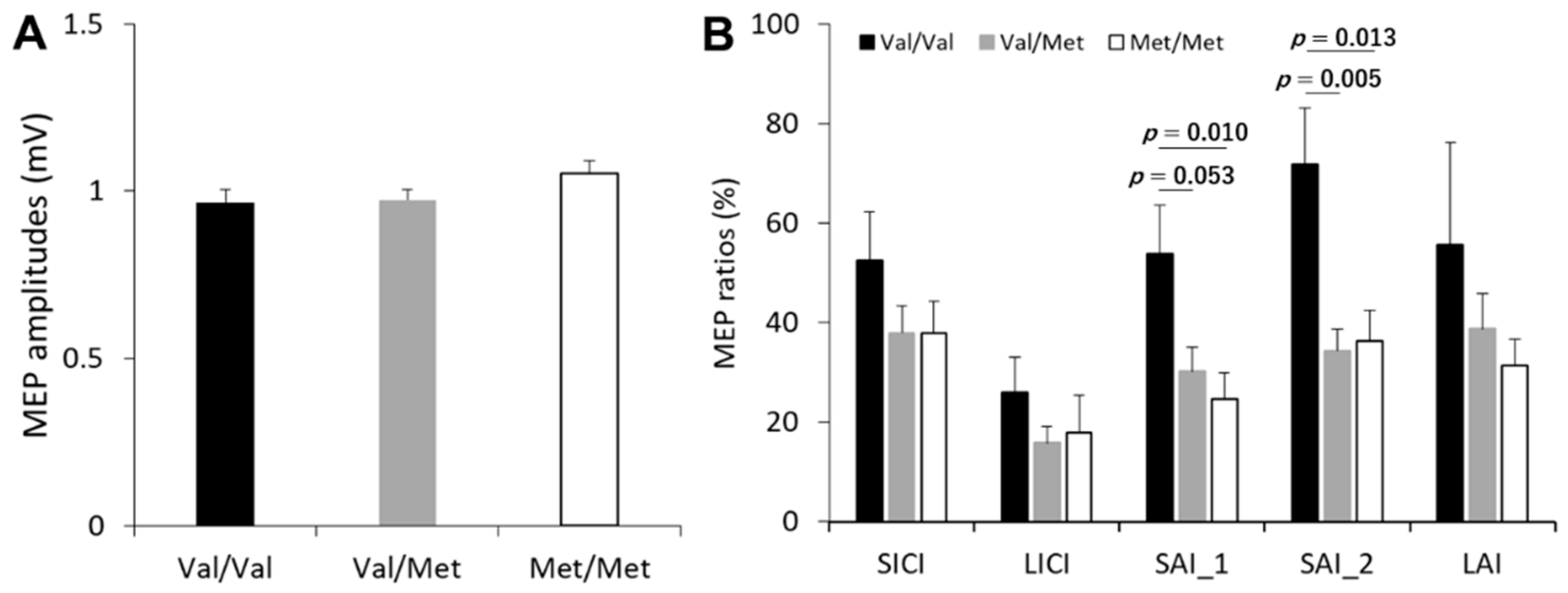
3.2. Single-Pulse MEP and Inhibitory TMS Conditions
3.3. Effect of BDNF Genotype on the Cortical Inhibitory Network
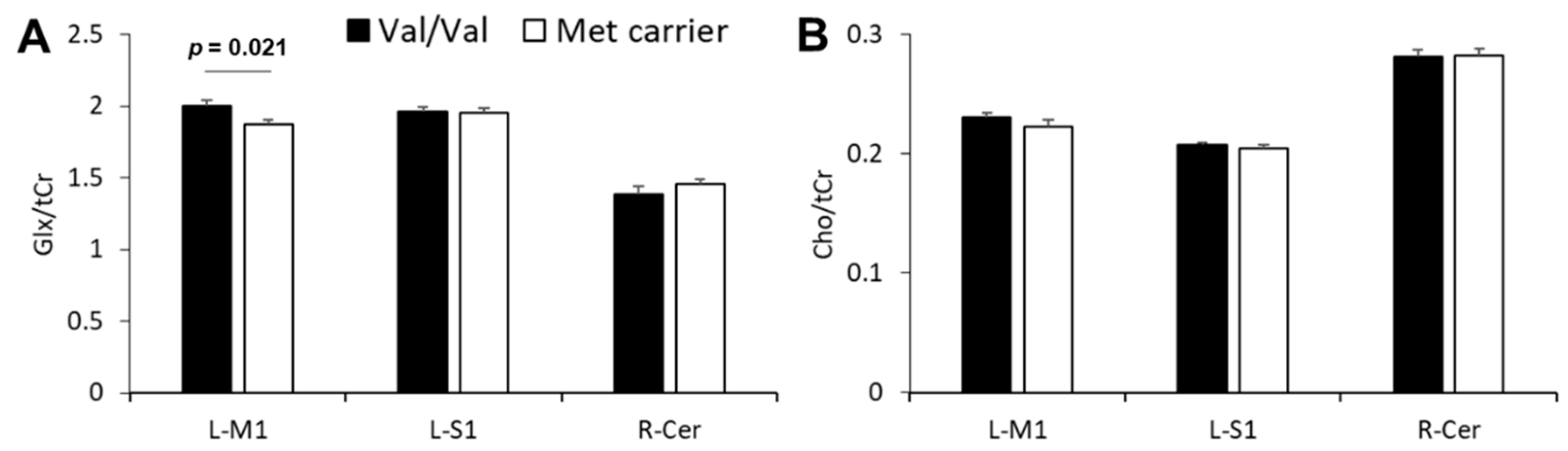
3.4. Effect of BDNF Genotype on Neurometabolite Concentrations
3.5. Relationship between SAI and Glx
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kujirai, T.; Caramia, M.D.; Rothwell, J.C.; Day, B.L.; Thompson, P.D.; Ferbert, A.; Wroe, S.; Asselman, P.; Marsden, C.D. Corticocortical inhibition in human motor cortex. J. Physiol. 1993, 471, 501–519. [Google Scholar] [CrossRef]
- Bauer, P.R.; de Goede, A.A.; Stern, W.M.; Pawley, A.D.; Chowdhury, F.A.; Helling, R.M.; Bouet, R.; Kalitzin, S.N.; Visser, G.H.; Sisodiya, S.M.; et al. Long-interval intracortical inhibition as biomarker for epilepsy: A transcranial magnetic stimulation study. Brain 2018, 141, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Corwell, B.; Hallett, M. Modulation of motor cortex excitability by median nerve and digit stimulation. Exp. Brain Res. 1999, 129, 77–86. [Google Scholar] [CrossRef]
- Tokimura, H.; Di Lazzaro, V.; Tokimura, Y.; Oliviero, A.; Profice, P.; Insola, A.; Mazzone, P.; Tonali, P.; Rothwell, J.C. Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J. Physiol. 2000, 523 Pt 2, 503–513. [Google Scholar] [CrossRef]
- Wassermann, E.M. Variation in the response to transcranial magnetic brain stimulation in the general population. Clin. Neurophysiol. 2002, 113, 1165–1171. [Google Scholar] [CrossRef]
- Yamada, K.; Mizuno, M.; Nabeshima, T. Role for brain-derived neurotrophic factor in learning and memory. Life Sci. 2002, 70, 735–744. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Jing, D.; Bath, K.G.; Ieraci, A.; Khan, T.; Siao, C.J.; Herrera, D.G.; Toth, M.; Yang, C.; McEwen, B.S.; et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science 2006, 314, 140–143. [Google Scholar] [CrossRef]
- Kleim, J.A.; Chan, S.; Pringle, E.; Schallert, K.; Procaccio, V.; Jimenez, R.; Cramer, S.C. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat. Neurosci. 2006, 9, 735–737. [Google Scholar] [CrossRef] [PubMed]
- Taschereau-Dumouchel, V.; Hetu, S.; Michon, P.E.; Vachon-Presseau, E.; Massicotte, E.; De Beaumont, L.; Fecteau, S.; Poirier, J.; Mercier, C.; Chagnon, Y.C.; et al. BDNF Val(66)Met polymorphism influences visuomotor associative learning and the sensitivity to action observation. Sci. Rep. 2016, 6, 34907. [Google Scholar] [CrossRef] [PubMed]
- Wens, I.; Keytsman, C.; Deckx, N.; Cools, N.; Dalgas, U.; Eijnde, B.O. Brain derived neurotrophic factor in multiple sclerosis: Effect of 24 weeks endurance and resistance training. Eur. J. Neurol. 2016, 23, 1028–1035. [Google Scholar] [CrossRef]
