Virtual Reality for Neurorehabilitation and Cognitive Enhancement
Abstract
1. Introduction
2. Types of Virtual Reality
2.1. Non-Immersive Virtual Reality
2.2. Fully Immersive Virtual Reality
2.3. Augmented Reality
2.4. Mixed Reality
2.5. Extended Reality
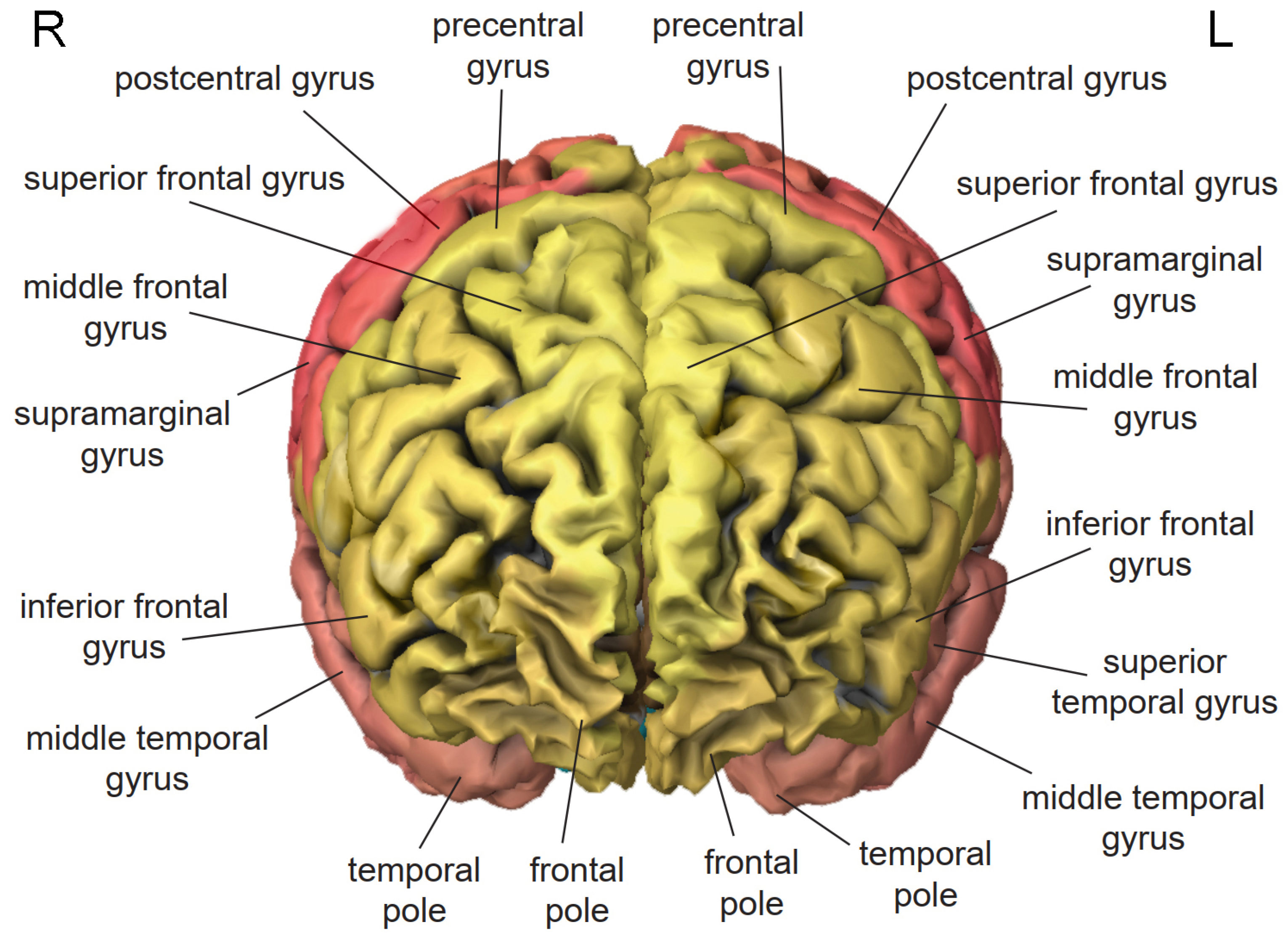
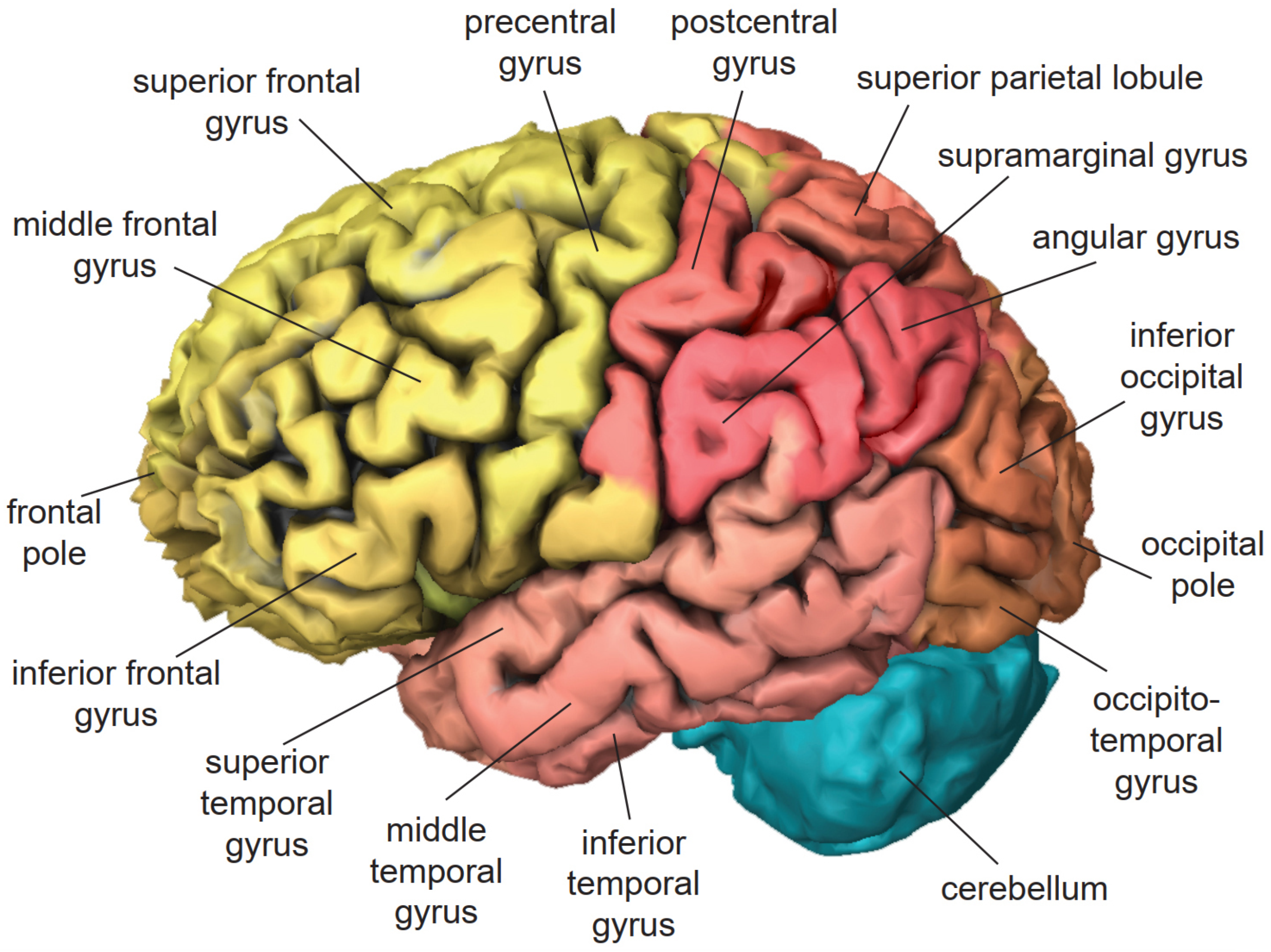
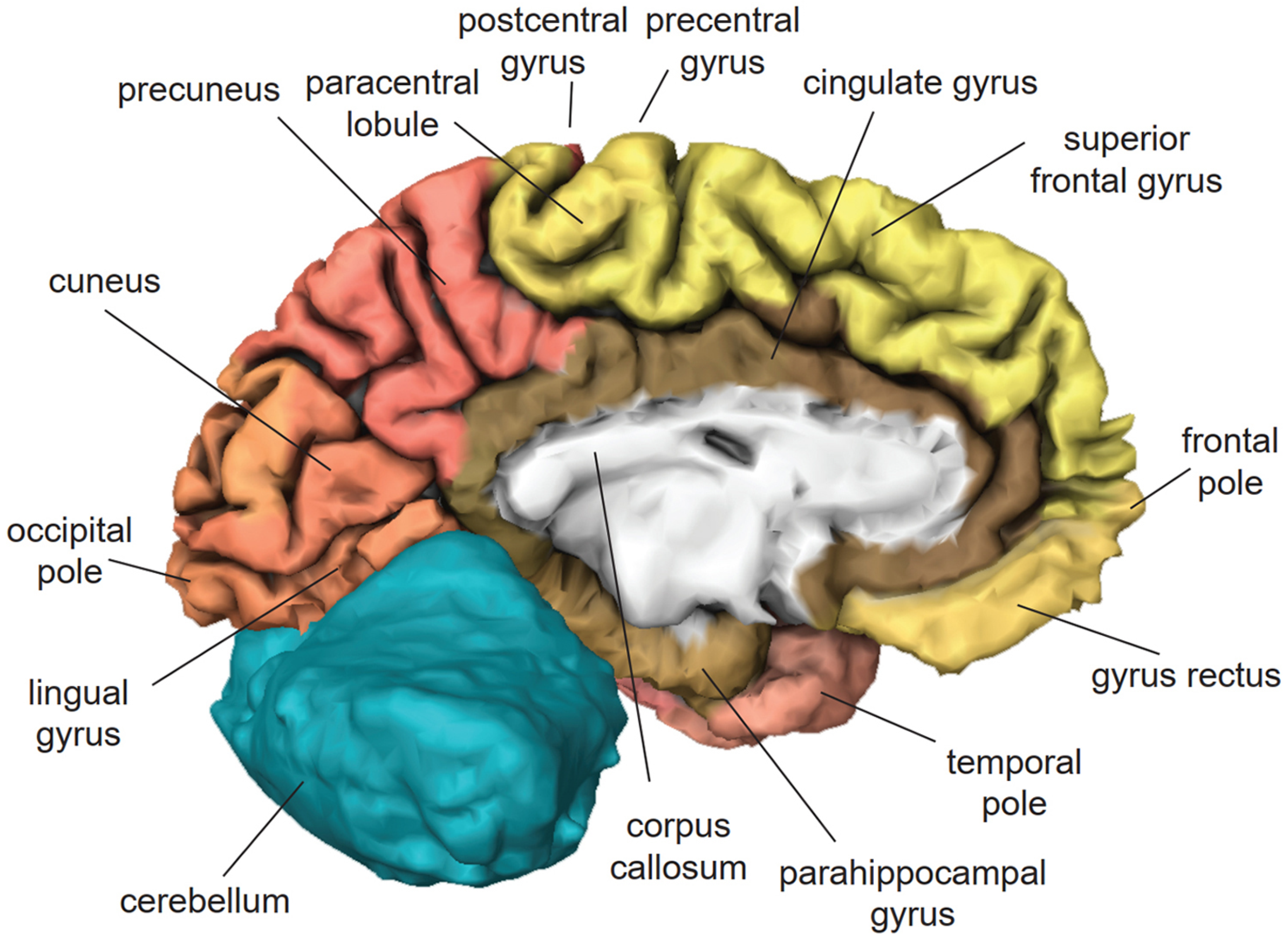
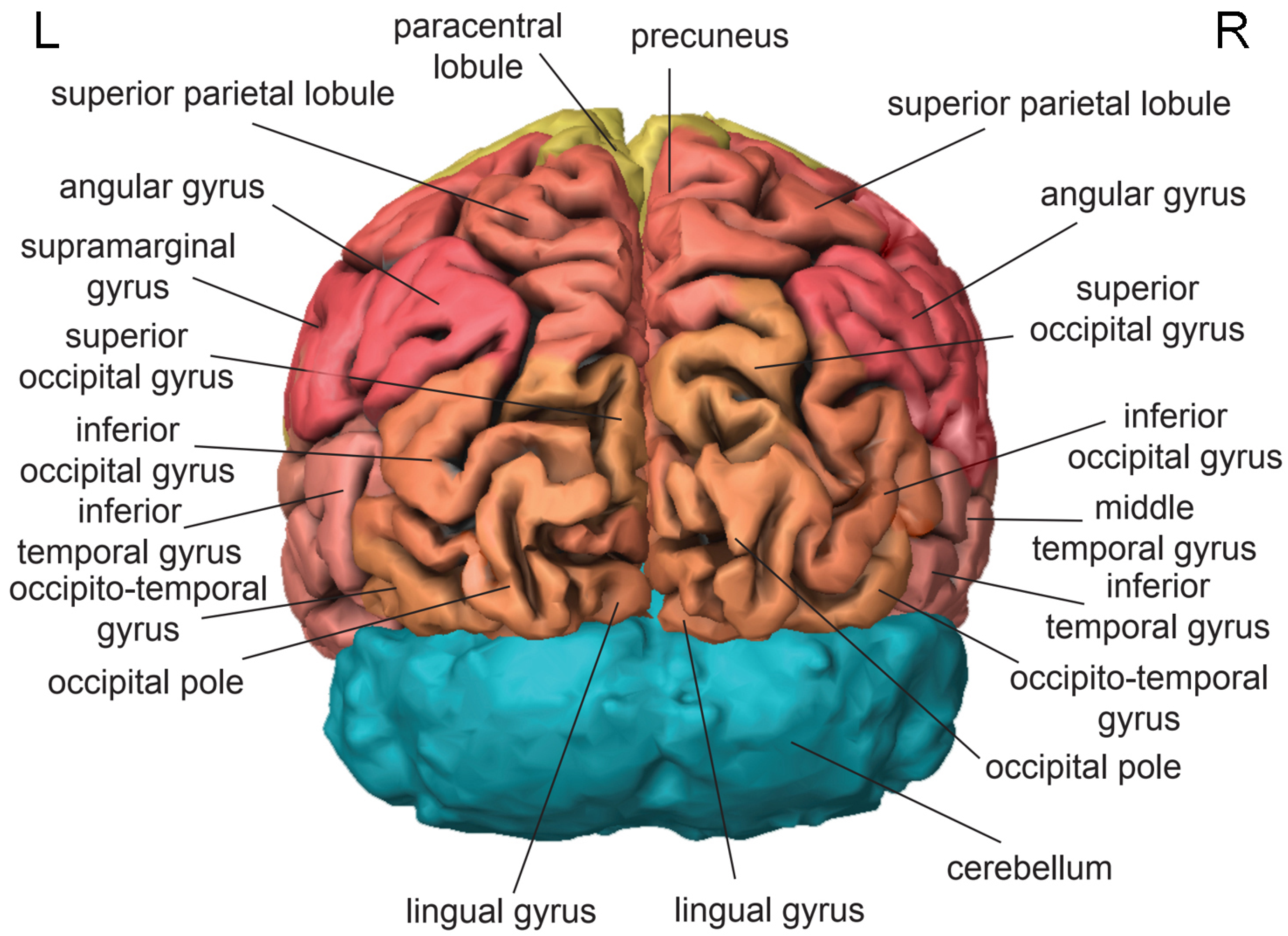
3. Cortical Localization of Cognitive Functions
3.1. Frontal Lobe
3.2. Temporal Lobe
3.3. Parietal Lobe
3.4. Occipital Lobe
4. Virtual Reality for Neurorehabilitation
4.1. Motor Rehabilitation
4.2. Cognitive Rehabilitation
4.3. Emotional Rehabilitation
4.4. Sensory Rehabilitation
5. Virtual Reality for Replacement of Function
5.1. Replacement of Motor Function
5.2. Replacement of Sensory Function
6. Virtual Reality for Self-Enhancement
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADL | activities of daily living |
| AR | augmented reality |
| AROM | active range of motion |
| ATM | automated teller machine |
| BCI | brain–computer interface |
| CCD | charge-coupled device |
| ECoG | electrocorticography |
| EEG | electroencephalography |
| HMD | head-mounted display |
| MEG | magnetoencephalography |
| MR | mixed reality |
| MRI | magnetic resonance imaging |
| NIRS | near-infrared spectroscopy |
| PET | positron emission tomography |
| SMR | sensorimotor rhythm |
| TBI | traumatic brain injury |
| TMS | transcranial magnetic stimulation |
| VR | virtual reality |
| XR | extended reality |
References
- Fenwick, T.; Edwards, R. Exploring the impact of digital technologies on professional responsibilities and education. Eur. Educ. Res. J. 2015, 15, 117–131. [Google Scholar] [CrossRef]
- Hilbert, M. Digital technology and social change: The digital transformation of society from a historical perspective. Dialogues Clin. Neurosci. 2020, 22, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Chassiakos, Y.R.; Radesky, J.; Christakis, D.; Moreno, M.A.; Cross, C. Children and adolescents and digital media. Pediatrics 2016, 138, e20162593. [Google Scholar] [CrossRef]
- Small, G.W.; Lee, J.; Kaufman, A.; Jalil, J.; Siddarth, P.; Gaddipati, H.; Moody, T.D.; Bookheimer, S.Y. Brain health consequences of digital technology use. Dialogues Clin. Neurosci. 2020, 22, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Ghahramani, F.; Wang, J. Impact of smartphones on quality of life: A health information behavior perspective. Inf. Syst. Front. 2020, 22, 1275–1290. [Google Scholar] [CrossRef]
- Cohen, J.; Bancilhon, J.M.; Grace, T. Digitally connected living and quality of life: An analysis of the Gauteng City-Region, South Africa. Electron. J. Inf. Syst. Dev. Ctries. 2018, 84, e12010. [Google Scholar] [CrossRef]
- Georgieva, I.; Georgiev, G.V. Redesign me: Virtual reality experience of the line of life and its connection to a healthier self. Behav. Sci. 2019, 9, 111. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, I.; Georgiev, G.V. Reconstructing personal stories in virtual reality as a mechanism to recover the self. Int. J. Environ. Res. Public Health 2020, 17, 26. [Google Scholar] [CrossRef]
- Lee, L.N.; Kim, M.J.; Hwang, W.J. Potential of augmented reality and virtual reality technologies to promote wellbeing in older adults. Appl. Sci. 2019, 9, 3556. [Google Scholar] [CrossRef]
- Cortés-Pérez, I.; Nieto-Escamez, F.A.; Obrero-Gaitán, E. Immersive virtual reality in stroke patients as a new approach for reducing postural disabilities and falls risk: A case series. Brain Sci. 2020, 10, 296. [Google Scholar] [CrossRef] [PubMed]
- Aulisio, M.C.; Han, D.Y.; Glueck, A.C. Virtual reality gaming as a neurorehabilitation tool for brain injuries in adults: A systematic review. Brain Inj. 2020, 34, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, L.R.; Serruya, M.D.; Friehs, G.M.; Mukand, J.A.; Saleh, M.; Caplan, A.H.; Branner, A.; Chen, D.; Penn, R.D.; Donoghue, J.P. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature 2006, 442, 164–171. [Google Scholar] [CrossRef]
- Onose, G.; Grozea, C.; Anghelescu, A.; Daia, C.; Sinescu, C.J.; Ciurea, A.V.; Spircu, T.; Mirea, A.; Andone, I.; Spânu, A.; et al. On the feasibility of using motor imagery EEG-based brain–computer interface in chronic tetraplegics for assistive robotic arm control: A clinical test and long-term post-trial follow-up. Spinal Cord 2012, 50, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Vansteensel, M.J.; Pels, E.G.M.; Bleichner, M.G.; Branco, M.P.; Denison, T.; Freudenburg, Z.V.; Gosselaar, P.; Leinders, S.; Ottens, T.H.; Van Den Boom, M.A.; et al. Fully implanted brain-computer interface in a locked-in patient with ALS. N. Engl. J. Med. 2016, 375, 2060–2066. [Google Scholar] [CrossRef]
- Pandarinath, C.; Nuyujukian, P.; Blabe, C.H.; Sorice, B.L.; Saab, J.; Willett, F.R.; Hochberg, L.R.; Shenoy, K.V.; Henderson, J.M. High performance communication by people with paralysis using an intracortical brain-computer interface. eLife 2017, 6, e18554. [Google Scholar] [CrossRef]
- Leeb, R.; Perez-Marcos, D. Brain-computer interfaces and virtual reality for neurorehabilitation. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 168, pp. 183–197. [Google Scholar] [CrossRef]
- Hwang, J.; Lee, S. The effect of virtual reality program on the cognitive function and balance of the people with mild cognitive impairment. J. Phys. Ther. Sci. 2017, 29, 1283–1286. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.C.M.; Andringa, G. The potential of immersive virtual reality for cognitive training in elderly. Gerontology 2020, 66, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Gamito, P.; Oliveira, J.; Alves, C.; Santos, N.; Coelho, C.; Brito, R. Virtual reality-based cognitive stimulation to improve cognitive functioning in community elderly: A controlled study. Cyberpsychol. Behav. Soc. Netw. 2020, 23, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.Y.; Tseng, H.Y.; Lin, Y.J.; Wang, C.J.; Hsu, W.C. Using virtual reality-based training to improve cognitive function, instrumental activities of daily living and neural efficiency in older adults with mild cognitive impairment. Eur. J. Phys. Rehabil. Med. 2020, 56, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, V.; Stramba-Badiale, C.; Cavedoni, S.; Pedroli, E.; Cipresso, P.; Riva, G. Virtual reality meets non-invasive brain stimulation: Integrating two methods for cognitive rehabilitation of mild cognitive impairment. Front. Neurol. 2020, 11, 1117. [Google Scholar] [CrossRef]
- Thapa, N.; Park, H.J.; Yang, J.G.; Son, H.; Jang, M.; Lee, J.; Kang, S.W.; Park, K.W.; Park, H. The effect of a virtual reality-based intervention program on cognition in older adults with mild cognitive impairment: A randomized control trial. J. Clin. Med. 2020, 9, 1283. [Google Scholar] [CrossRef] [PubMed]
- Citri, A.; Malenka, R.C. Synaptic plasticity: Multiple forms, functions, and mechanisms. Neuropsychopharmacology 2007, 33, 18–41. [Google Scholar] [CrossRef]
- Hortsch, M.; Umemori, H. The Sticky Synapse: Cell Adhesion Molecules and Their Role in Synapse Formation and Maintenance; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar] [CrossRef]
