Anti-Cholinesterase Combination Drug Therapy as a Potential Treatment for Alzheimer’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Enzymatic Activity Assays
AChE Assay Using Neuroblastoma Cells
2.3. Computational Analysis
2.4. Cytotoxicity Assays
2.5. Statistical Analysis
3. Results
3.1. Drug Inhibition of AChE Protein In Vitro
3.2. Drug Inhibition of AChE within Differentiated Neurons
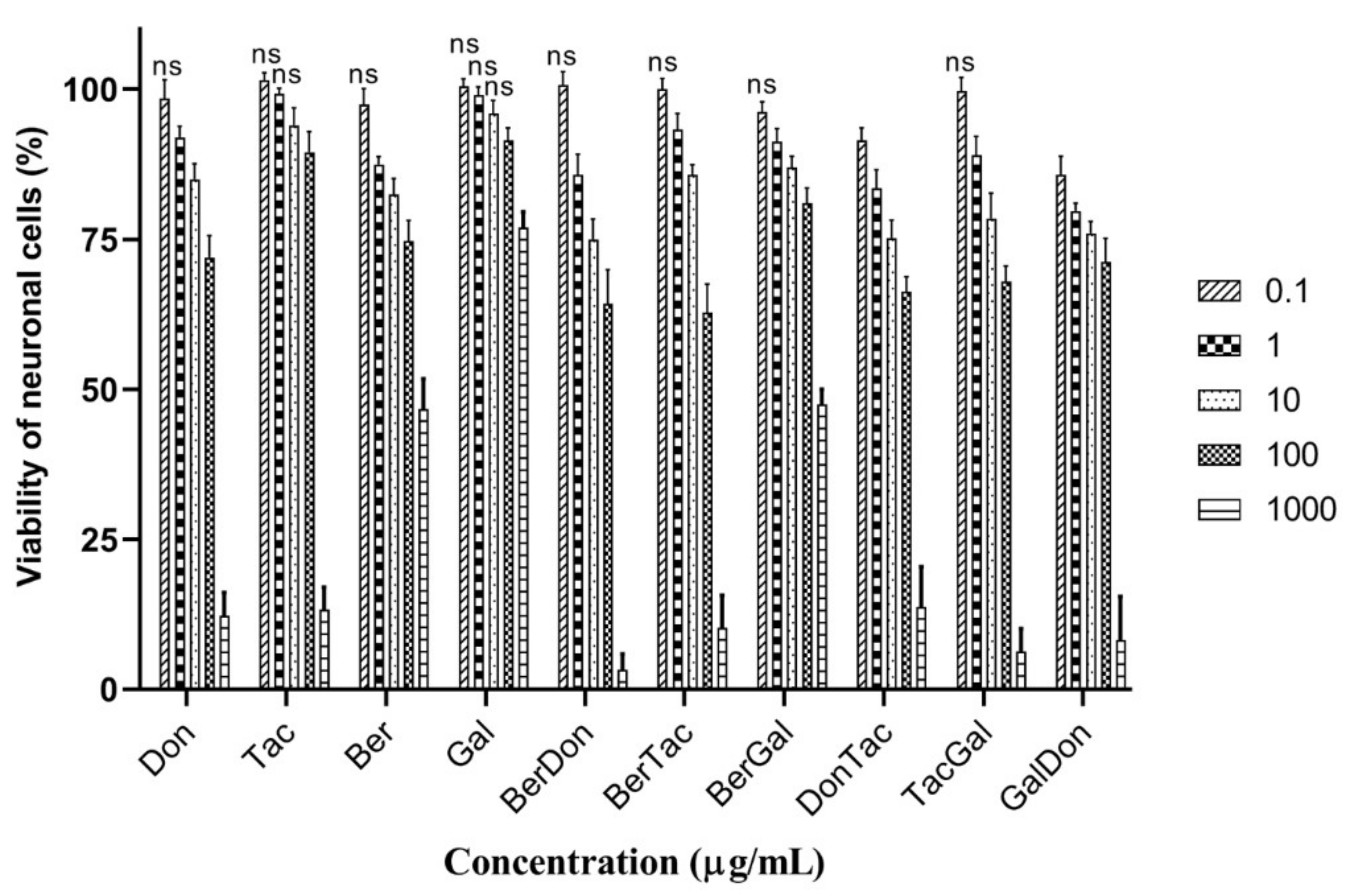
3.3. Cytotoxicity of Individual and Dual-Drug ChEI Combinations to Neuronal Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. 10 Facts on Dementia, WHO Media Centre. 2009. Available online: https://www.who.int/features/factfiles/dementia/en/ (accessed on 26 December 2020).