- Yu, H.; Chen, Z.Y. The role of BDNF in depression on the basis of its location in the neural circuitry. Acta Pharmacol. Sin. 2011, 32, 3–11. [Google Scholar] [CrossRef]
- Sochal, M.; Małecka-Panas, E.; Gabryelska, A.; Fichna, J.; Talar-Wojnarowska, R.; Szmyd, B.; Białasiewicz, P. Brain-derived neurotrophic factor is elevated in the blood serum of Crohn’s disease patients, but is not influenced by anti-TNF-α treatment-A pilot study. Neurogastroenterol. Motil. 2020, 49, e13978. [Google Scholar] [CrossRef]
- Pattwell, S.S.; Bath, K.G.; Perez-Castro, R.; Lee, F.S.; Chao, M.V.; Ninan, I. The BDNF Val66Met polymorphism impairs synaptic transmission and plasticity in the infralimbic medial prefrontal cortex. J. Neurosci. 2012, 32, 2410–2421. [Google Scholar] [CrossRef]
- Ninan, I.; Bath, K.G.; Dagar, K.; Perez-Castro, R.; Plummer, M.R.; Lee, F.S.; Chao, M.V. The BDNF Val66Met polymorphism impairs NMDA receptor-dependent synaptic plasticity in the hippocampus. J. Neurosci. 2010, 30, 8866–8870. [Google Scholar] [CrossRef]
- Ferland, M.C.; Therrien-Blanchet, J.M.; Lefebvre, G.; Klees-Themens, G.; Proulx, S.; Theoret, H. Longitudinal assessment of (1)H-MRS (GABA and Glx) and TMS measures of cortical inhibition and facilitation in the sensorimotor cortex. Exp. Brain Res. 2019, 237, 3461–3474. [Google Scholar] [CrossRef] [PubMed]
- Stagg, C.J.; Bachtiar, V.; Johansen-Berg, H. The role of GABA in human motor learning. Curr. Biol. 2011, 21, 480–484. [Google Scholar] [CrossRef]
- Dyke, K.; Pepes, S.E.; Chen, C.; Kim, S.; Sigurdsson, H.P.; Draper, A.; Husain, M.; Nachev, P.; Gowland, P.A.; Morris, P.G.; et al. Comparing GABA-dependent physiological measures of inhibition with proton magnetic resonance spectroscopy measurement of GABA using ultra-high-field MRI. Neuroimage 2017, 152, 360–370. [Google Scholar] [CrossRef]
- Tremblay, S.; Beaule, V.; Proulx, S.; de Beaumont, L.; Marjanska, M.; Doyon, J.; Pascual-Leone, A.; Lassonde, M.; Theoret, H. Relationship between transcranial magnetic stimulation measures of intracortical inhibition and spectroscopy measures of GABA and glutamate + glutamine. J. Neurophysiol. 2013, 109, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, U.; Reis, J.; Schwenkreis, P.; Rosanova, M.; Strafella, A.; Badawy, R.; Müller-Dahlhaus, F. TMS and drugs revisited 2014. Clin. Neurophysiol. 2015, 126, 1847–1868. [Google Scholar] [CrossRef] [PubMed]
- Triesch, J.; Zrenner, C.; Ziemann, U. Modeling TMS-induced I-waves in human motor cortex. Prog. Brain Res. 2015, 222, 105–124. [Google Scholar]