- Hering, H.; Sheng, M. Dentritic spines: Structure, dynamics and regulation. Nat. Rev. Neurosci. 2001, 2, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Bock, J.; Gruss, M.; Becker, S.; Braun, K. Experience-induced changes of dendritic spine densities in the prefrontal and sensory cortex: Correlation with developmental time windows. Cereb. Cortex 2005, 15, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Holtmaat, A.J.G.D.; Trachtenberg, J.T.; Wilbrecht, L.; Shepherd, G.M.; Zhang, X.; Knott, G.W.; Svoboda, K. Transient and persistent dendritic spines in the neocortex in vivo. Neuron 2005, 45, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lai, C.S.W.; Bai, Y.; Li, W.; Zhao, R.; Yang, G.; Frank, M.G.; Gan, W.B. REM sleep promotes experience-dependent dendritic spine elimination in the mouse cortex. Nat. Commun. 2020, 11, 4819. [Google Scholar] [CrossRef] [PubMed]
- Chklovskii, D.B. Synaptic connectivity and neuronal morphology: Two sides of the same coin. Neuron 2004, 43, 609–617. [Google Scholar] [CrossRef]
- Markham, J.A.; Greenough, W.T. Experience-driven brain plasticity: Beyond the synapse. Neuron Glia Biol. 2004, 1, 351–363. [Google Scholar] [CrossRef]
- Hamilton, D.A.; Silasi, G.; Magcalas, C.M.; Pellis, S.M.; Kolb, B. Social and olfactory experiences modify neuronal morphology of orbital frontal cortex. Behav. Neurosci. 2020, 134, 59–68. [Google Scholar] [CrossRef]
- Zhang, W.; Linden, D.J. The other side of the engram: Experience-driven changes in neuronal intrinsic excitability. Nat. Rev. Neurosci. 2003, 4, 885–900. [Google Scholar] [CrossRef] [PubMed]
- Schulz, D.J. Plasticity and stability in neuronal output via changes in intrinsic excitability: It’s what’s inside that counts. J. Exp. Biol. 2006, 209, 4821–4827. [Google Scholar] [CrossRef]
- McKay, B.M.; Matthews, E.A.; Oliveira, F.A.; Disterhoft, J.F. Intrinsic neuronal excitability is reversibly altered by a single experience in fear conditioning. J. Neurophysiol. 2009, 102, 2763–2770. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Kastner, S. Promises and limitations of human intracranial electroencephalography. Nat. Neurosci. 2018, 21, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Racz, F.S.; Stylianou, O.; Mukli, P.; Eke, A. Multifractal and entropy analysis of resting-state electroencephalography reveals spatial organization in local dynamic functional connectivity. Sci. Rep. 2019, 9, 13474. [Google Scholar] [CrossRef]
- Hill, R.M.; Boto, E.; Holmes, N.; Hartley, C.; Seedat, Z.A.; Leggett, J.; Roberts, G.; Shah, V.; Tierney, T.M.; Woolrich, M.W.; et al. A tool for functional brain imaging with lifespan compliance. Nat. Commun. 2019, 10, 4785. [Google Scholar] [CrossRef] [PubMed]
- Tierney, T.M.; Mellor, S.; O’Neill, G.C.; Holmes, N.; Boto, E.; Roberts, G.; Hill, R.M.; Leggett, J.; Bowtell, R.; Brookes, M.J.; et al. Pragmatic spatial sampling for wearable MEG arrays. Sci. Rep. 2020, 10, 21609. [Google Scholar] [CrossRef] [PubMed]
- Quaresima, V.; Ferrari, M. Functional near-infrared spectroscopy (fNIRS) for assessing cerebral cortex function during human behavior in natural/social situations: A concise review. Organ. Res. Methods 2016, 22, 46–68. [Google Scholar] [CrossRef]
- Causse, M.; Chua, Z.; Peysakhovich, V.; Del Campo, N.; Matton, N. Mental workload and neural efficiency quantified in the prefrontal cortex using fNIRS. Sci. Rep. 2017, 7, 5222. [Google Scholar] [CrossRef] [PubMed]
- Varvatsoulias, G. The physiological processes underpinning PET and fMRI techniques with an emphasis on the temporal and spatial resolution of these methods. Psychol. Thought 2013, 6, 173–195. [Google Scholar] [CrossRef]
- Wehrl, H.F.; Hossain, M.; Lankes, K.; Liu, C.C.; Bezrukov, I.; Martirosian, P.; Schick, F.; Reischl, G.; Pichler, B.J. Simultaneous PET-MRI reveals brain function in activated and resting state on metabolic, hemodynamic and multiple temporal scales. Nat. Med. 2013, 19, 1184–1189. [Google Scholar] [CrossRef]
- Jamadar, S.D.; Ward, P.G.D.; Close, T.G.; Fornito, A.; Premaratne, M.; O’Brien, K.; Stäb, D.; Chen, Z.; Shah, N.J.; Egan, G.F. Simultaneous BOLD-fMRI and constant infusion FDG-PET data of the resting human brain. Sci. Data 2020, 7, 363. [Google Scholar] [CrossRef]
- Raichle, M.E. A brief history of human brain mapping. Trends Neurosci. 2009, 32, 118–126. [Google Scholar] [CrossRef]
- Bijsterbosch, J.; Harrison, S.J.; Jbabdi, S.; Woolrich, M.; Beckmann, C.; Smith, S.; Duff, E.P. Challenges and future directions for representations of functional brain organization. Nat. Neurosci. 2020, 23, 1484–1495. [Google Scholar] [CrossRef]
- Tian, Y.; Margulies, D.S.; Breakspear, M.; Zalesky, A. Topographic organization of the human subcortex unveiled with functional connectivity gradients. Nat. Neurosci. 2020, 23, 1421–1432. [Google Scholar] [CrossRef]
- Ventura, S.; Brivio, E.; Riva, G.; Baños, R.M. Immersive versus non-immersive experience: Exploring the feasibility of memory assessment through 360° technology. Front. Psychol. 2019, 10, 2509. [Google Scholar] [CrossRef]
- Sanchez-Vives, M.V.; Slater, M. From presence to consciousness through virtual reality. Nat. Rev. Neurosci. 2005, 6, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Bohil, C.J.; Alicea, B.; Biocca, F.A. Virtual reality in neuroscience research and therapy. Nat. Rev. Neurosci. 2011, 12, 752–762. [Google Scholar] [CrossRef]
- Matthews, D. Virtual-reality applications give science a new dimension. Nature 2018, 557, 127–128. [Google Scholar] [CrossRef]
- Porter, M.E.; Heppelmann, J.E. Why every organization needs an augmented reality strategy. Harv. Bus. Rev. 2017, 95, 46–57. [Google Scholar]
- Invitto, S.; Spada, I.; De Paolis, L.T. Augmented reality, embodied cognition and learning. In Augmented and Virtual Reality; Lecture Notes in Computer, Science; De Paolis, L.T., Mongelli, A., Eds.; Springer: Cham, Switzerland, 2015; pp. 125–134. [Google Scholar] [CrossRef]
- Hu, X.; Georgiev, G.V.; Casakin, H. Mitigating design fixation with evolving extended reality technology: An emerging opportunity. Proc. Des. Soc. Des. Conf. 2020, 1, 1305–1314. [Google Scholar] [CrossRef]
- Hu, X.; Georgiev, G.V. Opportunities with uncertainties: The outlook of virtual reality in the early stages of design. In Proceedings of the Sixth International Conference on Design Creativity (ICDC 2020); Boujut, J.F., Cascini, G., Ahmed-Kristensen, S., Georgiev, G.V., Iivari, N., Eds.; The Design Society: Oulu, Finland, 2020; pp. 215–222. [Google Scholar] [CrossRef]
- Park, E.; Yun, B.J.; Min, Y.S.; Lee, Y.S.; Moon, S.J.; Huh, J.W.; Cha, H.; Chang, Y.; Jung, T.D. Effects of a mixed reality-based cognitive training system compared to a conventional computer-assisted cognitive training system on mild cognitive impairment: A pilot study. Cogn. Behav. Neurol. 2019, 32, 172–178. [Google Scholar] [CrossRef]
- Georgiev, D.D. Quantum Information and Consciousness: A Gentle Introduction; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar] [CrossRef]
- Georgiev, D.D. Inner privacy of conscious experiences and quantum information. Biosystems 2020, 187, 104051. [Google Scholar] [CrossRef]