- GBD 2016 Dementia Collaborators. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 88–106. [Google Scholar] [CrossRef]
- Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimers Dement. 2016, 12, 459–509. [Google Scholar]
- Burns, A.; Iliffe, S. Alzheimer’s disease. BMJ 2009, 338, b158. [Google Scholar] [CrossRef] [PubMed]
- Frisoni, G.B.; Laakso, M.P.; Beltramello, A.; Geroldi, C.; Bianchetti, A.; Soininen, H.; Trabucchi, M. Hippocampal and entorhinal cortex atrophy in frontotemporal dementia and Alzheimer’s disease. Neurology 1999, 52, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Dugger, B.N.; Dickson, D.W. Pathology of neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef] [PubMed]
- Bird, C.M.; Burgess, N. The hippocampus and memory: Insights from spatial processing. Nat. Rev. Neurosci. 2008, 9, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Terry, A.V.; Buccafusco, J.J. The cholinergic hypothesis of age and Alzheimer’s Disease-related cognitive deficits: Recent challenges and their implications for novel drug development. J. Pharm. Exp. Ther. 2003, 306, 821–827. [Google Scholar] [CrossRef]
- Schleibs, R.; Arendt, T. The cholinergic system in aging and neuronal degeneration. Behav. Brain Res. 2011, 221, 555–563. [Google Scholar] [CrossRef]
- Grossberg, G.T. Cholinesterase inhibitors for the treatment of Alzheimer’s disease: Getting on and staying on. Curr. Ther. Res. Clin. Exp. 2003, 64, 216–235. [Google Scholar] [CrossRef]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Available online: https://www.nice.org.uk/guidance/ta217/chapter/1-guidance (accessed on 26 December 2020).
- Perrier, A.L.; Massoulie, J.; Krejci, E. PRiMA: The membrane anchor of acetylcholinesterase in the brain. Neuron 2002, 33, 275–285. [Google Scholar] [CrossRef]
- Blotnick-Rubin, E.; Anglister, L. Fine Localization of Acetylcholinesterase in the Synaptic Cleft of the Vertebrate Neuromuscular Junction. Front. Mol. Neurosci. 2018, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.M. Cholinesterase inhibitors in the treatment of dementia. JAOA 2005, 105, 145–158. [Google Scholar] [PubMed]
- Birks, J.S. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst. Rev. 2006, CD005593. [Google Scholar] [CrossRef]
- Ahmed, F.; Ghalib, R.M.; Sasikala, P.; Ahmed, K.K. Cholinesterase inhibitors from botanicals. Pharm. Rev. 2013, 7, 121–130. [Google Scholar] [CrossRef]
- Nwidu, L.L.; Elmorsy, E.; Thornton, J.; Wijamunige, B.; Wijesekara, A.; Tarbox, R.; Carter, W.G. Anti-acetylcholinesterase activity and antioxidant properties of extracts and fractions of Carpolobia lutea. Pharm. Biol. 2017, 55, 1875–1883. [Google Scholar] [CrossRef]
- Nwidu, L.L.; Elmorsy, E.; Aprioku, J.S.; Siminialayi, I.; Carter, W.G. In vitro anti-cholinesterase and antioxidant activity of extracts of Moringa oleifera plants from Rivers State, Niger Delta, Nigeria. Medicines 2018, 5, 71. [Google Scholar] [CrossRef]
- Jabir, N.R.; Khan, F.R.; Tabrez, S. Cholinesterase targeting by polyphenols: A therapeutic approach for the treatment of Alzheimer’s disease. CNS Neurosci. Ther. 2018, 24, 753–762. [Google Scholar] [CrossRef]