- Cash, R.F.; Isayama, R.; Gunraj, C.A.; Ni, Z.; Chen, R. The influence of sensory afferent input on local motor cortical excitatory circuitry in humans. J. Physiol. 2015, 593, 1667–1684. [Google Scholar] [CrossRef] [PubMed]
- Wagle-Shukla, A.; Ni, Z.; Gunraj, C.A.; Bahl, N.; Chen, R. Effects of short interval intracortical inhibition and intracortical facilitation on short interval intracortical facilitation in human primary motor cortex. J. Physiol. 2009, 587, 5665–5678. [Google Scholar] [CrossRef]
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009, 120, 2008–2039. [Google Scholar] [CrossRef]
- Onishi, H.; Otsuru, N.; Kojima, S.; Miyaguchi, S.; Saito, K.; Inukai, Y.; Yamashiro, K.; Sato, D.; Tamaki, H.; Shirozu, H.; et al. Variability and reliability of paired-pulse depression and cortical oscillation induced by median nerve stimulation. Brain Topogr. 2018, 31, 780–794. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Gunraj, C.; Wagle-Shukla, A.; Udupa, K.; Mazzella, F.; Lozano, A.M.; Chen, R. Direct demonstration of inhibitory interactions between long interval intracortical inhibition and short interval intracortical inhibition. J. Physiol. 2011, 589, 2955–2962. [Google Scholar] [CrossRef]
- Hamada, M.; Strigaro, G.; Murase, N.; Sadnicka, A.; Galea, J.M.; Edwards, M.J.; Rothwell, J.C. Cerebellar modulation of human associative plasticity. J. Physiol. 2012, 590, 2365–2374. [Google Scholar] [CrossRef]
- Turco, C.V.; El-Sayes, J.; Locke, M.B.; Chen, R.; Baker, S.; Nelson, A.J. Effects of lorazepam and baclofen on short- and long-latency afferent inhibition. J. Physiol. 2018, 596, 5267–5280. [Google Scholar] [CrossRef]
- Provencher, S.W. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med. 1993, 30, 672–679. [Google Scholar] [CrossRef]
- Nwaroh, C.; Giuffre, A.; Cole, L.; Bell, T.; Carlson, H.L.; MacMaster, F.P.; Kirton, A.; Harris, A.D. Effects of transcranial direct current stimulation on GABA and Glx in children: A pilot study. PLoS ONE 2020, 15, e0222620. [Google Scholar] [CrossRef] [PubMed]
- Stagg, C.J.; Bestmann, S.; Constantinescu, A.O.; Moreno, L.M.; Allman, C.; Mekle, R.; Woolrich, M.; Near, J.; Johansen-Berg, H.; Rothwell, J.C. Relationship between physiological measures of excitability and levels of glutamate and GABA in the human motor cortex. J. Physiol. 2011, 589, 5845–5855. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Wang, H.; Lu, N.; Chen, T.; He, H.; Lu, Y.; Tu, X.M. Log-transformation and its implications for data analysis. Shanghai Arch. Psychiatry 2014, 26, 105–109. [Google Scholar]