- Georgiev, D.D. Quantum information theoretic approach to the mind–brain problem. Prog. Biophys. Mol. Biol. 2020, 158, 16–32. [Google Scholar] [CrossRef]
- Van Essen, D.C.; Donahue, C.J.; Glasser, M.F. Development and evolution of cerebral and cerebellar cortex. Brain Behav. Evol. 2018, 91, 158–169. [Google Scholar] [CrossRef]
- Georgiev, D.D.; Kolev, S.K.; Cohen, E.; Glazebrook, J.F. Computational capacity of pyramidal neurons in the cerebral cortex. Brain Res. 2020, 1748, 147069. [Google Scholar] [CrossRef]
- Popper, K.R.; Eccles, J.C. The Self and Its Brain: An Argument for Interactionism; Routledge & Kegan Paul: London, UK, 1983. [Google Scholar] [CrossRef]
- Eccles, J.C. Facing Reality: Philosophical Adventures by a Brain Scientist; Heidelberg Science Library; Springer: Berlin, Germany, 1970; Volume 13. [Google Scholar] [CrossRef]
- Phillips, C.G.; Zeki, S.; Barlow, H.B. Localization of function in the cerebral cortex: Past, present and future. Brain 1984, 107, 328–361. [Google Scholar] [CrossRef]
- Gross, C.G. A Hole in the Head: More Tales in the History of Neuroscience; MIT Press: Cambridge, MA, USA, 2009. [Google Scholar] [CrossRef]
- Posner, M.I.; Petersen, S.E.; Fox, P.T.; Raichle, M.E. Localization of cognitive operations in the human brain. Science 1988, 240, 1627–1631. [Google Scholar] [CrossRef]
- Ross, E.D. Cerebral localization of functions and the neurology of language: Fact versus fiction or is it something else? Neuroscientist 2010, 16, 222–243. [Google Scholar] [CrossRef] [PubMed]
- Vourvopoulos, A.; Bermudez, I.B.S. Motor priming in virtual reality can augment motor-imagery training efficacy in restorative brain-computer interaction: A within-subject analysis. J. Neuroeng. Rehabil. 2016, 13, 69. [Google Scholar] [CrossRef]
- Vourvopoulos, A.; Pardo, O.M.; Lefebvre, S.; Neureither, M.; Saldana, D.; Jahng, E.; Liew, S.L. Effects of a brain-computer interface with virtual reality (VR) neurofeedback: A pilot study in chronic stroke patients. Front. Hum. Neurosci. 2019, 13, 210. [Google Scholar] [CrossRef]
- Wiederhold, B.K.; Wiederhold, M.D. Virtual reality with fMRI: A breakthrough cognitive treatment tool. Virtual Real. 2008, 12, 259–267. [Google Scholar] [CrossRef]
- Calabrò, R.S.; Naro, A.; Russo, M.; Leo, A.; De Luca, R.; Balletta, T.; Buda, A.; La Rosa, G.; Bramanti, A.; Bramanti, P. The role of virtual reality in improving motor performance as revealed by EEG: A randomized clinical trial. J. Neuroeng. Rehabil. 2017, 14, 53. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, I.I.; Harel, M.; Malach, R. When the brain loses its self: Prefrontal inactivation during sensorimotor processing. Neuron 2006, 50, 329–339. [Google Scholar] [CrossRef]
- Fried, I.; Wilson, C.L.; MacDonald, K.A.; Behnke, E.J. Electric current stimulates laughter. Nature 1998, 391, 650. [Google Scholar] [CrossRef] [PubMed]
- Kübler, A.; Dixon, V.; Garavan, H. Automaticity and reestablishment of executive control—An fMRI study. J. Cogn. Neurosci. 2006, 18, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Raye, C.L.; Johnson, M.K.; Mitchell, K.J.; Reeder, J.A.; Greene, E.J. Neuroimaging a single thought: Dorsolateral PFC activity associated with refreshing just-activated information. NeuroImage 2002, 15, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.X.; Leung, H.C.; Johnson, M.K. Frontal activations associated with accessing and evaluating information in working memory: An fMRI study. NeuroImage 2003, 20, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Babiloni, C.; Ferretti, A.; Del Gratta, C.; Carducci, F.; Vecchio, F.; Romani, G.L.; Rossini, P.M. Human cortical responses during one-bit delayed-response tasks: An fMRI study. Brain Res. Bull. 2005, 65, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Dronkers, N.F.; Plaisant, O.; Iba-Zizen, M.T.; Cabanis, E.A. Paul Broca’s historic cases: High resolution MR imaging of the brains of Leborgne and Lelong. Brain 2007, 130, 1432–1441. [Google Scholar] [CrossRef]
- Kwan, C.L.; Crawley, A.P.; Mikulis, D.J.; Davis, K.D. An fMRI study of the anterior cingulate cortex and surrounding medial wall activations evoked by noxious cutaneous heat and cold stimuli. Pain 2000, 85, 359–374. [Google Scholar] [CrossRef]
- Foland-Ross, L.C.; Hamilton, P.; Sacchet, M.D.; Furman, D.J.; Sherdell, L.; Gotlib, I.H. Activation of the medial prefrontal and posterior cingulate cortex during encoding of negative material predicts symptom worsening in major depression. Neuroreport 2014, 25, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Shackman, A.J.; Salomons, T.V.; Slagter, H.A.; Fox, A.S.; Winter, J.J.; Davidson, R.J. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat. Rev. Neurosci. 2011, 12, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Binney, R.J.; Ralph, M.A.L. Using a combination of fMRI and anterior temporal lobe rTMS to measure intrinsic and induced activation changes across the semantic cognition network. Neuropsychologia 2015, 76, 170–181. [Google Scholar] [CrossRef]
- Bréchet, L.; Mange, R.; Herbelin, B.; Theillaud, Q.; Gauthier, B.; Serino, A.; Blanke, O. First-person view of one’s body in immersive virtual reality: Influence on episodic memory. PLoS ONE 2019, 14, e0197763. [Google Scholar] [CrossRef] [PubMed]
- Price, C.J.; Price, C.J.; Wise, R.J.S.; Warburton, E.A.; Moore, C.J.; Howard, D.; Patterson, K.; Frackowiak, R.S.J.; Friston, K.J. Hearing and saying: The functional neuro-anatomy of auditory word processing. Brain 1996, 119, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, J.; Fan, L.; Li, H.; Zhang, W.; Hu, Q.; Jiang, T. Tractography-based parcellation of the human middle temporal gyrus. Sci. Rep. 2015, 5, 18883. [Google Scholar] [CrossRef]
- Buckner, R.L.; Koutstaal, W.; Schacter, D.L.; Rosen, B.R. Functional MRI evidence for a role of frontal and inferior temporal cortex in amodal components of priming. Brain 2000, 123, 620–640. [Google Scholar] [CrossRef] [PubMed]
- Nasr, S.; Tootell, R.B.H. Role of fusiform and anterior temporal cortical areas in facial recognition. NeuroImage 2012, 63, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, E.; Ohki, K.; Miyashita, Y. The role of the parahippocampal gyrus in source memory for external and internal events. NeuroReport 2002, 13, 1951–1956. [Google Scholar] [CrossRef]
- Ehrsson, H.H.; Holmes, N.P.; Passingham, R.E. Touching a rubber hand: Feeling of body ownership is associated with activity in multisensory brain areas. J. Neurosci. 2005, 25, 10564–10573. [Google Scholar] [CrossRef]
- Slater, M.; Pérez Marcos, D.; Ehrsson, H.; Sanchez-Vives, M. Inducing illusory ownership of a virtual body. Front. Neurosci. 2009, 3, 29. [Google Scholar] [CrossRef] [PubMed]
- Kilteni, K.; Groten, R.; Slater, M. The sense of embodiment in virtual reality. Presence 2012, 21, 373–387. [Google Scholar] [CrossRef]
- Clemente, M.; Rey, B.; Rodríguez-Pujadas, A.; Barros-Loscertales, A.; Baños, R.M.; Botella, C.; Alcañiz, M.; Ávila, C. An fMRI study to analyze neural correlates of presence during virtual reality experiences. Interact. Comput. 2014, 26, 269–284. [Google Scholar] [CrossRef]
- Brecht, M. The body model theory of somatosensory cortex. Neuron 2017, 94, 985–992. [Google Scholar] [CrossRef]
- Andersson, P.; Ragni, F.; Lingnau, A. Visual imagery during real-time fMRI neurofeedback from occipital and superior parietal cortex. NeuroImage 2019, 200, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Bonda, E.; Petrides, M.; Frey, S.; Evans, A. Neural correlates of mental transformations of the body-in-space. Proc. Natl. Acad. Sci. USA 1995, 92, 11180–11184. [Google Scholar] [CrossRef] [PubMed]
- Wadden, K.P.; Snow, N.J.; Sande, P.; Slawson, S.; Waller, T.; Boyd, L.A. Yoga practitioners uniquely activate the superior parietal lobule and supramarginal gyrus during emotion regulation. Front. Integr. Neurosci. 2018, 12, 60. [Google Scholar] [CrossRef]
- Ben-Shabat, E.; Matyas, T.A.; Pell, G.S.; Brodtmann, A.; Carey, L.M. The right supramarginal gyrus is important for proprioception in healthy and stroke-affected participants: A functional MRI study. Front. Neurol. 2015, 6, 248. [Google Scholar] [CrossRef] [PubMed]
- Celsis, P.; Boulanouar, K.; Doyon, B.; Ranjeva, J.P.; Berry, I.; Nespoulous, J.L.; Chollet, F. Differential fMRI responses in the left posterior superior temporal gyrus and left supramarginal gyrus to habituation and change detection in syllables and tones. NeuroImage 1999, 9, 135–144. [Google Scholar] [CrossRef]
- Oberhuber, M.; Hope, T.M.H.; Seghier, M.L.; Parker Jones, O.; Prejawa, S.; Green, D.W.; Price, C.J. Four functionally distinct regions in the left supramarginal gyrus support word processing. Cereb. Cortex 2016, 26, 4212–4226. [Google Scholar] [CrossRef]
- Stanescu-Cosson, R.; Pinel, P.; van de Moortele, P.F.; Le Bihan, D.; Cohen, L.; Dehaene, S. Understanding dissociations in dyscalculia: A brain imaging study of the impact of number size on the cerebral networks for exact and approximate calculation. Brain 2000, 123, 2240–2255. [Google Scholar] [CrossRef]
- van der Linden, M.; Berkers, R.M.W.J.; Morris, R.G.M.; Fernández, G. Angular gyrus involvement at encoding and retrieval is associated with durable but less specific memories. J. Neurosci. 2017, 37, 9474–9485. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Kirino, E. Increased functional connectivity of the angular gyrus during imagined music performance. Front. Hum. Neurosci. 2019, 13, 92. [Google Scholar] [CrossRef]
- Cavanna, A.E.; Trimble, M.R. The precuneus: A review of its functional anatomy and behavioural correlates. Brain 2006, 129, 564–583. [Google Scholar] [CrossRef] [PubMed]
- Glickstein, M. The discovery of the visual cortex. Sci. Am. 1988, 259, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Bekrater-Bodmann, R.; Foell, J.; Diers, M.; Kamping, S.; Rance, M.; Kirsch, P.; Trojan, J.; Fuchs, X.; Bach, F.; Çakmak, H.K.; et al. The importance of synchrony and temporal order of visual and tactile input for illusory limb ownership experiences—An fMRI study applying virtual reality. PLoS ONE 2014, 9, e87013. [Google Scholar] [CrossRef]
- Tootell, R.B.H.; Hadjikhani, N.K.; Vanduffel, W.; Liu, A.K.; Mendola, J.D.; Sereno, M.I.; Dale, A.M. Functional analysis of primary visual cortex (V1) in humans. Proc. Natl. Acad. Sci. USA 1998, 95, 811–817. [Google Scholar] [CrossRef]
- Bridge, H. Mapping the visual brain: How and why. Eye 2011, 25, 291–296. [Google Scholar] [CrossRef]
- Kawachi, J. Brodmann areas 17, 18, and 19 in the human brain: An overview. Brain Nerve 2017, 69, 397–410. [Google Scholar] [CrossRef]
- Vanni, S.; Tanskanen, T.; Seppä, M.; Uutela, K.; Hari, R. Coinciding early activation of the human primary visual cortex and anteromedial cuneus. Proc. Natl. Acad. Sci. USA 2001, 98, 2776–2780. [Google Scholar] [CrossRef]
- Yang, Y.L.; Deng, H.X.; Xing, G.Y.; Xia, X.L.; Li, H.F. Brain functional network connectivity based on a visual task: Visual information processing-related brain regions are significantly activated in the task state. Neural Regen. Res. 2015, 10, 298–307. [Google Scholar] [CrossRef]
- Mechelli, A.; Humphreys, G.W.; Mayall, K.; Olson, A.; Price, C.J. Differential effects of word length and visual contrast in the fusiform and lingual gyri during reading. Proc. R. Soc. Lond. Ser. B 2000, 267, 1909–1913. [Google Scholar] [CrossRef]
- Dong, Y.; Fukuyama, H.; Honda, M.; Okada, T.; Hanakawa, T.; Nakamura, K.; Nagahama, Y.; Nagamine, T.; Konishi, J.; Shibasaki, H. Essential role of the right superior parietal cortex in Japanese kana mirror reading: An fMRI study. Brain 2000, 123, 790–799. [Google Scholar] [CrossRef][Green Version]
- Faul, M.; Coronado, V. Epidemiology of traumatic brain injury. In Handbook of Clinical Neurology; Grafman, J., Salazar, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 127, Chapter 1; pp. 3–13. [Google Scholar] [CrossRef]
- Guzik, A.; Bushnell, C. Stroke epidemiology and risk factor management. Contin. Lifelong Learn. Neurol. 2017, 23, 15–39. [Google Scholar] [CrossRef]
- Castor, N.; El Massioui, F. Traumatic brain injury and stroke: Does recovery differ? Brain Inj. 2018, 32, 1803–1810. [Google Scholar] [CrossRef]
- Weiss, P.L.T.; Keshner, E.A.; Levin, M.F. Virtual Reality for Physical and Motor Rehabilitation; Virtual Reality Technologies for Health and Clinical Applications; Springer: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Aida, J.; Chau, B.; Dunn, J. Immersive virtual reality in traumatic brain injury rehabilitation: A literature review. NeuroRehabilitation 2018, 42, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Levac, D.; Miller, P.; Missiuna, C. Usual and virtual reality video game-based physiotherapy for children and youth with acquired brain injuries. Phys. Occup. Ther. Pediatr. 2012, 32, 180–195. [Google Scholar] [CrossRef] [PubMed]
- Piron, L.; Cenni, F.; Tonin, P.; Dam, M. Virtual Reality as an assessment tool for arm motor deficits after brain lesions. Stud. Health Technol. Inform. 2001, 81, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Gatica-Rojas, V.; Mendez-Rebolledo, G. Virtual reality interface devices in the reorganization of neural networks in the brain of patients with neurological diseases. Neural Regen. Res. 2014, 9, 888–896. [Google Scholar] [CrossRef]
- Saposnik, G.; Teasell, R.; Mamdani, M.; Hall, J.; McIlroy, W.; Cheung, D.; Thorpe Kevin, E.; Cohen Leonardo, G.; Bayley, M. Effectiveness of virtual reality using Wii gaming technology in stroke rehabilitation. Stroke 2010, 41, 1477–1484. [Google Scholar] [CrossRef]
- Fernandes, A.B.; Passos, J.O.; Brito, D.P.; Campos, T.F. Comparison of the immediate effect of the training with a virtual reality game in stroke patients according side brain injury. NeuroRehabilitation 2014, 35, 39–45. [Google Scholar] [CrossRef]
- Keller, J.; Stetkarova, I.; Macri, V.; Kuhn, S.; Petioky, J.; Gualeni, S.; Simmons, C.D.; Arthanat, S.; Zilber, P. Virtual reality-based treatment for regaining upper extremity function induces cortex grey matter changes in persons with acquired brain injury. J. Neuroeng. Rehabil. 2020, 17, 127. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, Y.M.; Lee, B.H. Effects of virtual reality-based bilateral upper-extremity training on brain activity in post-stroke patients. J. Phys. Ther. Sci. 2015, 27, 2285–2287. [Google Scholar] [CrossRef]