- Opie, G.M.; Pourmajidian, M.; Ziemann, U.; Semmler, J.G. Investigating the influence of paired-associative stimulation on multi-session skill acquisition and retention in older adults. Clin. Neurophysiol. 2020, 131, 1497–1507. [Google Scholar] [CrossRef]
- Opie, G.M.; Hand, B.J.; Coxon, J.P.; Ridding, M.C.; Ziemann, U.; Semmler, J.G. Visuomotor task acquisition is reduced by priming paired associative stimulation in older adults. Neurobiol. Aging 2019, 81, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Boroojerdi, B.; Kopylev, L.; Battaglia, F.; Facchini, S.; Ziemann, U.; Muellbacher, W.; Cohen, L.G. Reproducibility of intracortical inhibition and facilitation using the paired-pulse paradigm. Muscle Nerve. 2000, 23, 1594–1597. [Google Scholar] [CrossRef]
- Goldsworthy, M.R.; Hordacre, B.; Ridding, M.C. Minimum number of trials required for within- and between-session reliability of TMS measures of corticospinal excitability. Neuroscience 2016, 320, 205–209. [Google Scholar] [CrossRef]
- Mizoguchi, Y.; Ishibashi, H.; Nabekura, J. The action of BDNF on GABA(A) currents changes from potentiating to suppressing during maturation of rat hippocampal CA1 pyramidal neurons. J. Physiol. 2003, 548, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Di Lazzaro, V.; Oliviero, A.; Profice, P.; Pennisi, M.A.; Di Giovanni, S.; Zito, G.; Tonali, P.; Rothwell, J.C. Muscarinic receptor blockade has differential effects on the excitability of intracortical circuits in the human motor cortex. Exp. Brain Res. 2000, 135, 455–461. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Pilato, F.; Dileone, M.; Tonali, P.A.; Ziemann, U. Dissociated effects of diazepam and lorazepam on short-latency afferent inhibition. J. Physiol. 2005, 569, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, U.; Chen, R.; Cohen, L.G.; Hallett, M. Dextromethorphan decreases the excitability of the human motor cortex. Neurology 1998, 51, 1320–1324. [Google Scholar] [CrossRef]
- Schwenkreis, P.; Witscher, K.; Janssen, F.; Addo, A.; Dertwinkel, R.; Zenz, M.; Malin, J.P.; Tegenthoff, M. Influence of the N-methyl-D-aspartate antagonist memantine on human motor cortex excitability. Neurosci. Lett. 1999, 270, 137–140. [Google Scholar] [CrossRef]
- Ilic, T.V.; Meintzschel, F.; Cleff, U.; Ruge, D.; Kessler, K.R.; Ziemann, U. Short-interval paired-pulse inhibition and facilitation of human motor cortex: The dimension of stimulus intensity. J. Physiol. 2002, 545, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Gruber, O.; Hasan, A.; Scherk, H.; Wobrock, T.; Schneider-Axmann, T.; Ekawardhani, S.; Schmitt, A.; Backens, M.; Reith, W.; Meyer, J.; et al. Association of the brain-derived neurotrophic factor val66met polymorphism with magnetic resonance spectroscopic markers in the human hippocampus: In vivo evidence for effects on the glutamate system. Eur. Arch. Psychiatry Clin. Neurosci. 2012, 262, 23–31. [Google Scholar] [CrossRef]