- Jung, S.M.; Choi, W.H. Effects of virtual reality intervention on upper limb motor function and activity of daily living in patients with lesions in different regions of the brain. J. Phys. Ther. Sci. 2017, 29, 2103–2106. [Google Scholar] [CrossRef][Green Version]
- Bonuzzi, G.M.G.; de Freitas, T.B.; Palma, G.; Soares, M.A.A.; Lange, B.; Pompeu, J.E.; Torriani-Pasin, C. Effects of the brain-damaged side after stroke on the learning of a balance task in a non-immersive virtual reality environment. Physiother. Theory Pract. 2020. [Google Scholar] [CrossRef]
- Maggio, M.G.; Torrisi, M.; Buda, A.; De Luca, R.; Piazzitta, D.; Cannavo, A.; Leo, A.; Milardi, D.; Manuli, A.; Calabrò, R.S. Effects of robotic neurorehabilitation through Lokomat plus virtual reality on cognitive function in patients with traumatic brain injury: A retrospective case-control study. Int. J. Neurosci. 2020, 130, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Biffi, E.; Beretta, E.; Cesareo, A.; Maghini, C.; Turconi, A.C.; Reni, G.; Strazzer, S. An immersive virtual reality platform to enhance walking ability of children with acquired brain injuries. Methods Inf. Med. 2017, 56, 119–126. [Google Scholar] [CrossRef]
- Luu, T.P.; He, Y.; Brown, S.; Nakagame, S.; Contreras-Vidal, J.L. Gait adaptation to visual kinematic perturbations using a real-time closed-loop brain-computer interface to a virtual reality avatar. J. Neural Eng. 2016, 13, 036006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Abreu, B.C.; Masel, B.; Scheibel, R.S.; Christiansen, C.H.; Huddleston, N.; Ottenbacher, K.J. Virtual reality in the assessment of selected cognitive function after brain injury. Am. J. Phys. Med. Rehabil. 2001, 80, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Abreu, B.C.; Seale, G.S.; Masel, B.; Christiansen, C.H.; Ottenbacher, K.J. A virtual reality environment for evaluation of a daily living skill in brain injury rehabilitation: Reliability and validity. Arch. Phys. Med. Rehabil. 2003, 84, 1118–1124. [Google Scholar] [CrossRef]
- Besnard, J.; Richard, P.; Banville, F.; Nolin, P.; Aubin, G.; Le Gall, D.; Richard, I.; Allain, P. Virtual reality and neuropsychological assessment: The reliability of a virtual kitchen to assess daily-life activities in victims of traumatic brain injury. Appl. Neuropsychol. Adult 2016, 23, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Maggio, M.G.; De Luca, R.; Molonia, F.; Porcari, B.; Destro, M.; Casella, C.; Salvati, R.; Bramanti, P.; Calabrò, R.S. Cognitive rehabilitation in patients with traumatic brain injury: A narrative review on the emerging use of virtual reality. J. Clin. Neurosci. 2019, 61, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Canty, A.L.; Fleming, J.; Patterson, F.; Green, H.J.; Man, D.; Shum, D.H. Evaluation of a virtual reality prospective memory task for use with individuals with severe traumatic brain injury. Neuropsychol. Rehabil. 2014, 24, 238–265. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.A.; Rose, F.D.; Rushton, S.; Pentland, B.; Attree, E.A. Virtual reality: A new prosthesis for brain injury rehabilitation. Scott. Med. J. 1998, 43, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Allain, P.; Foloppe, D.A.; Besnard, J.; Yamaguchi, T.; Etcharry-Bouyx, F.; Le Gall, D.; Nolin, P.; Richard, P. Detecting everyday action deficits in Alzheimer’s disease using a nonimmersive virtual reality kitchen. J. Int. Neuropsychol. Soc. 2014, 20, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Fong, K.N.; Chow, K.Y.; Chan, B.C.; Lam, K.C.; Lee, J.C.; Li, T.H.; Yan, E.W.; Wong, A.T. Usability of a virtual reality environment simulating an automated teller machine for assessing and training persons with acquired brain injury. J. Neuroeng. Rehabil. 2010, 7, 19. [Google Scholar] [CrossRef][Green Version]
- Levy, C.E.; Miller, D.M.; Akande, C.A.; Lok, B.; Marsiske, M.; Halan, S. V-Mart, a virtual reality grocery store: A focus group study of a promising intervention for mild traumatic brain injury and posttraumatic stress disorder. Am. J. Phys. Med. Rehabil. 2019, 98, 191–198. [Google Scholar] [CrossRef]
- Yip, B.C.; Man, D.W. Virtual reality-based prospective memory training program for people with acquired brain injury. NeuroRehabilitation 2013, 32, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Grealy, M.A.; Johnson, D.A.; Rushton, S.K. Improving cognitive function after brain injury: The use of exercise and virtual reality. Arch. Phys. Med. Rehabil. 1999, 80, 661–667. [Google Scholar] [CrossRef]
- De Luca, R.; Maggio, M.G.; Maresca, G.; Latella, D.; Cannavo, A.; Sciarrone, F.; Lo Voi, E.; Accorinti, M.; Bramanti, P.; Calabrò, R.S. Improving cognitive function after traumatic brain injury: A clinical trial on the potential use of the semi-immersive virtual reality. Behav. Neurol. 2019, 2019, 9268179. [Google Scholar] [CrossRef]
- Man, D.W.; Poon, W.S.; Lam, C. The effectiveness of artificial intelligent 3-D virtual reality vocational problem-solving training in enhancing employment opportunities for people with traumatic brain injury. Brain Inj. 2013, 27, 1016–1025. [Google Scholar] [CrossRef]
- Mysiw, W.J.; Jackson, R.D. Tricyclic antidepressant therapy after traumatic brain injury. J. Head Trauma Rehabil. 1987, 2, 34–42. [Google Scholar] [CrossRef]
- Kalra, I.D.; Watanabe, T.K. Mood stabilizers for traumatic brain injury-related agitation. J. Head Trauma Rehabil. 2017, 32, E61–E64. [Google Scholar] [CrossRef] [PubMed]
- Neumann, D. Treatments for emotional issues after traumatic brain injury. J. Head Trauma Rehabil. 2017, 32, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Neumann, D.; Malec, J.F.; Hammond, F.M. Reductions in alexithymia and emotion dysregulation after training emotional self-awareness following traumatic brain injury: A phase I trial. J. Head Trauma Rehabil. 2017, 32, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Clemenson, G.D.; Stark, C.E.L. Virtual environmental enrichment through video games improves hippocampal-associated memory. J. Neurosci. 2015, 35, 16116–16125. [Google Scholar] [CrossRef] [PubMed]
- Toda, T.; Parylak, S.L.; Linker, S.B.; Gage, F.H. The role of adult hippocampal neurogenesis in brain health and disease. Mol. Psychiatry 2019, 24, 67–87. [Google Scholar] [CrossRef]
- Berdugo-Vega, G.; Arias-Gil, G.; López-Fernández, A.; Artegiani, B.; Wasielewska, J.M.; Lee, C.C.; Lippert, M.T.; Kempermann, G.; Takagaki, K.; Calegari, F. Increasing neurogenesis refines hippocampal activity rejuvenating navigational learning strategies and contextual memory throughout life. Nat. Commun. 2020, 11, 135. [Google Scholar] [CrossRef]
- Cameron, H.A.; Glover, L.R. Adult neurogenesis: Beyond learning and memory. Annu. Rev. Psychol. 2015, 66, 53–81. [Google Scholar] [CrossRef]
- Malberg, J.E.; Eisch, A.J.; Nestler, E.J.; Duman, R.S. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J. Neurosci. 2000, 20, 9104–9110. [Google Scholar] [CrossRef]