- Kotlęga, D.; Peda, B.; Zembroń-Łacny, A.; Gołąb-Janowska, M.; Nowacki, P. The role of brain-derived neurotrophic factor and its single nucleotide polymorphisms in stroke patients. Neurol. Neurochir. Pol. 2017, 51, 240–246. [Google Scholar] [CrossRef]
- Chang, W.H.; Bang, O.Y.; Shin, Y.I.; Lee, A.; Pascual-Leone, A.; Kim, Y.H. BDNF polymorphism and differential rTMS effects on motor recovery of stroke patients. Brain Stimul. 2014, 7, 553–558. [Google Scholar] [CrossRef]
- Bailey, A.Z.; Asmussen, M.J.; Nelson, A.J. Short-latency afferent inhibition determined by the sensory afferent volley. J. Neurophysiol. 2016, 116, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Zarzecki, P.; Shinoda, Y.; Asanuma, H. Projection from area 3a to the motor cortex by neurons activated from group I muscle afferents. Exp. Brain Res. 1978, 33, 269–282. [Google Scholar] [CrossRef]
- Ho, B.C.; Milev, P.; O’Leary, D.S.; Librant, A.; Andreasen, N.C.; Wassink, T.H. Cognitive and magnetic resonance imaging brain morphometric correlates of brain-derived neurotrophic factor Val66Met gene polymorphism in patients with schizophrenia and healthy volunteers. Arch. Gen. Psychiatry 2006, 63, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Poo, M.M. Neurotrophins as synaptic modulators. Nat. Rev. Neurosci. 2001, 2, 24–32. [Google Scholar] [CrossRef]
- Gallinat, J.; Schubert, F.; Bruhl, R.; Hellweg, R.; Klar, A.A.; Kehrer, C.; Wirth, C.; Sander, T.; Lang, U.E. Met carriers of BDNF Val66Met genotype show increased N-acetylaspartate concentration in the anterior cingulate cortex. Neuroimage 2010, 49, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Di Lazzaro, V.; Pilato, F.; Dileone, M.; Profice, P.; Ranieri, F.; Ricci, V.; Bria, P.; Tonali, P.A.; Ziemann, U. Segregating two inhibitory circuits in human motor cortex at the level of GABAA receptor subtypes: A TMS study. Clin. Neurophysiol. 2007, 118, 2207–2214. [Google Scholar] [CrossRef]
- McDonnell, M.N.; Orekhov, Y.; Ziemann, U. The role of GABA(B) receptors in intracortical inhibition in the human motor cortex. Exp. Brain Res. 2006, 173, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Cassady, K.; Gagnon, H.; Lalwani, P.; Simmonite, M.; Foerster, B.; Park, D.; Peltier, S.J.; Petrou, M.; Taylor, S.F.; Weissman, D.H.; et al. Sensorimotor network segregation declines with age and is linked to GABA and to sensorimotor performance. Neuroimage 2019, 186, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Baeshen, A.; Wyss, P.O.; Henning, A.; O’Gorman, R.L.; Piccirelli, M.; Kollias, S.; Michels, L. Test-retest reliability of the brain metabolites GABA and Glx with JPRESS, PRESS, and MEGA-PRESS MRS sequences in vivo at 3T. J. Magn. Reson. Imaging 2020, 51, 1181–1191. [Google Scholar] [CrossRef] [PubMed]


| Val/Val | Val/Met | Met/Met | |
|---|---|---|---|
| TMS (n = 45) | |||
| Participants | 14 | 19 | 12 |
| Age | 21.9 ± 1.7 | 21.8 ± 1.3 | 22.8 ± 3.0 |
| TS1 mV | 61.7 ± 8.3 | 63.1 ± 7.3 | 58.7 ± 9.0 |
| RMT | 48.9 ± 9.1 | 52.8 ± 6.3 | 49.3 ± 7.1 |
| Electrial stimulation | 10.3 ± 2.2 | 10.7 ± 2.1 | 9.0 ± 2.0 |
| MR (n = 30) | |||
| Participants | 14 | 11 | 5 |
| Age | 22.1 ± 2.1 | 22.2 ± 1.7 | 21.6 ± 0.5 |
| Both Sessions | Session 1 | Session 2 | |
|---|---|---|---|
| Single-pulse | 0.99 ± 0.02 | 1.01 ± 0.02 | 0.98 ± 0.02 |
| SICI | 0.43 ± 0.04 | 0.44 ± 0.05 | 0.41 ± 0.04 |
| LICI | 0.19 ± 0.03 | 0.20 ± 0.04 | 0.19 ± 0.03 |
| SAI_1 | 0.36 ± 0.05 | 0.36 ± 0.04 | 0.36 ± 0.06 |
| SAI_2 | 0.47 ± 0.05 | 0.48 ± 0.06 | 0.45 ± 0.06 |
| LAI | 0.43 ± 0.08 | 0.43 ± 0.07 | 0.43 ± 0.09 |
| Left M1 | Left S1 | Right Cerebellum | |
|---|---|---|---|
| Glx/tCr | 1.93 ± 0.03 | 1.96 ± 0.02 | 1.42 ± 0.03 |
| Cho/tCr | 0.23 ± 0.00 | 0.21 ± 0.00 | 0.28 ± 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasaki, R.; Otsuru, N.; Miyaguchi, S.; Kojima, S.; Watanabe, H.; Ohno, K.; Sakurai, N.; Kodama, N.; Sato, D.; Onishi, H. Influence of Brain-Derived Neurotrophic Factor Genotype on Short-Latency Afferent Inhibition and Motor Cortex Metabolites. Brain Sci. 2021, 11, 395. https://doi.org/10.3390/brainsci11030395
Sasaki R, Otsuru N, Miyaguchi S, Kojima S, Watanabe H, Ohno K, Sakurai N, Kodama N, Sato D, Onishi H. Influence of Brain-Derived Neurotrophic Factor Genotype on Short-Latency Afferent Inhibition and Motor Cortex Metabolites. Brain Sciences. 2021; 11(3):395. https://doi.org/10.3390/brainsci11030395
Chicago/Turabian StyleSasaki, Ryoki, Naofumi Otsuru, Shota Miyaguchi, Sho Kojima, Hiraku Watanabe, Ken Ohno, Noriko Sakurai, Naoki Kodama, Daisuke Sato, and Hideaki Onishi. 2021. "Influence of Brain-Derived Neurotrophic Factor Genotype on Short-Latency Afferent Inhibition and Motor Cortex Metabolites" Brain Sciences 11, no. 3: 395. https://doi.org/10.3390/brainsci11030395
APA StyleSasaki, R., Otsuru, N., Miyaguchi, S., Kojima, S., Watanabe, H., Ohno, K., Sakurai, N., Kodama, N., Sato, D., & Onishi, H. (2021). Influence of Brain-Derived Neurotrophic Factor Genotype on Short-Latency Afferent Inhibition and Motor Cortex Metabolites. Brain Sciences, 11(3), 395. https://doi.org/10.3390/brainsci11030395