- Santarelli, L.; Saxe, M.; Gross, C.; Surget, A.; Battaglia, F.; Dulawa, S.; Weisstaub, N.; Lee, J.; Duman, R.; Arancio, O.; et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003, 301, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Eisch, A.J.; Petrik, D. Depression and hippocampal neurogenesis: A road to remission? Science 2012, 338, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.K.; Ding, V.Y.; Ball, R.L.; Dolce, D.L.; Henderson, J.M.; Halpern, C.H. Novel application of virtual reality in patient engagement for deep brain stimulation: A pilot study. Brain Stimul. 2018, 11, 935–937. [Google Scholar] [CrossRef]
- Grealy, M.A.; Heffernan, D. The rehabilitation of brain injured children: The case for including physical exercise and virtual reality. Pediatr. Rehabil. 2000, 4, 41–49. [Google Scholar] [CrossRef]
- Fordyce, D.E.; Farrar, R.P. Enhancement of spatial learning in F344 rats by physical activity and related learning-associated alterations in hippocampal and cortical cholinergic functioning. Behav. Brain Res. 1991, 46, 123–133. [Google Scholar] [CrossRef]
- Etnier, J.L.; Landers, D.M. Brain function and exercise. Current perspectives. Sport. Med. 1995, 19, 81–85. [Google Scholar] [CrossRef]
- Etnier, J.L.; Salazar, W.; Landers, D.M.; Petruzzello, S.J.; Han, M.; Nowell, P. The influence of physical fitness and exercise upon cognitive functioning: A meta-analysis. J. Sport Exerc. Psychol. 1997, 19, 249–277. [Google Scholar] [CrossRef]
- Lin, T.W.; Kuo, Y.M. Exercise benefits brain function: The monoamine connection. Brain Sci. 2013, 3, 39–53. [Google Scholar] [CrossRef]
- Basso, J.C.; Suzuki, W.A. The effects of acute exercise on mood, cognition, neurophysiology, and neurochemical pathways: A review. Brain Plast. 2017, 2, 127–152. [Google Scholar] [CrossRef]
- Loonen, A.J.M.; Ivanova, S.A. Circuits regulating pleasure and happiness—Mechanisms of depression. Front. Hum. Neurosci. 2016, 10, 571. [Google Scholar] [CrossRef]
- Hoffman, H.G.; Richards, T.L.; Van Oostrom, T.; Coda, B.A.; Jensen, M.P.; Blough, D.K.; Sharar, S.R. The analgesic effects of opioids and immersive virtual reality distraction: Evidence from subjective and functional brain imaging assessments. Anesth. Analg. 2007, 105, 1776–1783. [Google Scholar] [CrossRef] [PubMed]
- Makin, T.R.; Scholz, J.; Filippini, N.; Henderson Slater, D.; Tracey, I.; Johansen-Berg, H. Phantom pain is associated with preserved structure and function in the former hand area. Nat. Commun. 2013, 4, 1570. [Google Scholar] [CrossRef]
- Ramachandran, V.S.; Rogers-Ramachandran, D. Synaesthesia in phantom limbs induced with mirrors. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1996, 263, 377–386. [Google Scholar] [CrossRef]
- Guenther, K. ‘It’s all done with mirrors’: V. S. Ramachandran and the material culture of phantom limb research. Med Hist. 2016, 60, 342–358. [Google Scholar] [CrossRef] [PubMed]
- Diers, M.; Kamping, S.; Kirsch, P.; Rance, M.; Bekrater-Bodmann, R.; Foell, J.; Trojan, J.; Fuchs, X.; Bach, F.; Maass, H.; et al. Illusion-related brain activations: A new virtual reality mirror box system for use during functional magnetic resonance imaging. Brain Res. 2015, 1594, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Prim. 2017, 3, 17018. [Google Scholar] [CrossRef]
- Brown, R.H.; Al-Chalabi, A. Amyotrophic lateral sclerosis. N. Engl. J. Med. 2017, 377, 162–172. [Google Scholar] [CrossRef]
- Gilhus, N.E. Myasthenia gravis. N. Engl. J. Med. 2016, 375, 2570–2581. [Google Scholar] [CrossRef] [PubMed]
- Mendell, J.R.; Campbell, K.; Rodino-Klapac, L.; Sahenk, Z.; Shilling, C.; Lewis, S.; Bowles, D.; Gray, S.; Li, C.; Galloway, G.; et al. Dystrophin immunity in Duchenne’s muscular dystrophy. N. Engl. J. Med. 2010, 363, 1429–1437. [Google Scholar] [CrossRef]
- Wolpaw, J.R.; Birbaumer, N.; McFarland, D.J.; Pfurtscheller, G.; Vaughan, T.M. Brain-computer interfaces for communication and control. Clin. Neurophysiol. 2002, 113, 767–791. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Ushiba, J.; Kimura, A.; Liu, M.; Tomita, Y. Change in brain activity through virtual reality-based brain-machine communication in a chronic tetraplegic subject with muscular dystrophy. BMC Neurosci. 2010, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Rudrappa, S.S.; Wilkinson, D.J.; Greenhaff, P.L.; Smith, K.; Idris, I.; Atherton, P.J. Human skeletal muscle disuse atrophy: Effects on muscle protein synthesis, breakdown, and insulin resistance—A qualitative review. Front. Physiol. 2016, 7, 361. [Google Scholar] [CrossRef] [PubMed]
- Leinders, S.; Vansteensel, M.J.; Branco, M.P.; Freudenburg, Z.V.; Pels, E.G.M.; Van der Vijgh, B.; Van Zandvoort, M.J.E.; Ramsey, N.F.; Aarnoutse, E.J. Dorsolateral prefrontal cortex-based control with an implanted brain–computer interface. Sci. Rep. 2020, 10, 15448. [Google Scholar] [CrossRef] [PubMed]
- Skola, F.; Tinkova, S.; Liarokapis, F. Progressive training for motor imagery brain-computer interfaces using gamification and virtual reality embodiment. Front. Hum. Neurosci. 2019, 13, 329. [Google Scholar] [CrossRef] [PubMed]
- Coogan, C.G.; He, B. Brain-computer interface control in a virtual reality environment and applications for the internet of things. IEEE Access 2018, 6, 10840–10849. [Google Scholar] [CrossRef]
- Chapman, R.M.; Bragdon, H.R. Evoked responses to numerical and non-numerical visual stimuli while problem solving. Nature 1964, 203, 1155–1157. [Google Scholar] [CrossRef] [PubMed]
- Rohani, D.A.; Sorensen, H.B.; Puthusserypady, S. Brain-computer interface using P300 and virtual reality: A gaming approach for treating ADHD. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2014, 2014, 3606–3609. [Google Scholar] [CrossRef] [PubMed]
- Regaçone, S.F.; Lima, D.D.B.; Banzato, M.S.; Gução, A.C.B.; Valenti, V.E.; Frizzo, A.C.F. Association between central auditory processing mechanism and cardiac autonomic regulation. Int. Arch. Med. 2014, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Ron-Angevin, R.; Diaz-Estrella, A. Brain-computer interface: Changes in performance using virtual reality techniques. Neurosci. Lett. 2009, 449, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Salisbury, D.B.; Dahdah, M.; Driver, S.; Parsons, T.D.; Richter, K.M. Virtual reality and brain computer interface in neurorehabilitation. Bayl. Univ. Med Cent. Proc. 2016, 29, 124–127. [Google Scholar] [CrossRef]
- Juliano, J.M.; Spicer, R.P.; Vourvopoulos, A.; Lefebvre, S.; Jann, K.; Ard, T.; Santarnecchi, E.; Krum, D.M.; Liew, S.L. Embodiment is related to better performance on a brain-computer interface in immersive virtual reality: A pilot study. Sensors 2020, 20, 1204. [Google Scholar] [CrossRef] [PubMed]
- Yee, N.; Bailenson, J. The Proteus effect: The effect of transformed self-representation on behavior. Hum. Commun. Res. 2007, 33, 271–290. [Google Scholar] [CrossRef]
- Azocar, A.F.; Mooney, L.M.; Duval, J.F.; Simon, A.M.; Hargrove, L.J.; Rouse, E.J. Design and clinical implementation of an open-source bionic leg. Nat. Biomed. Eng. 2020, 4, 941–953. [Google Scholar] [CrossRef]
- Graczyk, E.L.; Resnik, L.; Schiefer, M.A.; Schmitt, M.S.; Tyler, D.J. Home use of a neural-connected sensory prosthesis provides the functional and psychosocial experience of having a hand again. Sci. Rep. 2018, 8, 9866. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Catalan, M.; Mastinu, E.; Sassu, P.; Aszmann, O.; Brånemark, R. Self-contained neuromusculoskeletal arm prostheses. N. Engl. J. Med. 2020, 382, 1732–1738. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Kim, W.S.; Ku, J. Transcranial direct current stimulation effect on virtual hand illusion. Cyberpsychol. Behav. Soc. Netw. 2020, 23, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Bassolino, M.; Franza, M.; Bello Ruiz, J.; Pinardi, M.; Schmidlin, T.; Stephan, M.A.; Solca, M.; Serino, A.; Blanke, O. Non-invasive brain stimulation of motor cortex induces embodiment when integrated with virtual reality feedback. Eur. J. Neurosci. 2018, 47, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Banakou, D.; Kishore, S.; Slater, M. Virtually being Einstein results in an improvement in cognitive task performance and a decrease in age bias. Front. Psychol. 2018, 9, 917. [Google Scholar] [CrossRef] [PubMed]
- Cebolla, A.; Herrero, R.; Ventura, S.; Miragall, M.; Bellosta-Batalla, M.; Llorens, R.; Baños, R.M. Putting oneself in the body of others: A pilot study on the efficacy of an embodied virtual reality system to generate self-compassion. Front. Psychol. 2019, 10, 1521. [Google Scholar] [CrossRef] [PubMed]
- Serino, S.; Pedroli, E.; Keizer, A.; Triberti, S.; Dakanalis, A.; Pallavicini, F.; Chirico, A.; Riva, G. Virtual reality body swapping: A tool for modifying the allocentric memory of the body. Cyberpsychol. Behav. Soc. Netw. 2015, 19, 127–133. [Google Scholar] [CrossRef]
- Serino, S.; Polli, N.; Riva, G. From avatars to body swapping: The use of virtual reality for assessing and treating body-size distortion in individuals with anorexia. J. Clin. Psychol. 2019, 75, 313–322. [Google Scholar] [CrossRef]
- Tacikowski, P.; Weijs, M.L.; Ehrsson, H.H. Perception of our own body influences self-concept and self-incoherence impairs episodic memory. iScience 2020, 23, 101429. [Google Scholar] [CrossRef] [PubMed]
- Slater, M.; Pérez Marcos, D.; Ehrsson, H.; Sanchez-Vives, M. Towards a digital body: The virtual arm illusion. Front. Hum. Neurosci. 2008, 2, 6. [Google Scholar] [CrossRef]
- Birbaumer, N.; Murguialday, A.R.; Cohen, L. Brain–computer interface in paralysis. Curr. Opin. Neurol. 2008, 21, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Okahara, Y.; Takano, K.; Nagao, M.; Kondo, K.; Iwadate, Y.; Birbaumer, N.; Kansaku, K. Long-term use of a neural prosthesis in progressive paralysis. Sci. Rep. 2018, 8, 16787. [Google Scholar] [CrossRef]
- Penfield, W. The Mystery of the Mind: A Critical Study of Consciousness and the Human Brain; Princeton University Press: Princeton, NJ, USA, 1978. [Google Scholar]
- Lewis, P.M.; Ackland, H.M.; Lowery, A.J.; Rosenfeld, J.V. Restoration of vision in blind individuals using bionic devices: A review with a focus on cortical visual prostheses. Brain Res. 2015, 1595, 51–73. [Google Scholar] [CrossRef] [PubMed]
- Dobelle, W.H. Artificial vision for the blind by connecting a television camera to the visual cortex. ASAIO J. 2000, 46, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Yoshor, D.; Bosking, W.H.; Ghose, G.M.; Maunsell, J.H.R. Receptive fields in human visual cortex mapped with surface electrodes. Cereb. Cortex 2007, 17, 2293–2302. [Google Scholar] [CrossRef]
- Bosking, W.H.; Sun, P.; Ozker, M.; Pei, X.; Foster, B.L.; Beauchamp, M.S.; Yoshor, D. Saturation in phosphene size with increasing current levels delivered to human visual cortex. J. Neurosci. 2017, 37, 7188–7197. [Google Scholar] [CrossRef] [PubMed]
- Bosking, W.H.; Beauchamp, M.S.; Yoshor, D. Electrical stimulation of visual cortex: Relevance for the development of visual cortical prosthetics. Annu. Rev. Vis. Sci. 2017, 3, 141–166. [Google Scholar] [CrossRef]
- Georgiev, D.D. Electric and magnetic fields inside neurons and their impact upon the cytoskeletal microtubules. In Rhythmic Oscillations in Proteins to Human Cognition; Bandyopadhyay, A., Ray, K., Eds.; Studies in Rhythm Engineering; Springer: Singapore, 2021; Chapter 3; pp. 51–102. [Google Scholar] [CrossRef]
- Georgiev, G.V.; Georgiev, D.D. Enhancing user creativity: Semantic measures for idea generation. Knowl. Based Syst. 2018, 151, 1–15. [Google Scholar] [CrossRef]
- Georgiev, G.V.; Georgiev, D.D. Semantic analysis approach to studying design problem solving. Proc. Des. Soc. Int. Conf. Eng. Des. 2019, 1, 1823–1832. [Google Scholar] [CrossRef]
- Georgiev, G.V.; Georgiev, D.D. Semantic analysis of engineering design conversations. Proc. Des. Soc. Des. Conf. 2020, 1, 1265–1274. [Google Scholar] [CrossRef]
- Gong, Z.; Georgiev, G.V. Literature review: Existing methods using VR to enhance creativity. In Proceedings of the Sixth International Conference on Design Creativity (ICDC 2020); Boujut, J.F., Cascini, G., Ahmed-Kristensen, S., Georgiev, G.V., Iivari, N., Eds.; The Design Society: Oulu, Finland, 2020; pp. 117–124. [Google Scholar] [CrossRef]
- Howett, D.; Castegnaro, A.; Krzywicka, K.; Hagman, J.; Marchment, D.; Henson, R.; Rio, M.; King, J.A.; Burgess, N.; Chan, D. Differentiation of mild cognitive impairment using an entorhinal cortex-based test of virtual reality navigation. Brain 2019, 142, 1751–1766. [Google Scholar] [CrossRef]
- Browning, M.H.E.M.; Mimnaugh, K.J.; van Riper, C.J.; Laurent, H.K.; LaValle, S.M. Can simulated nature support mental health? Comparing short, single-doses of 360-degree nature videos in virtual reality with the outdoors. Front. Psychol. 2020, 10, 2667. [Google Scholar] [CrossRef] [PubMed]
- Nijman, S.A.; Veling, W.; Greaves-Lord, K.; Vermeer, R.R.; Vos, M.; Zandee, C.E.R.; Zandstra, D.C.; Geraets, C.N.W.; Pijnenborg, G.H.M. Dynamic Interactive Social Cognition Training in Virtual Reality (DiSCoVR) for social cognition and social functioning in people with a psychotic disorder: Study protocol for a multicenter randomized controlled trial. BMC Psychiatry 2019, 19, 272. [Google Scholar] [CrossRef]
- Dakoure, C.; Ben Abdessalem, H.; Boukadida, M.; Cuesta, M.; Bruneau, M.A.; Belleville, S.; Frasson, C. Virtual savannah: An effective therapeutic and relaxing treatment for people with subjective cognitive decline. In Brain Function Assessment in Learning; Lecture Notes in Computer Science; Frasson, C., Bamidis, P., Vlamos, P., Eds.; Springer: Cham, Switzerland, 2020; Volume 12462, pp. 107–112. [Google Scholar] [CrossRef]
- Georgieva, I. The similarity between the virtual and the real self—how the virtual self can help the real self. Stud. Health Technol. Inform. 2011, 167, 20–25. [Google Scholar] [CrossRef]
- Georgieva, I. Trauma and self-narrative in virtual reality: Toward recreating a healthier mind. Front. ICT 2017, 4, 27. [Google Scholar] [CrossRef]
- Freeman, D.; Reeve, S.; Robinson, A.; Ehlers, A.; Clark, D.; Spanlang, B.; Slater, M. Virtual reality in the assessment, understanding, and treatment of mental health disorders. Psychol. Med. 2017, 47, 2393–2400. [Google Scholar] [CrossRef]
- Slater, M.; Sanchez-Vives, M.V. Enhancing our lives with immersive virtual reality. Front. Robot. AI 2016, 3, 74. [Google Scholar] [CrossRef]
- Heim, M. The Metaphysics of Virtual Reality; Oxford University Press: Oxford, UK, 1994. [Google Scholar]
- Goddard, M.N. Genealogies of immersive media and virtual reality (VR) as practical aesthetic machines. In Practical Aesthetics; Herzogenrath, B., Ed.; Bloomsbury Academic: London, UK, 2020; pp. 171–181. [Google Scholar] [CrossRef]
- Banakou, D. The impact of virtual embodiment on perception, attitudes, and behaviour. Ph.D. Thesis, Department of Clinical Psychology and Psychobiology, University of Barcelona, Barcelona, Spain, 2017. [Google Scholar]
- Lee, M.; Lee, S.A.; Jeong, M.; Oh, H. Quality of virtual reality and its impacts on behavioral intention. Int. J. Hosp. Manag. 2020, 90, 102595. [Google Scholar] [CrossRef]
- Martens, M.A.; Antley, A.; Freeman, D.; Slater, M.; Harrison, P.J.; Tunbridge, E.M. It feels real: Physiological responses to a stressful virtual reality environment and its impact on working memory. J. Psychopharmacol. 2019, 33, 1264–1273. [Google Scholar] [CrossRef]
- Schutte, N.S. The impact of virtual reality on curiosity and other positive characteristics. Int. J. Hum. Comput. Interact. 2019, 36, 661–668. [Google Scholar] [CrossRef]
- Jo, D.; Kim, K.; Welch, G.F.; Jeon, W.; Kim, Y.; Kim, K.H.; Kim, G.J. The impact of avatar-owner visual similarity on body ownership in immersive virtual reality. In Proceedings of the 23rd ACM Symposium on Virtual Reality Software and Technology; Association for Computing Machinery: Gothenburg, Sweden, 2017; p. 77. [Google Scholar] [CrossRef]
- Waltemate, T.; Gall, D.; Roth, D.; Botsch, M.; Latoschik, M.E. The impact of avatar personalization and immersion on virtual body ownership, presence, and emotional response. IEEE Trans. Vis. Comput. Graph. 2018, 24, 1643–1652. [Google Scholar] [CrossRef] [PubMed]
- Riva, G.; Mantovani, F.; Capideville, C.S.; Preziosa, A.; Morganti, F.; Villani, D.; Gaggioli, A.; Botella, C.; Alcañiz, M. Affective interactions using virtual reality: The link between presence and emotions. Cyberpsychol. Behav. 2007, 10, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Diemer, J.; Alpers, G.W.; Peperkorn, H.M.; Shiban, Y.; Mühlberger, A. The impact of perception and presence on emotional reactions: A review of research in virtual reality. Front. Psychol. 2015, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, G.; Melo, M.; Vasconcelos-Raposo, J.; Bessa, M. Impact of different sensory stimuli on presence in credible virtual environments. IEEE Trans. Vis. Comput. Graph. 2020, 26, 3231–3240. [Google Scholar] [CrossRef]
- Ochs, M.; Mestre, D.; de Montcheuil, G.; Pergandi, J.M.; Saubesty, J.; Lombardo, E.; Francon, D.; Blache, P. Training doctors’ social skills to break bad news: Evaluation of the impact of virtual environment displays on the sense of presence. J. Multimodal User Interfaces 2019, 13, 41–51. [Google Scholar] [CrossRef]
- Uhm, J.P.; Lee, H.W.; Han, J.W. Creating sense of presence in a virtual reality experience: Impact on neurophysiological arousal and attitude towards a winter sport. Sport Manag. Rev. 2020, 23, 588–600. [Google Scholar] [CrossRef]
- Olmos-Raya, E.; Ferreira-Cavalcanti, J.; Contero, M.; Castellanos, M.C.; Giglioli, I.A.C.; Alcañiz, M. Mobile virtual reality as an educational platform: A pilot study on the impact of immersion and positive emotion induction in the learning process. Eurasia J. Math. Sci. Technol. Educ. 2018, 14, 2045–2057. [Google Scholar] [CrossRef]
- Steed, A.; Pan, Y.; Zisch, F.; Steptoe, W. The impact of a self-avatar on cognitive load in immersive virtual reality. In Proceedings of the 2016 IEEE Virtual Reality (VR); Institute of Electrical and Electronics Engineers: Greenville, South Carolina, 2016; pp. 67–76. [Google Scholar] [CrossRef]
- Wiederhold, B.K. How will virtual reality impact our understanding of sexuality? Cyberpsychol. Behav. Soc. Netw. 2018, 21, 147–148. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.; Skadberg, R.; Moore, T. A pilot study of the impact of repeated sessions of virtual reality on chronic neuropathic pain. Int. J. Virtual Real. 2018, 18, 19–34. [Google Scholar] [CrossRef]
- Ahmadpour, N.; Randall, H.; Choksi, H.; Gao, A.; Vaughan, C.; Poronnik, P. Virtual reality interventions for acute and chronic pain management. Int. J. Biochem. Cell Biol. 2019, 114, 105568. [Google Scholar] [CrossRef]
- Van Ooteghem, G.; Geets, X. Virtual reality animations, a new strategy to reduce patients’ anxiety induced by radiotherapy. Radiother. Oncol. 2019, 133, S280. [Google Scholar] [CrossRef]
- Schutte, N.S.; Stilinović, E.J. Facilitating empathy through virtual reality. Motiv. Emot. 2017, 41, 708–712. [Google Scholar] [CrossRef]
- Howard, M.C. Virtual reality interventions for personal development: A meta-analysis of hardware and software. Hum. Comput. Interact. 2019, 34, 205–239. [Google Scholar] [CrossRef]
- Lambrakopoulos, G.; Begetis, N.; Katifori, A.; Karvounis, M.; Ioannidis, Y. Experimental evaluation of the impact of virtual reality on the sentiment of fear. In Proceedings of the 23rd International Conference on Virtual System & Multimedia (VSMM); Goodman, L., Addison, A., Eds.; Institute of Electrical and Electronics Engineers: Dublin, Ireland, 2017; pp. 20–26. [Google Scholar] [CrossRef]
- Pan, D.; Xu, Q.; Ma, S.; Zhang, K. The impact of fear of the sea on working memory performance: A research based on virtual reality. In Proceedings of the 24th ACM Symposium on Virtual Reality Software and Technology; Association for Computing Machinery: Tokyo, Japan, 2018; p. 38. [Google Scholar] [CrossRef]
- Vázquez, C.; Xia, L.; Aikawa, T.; Maes, P. Words in motion: Kinesthetic language learning in virtual reality. In Proceedings of the 18th International Conference on Advanced Learning Technologies (ICALT); Institute of Electrical and Electronics Engineers: Mumbai, India, 2018; pp. 272–276. [Google Scholar] [CrossRef]
- Schutte, N.S.; Bhullar, N.; Stilinović, E.J.; Richardson, K. The impact of virtual environments on restorativeness and affect. Ecopsychology 2017, 9, 1–7. [Google Scholar] [CrossRef]
- Singh, D.K.A.; Rahman, N.N.A.; Seffiyah, R.; Chang, S.Y.; Zainura, A.K.; Aida, S.R.; Rajwinder, K.H.S. Impact of virtual reality games on psychological well-being and upper limb performance in adults with physical disabilities: A pilot study. Med J. Malays. 2017, 72, 119–121. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgiev, D.D.; Georgieva, I.; Gong, Z.; Nanjappan, V.; Georgiev, G.V. Virtual Reality for Neurorehabilitation and Cognitive Enhancement. Brain Sci. 2021, 11, 221. https://doi.org/10.3390/brainsci11020221
Georgiev DD, Georgieva I, Gong Z, Nanjappan V, Georgiev GV. Virtual Reality for Neurorehabilitation and Cognitive Enhancement. Brain Sciences. 2021; 11(2):221. https://doi.org/10.3390/brainsci11020221
Chicago/Turabian StyleGeorgiev, Danko D., Iva Georgieva, Zhengya Gong, Vijayakumar Nanjappan, and Georgi V. Georgiev. 2021. "Virtual Reality for Neurorehabilitation and Cognitive Enhancement" Brain Sciences 11, no. 2: 221. https://doi.org/10.3390/brainsci11020221
APA StyleGeorgiev, D. D., Georgieva, I., Gong, Z., Nanjappan, V., & Georgiev, G. V. (2021). Virtual Reality for Neurorehabilitation and Cognitive Enhancement. Brain Sciences, 11(2), 221. https://doi.org/10.3390/brainsci11020221