- Nwidu, L.L.; Alikwe, P.C.N.; Elmorsy, E.; Carter, W.G. An Investigation of potential sources of nutraceuticals from the Niger Delta Areas, Nigeria for attenuating oxidative stress. Medicines 2019, 6, 15. [Google Scholar] [CrossRef]
- Amat-ur-Rasool, H.; Symes, F.; Tooth, D.; Schaffert, L.-N.; Elmorsy, E.; Ahmed, M.; Hasnain, S.; Carter, W.G. Potential nutraceutical properties of leaves from several commonly cultivated plants. Biomolecules 2020, 10, 1556. [Google Scholar] [CrossRef]
- Cummings, J.L.; Tong, G.; Ballard, C. Treatment combinations for Alzheimer’s disease: Current and future pharmacotherapy options. J. Alz. Dis. 2019, 67, 779–794. [Google Scholar] [CrossRef] [PubMed]
- Amat-Ur-Rasool, H.; Ahmed, M. Designing second generation anti-Alzheimer compounds as inhibitors of human acetylcholinesterase: Computational screening of synthetic molecules and dietary phytochemicals. PLoS ONE 2015, 10, e0136509. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.F.; Shen, L. Berberine: A potential multipotent natural product to combat Alzheimer’s disease. Molecules 2011, 16, 6732–6740. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Wang, C.; Yang, W. Role of berberine in Alzheimer’s disease. Neuropsy. Dis. Treat. 2016, 12, 2509–2520. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharm. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Chou, T.-C. The mass-action law based algorithms for quantitative econo-green bio-research. Integr. Biol. 2011, 3, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, R.; Solomon, S.; Liu, Y.; Zhao, B.; Santillo, M.F.; Xia, M. Identification of acetylcholinesterase inhibitors using homogenous cell-based assays in quantitative high-throughput screening platforms. Biotechnol. J. 2017, 12, 1600715. [Google Scholar] [CrossRef]
- Chou, T.; Martin, N. CompuSyn for Drug Combinations: PC Software and User’s Guide: A Computer Program for Quantitation of Synergism and Antagonism in Drug Combinations, and the Determination of IC50 and ED50 and LD50 Values; ComboSyn: Paramus, NJ, USA, 2005. [Google Scholar]
- Chou, T.-C. The combination index (CI < 1) as the definition of synergism and of synergy claims. Synergy 2018, 7, 49–50. [Google Scholar]
- Encinas, M.; Iglesias, M.; Liu, Y.; Wang, H.; Muhaisen, A.; Cena, V.; Gallego, C.; Comella, J.X. Sequential treatment of SH-SY5Y cells with retinoic acid and brain-derived neurotrophic factor gives rise to fully differentiated, neurotrophic factor-dependent, human neuron-like cells. J. Neurochem. 2000, 75, 991–1003. [Google Scholar] [CrossRef]
- Cheung, Y.T.; Lau, W.K.; Yu, M.S.; Lai, C.S.; Yeung, S.C.; So, K.F.; Chang, R.C. Effects of all-trans-retinoic acid on human SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research. Neurotoxicology 2009, 30, 127–135. [Google Scholar] [CrossRef]
- Agholme, L.; Lindstrom, T.; Kagedal, K.; Marcusson, J.; Hallbeck, M. An in vitro model for neuroscience: Differentiation of SH-SY5Y cells into cells with morphological and biochemical characteristics of mature neurons. J. Alzheimers Dis. 2010, 20, 1069–1082. [Google Scholar] [CrossRef]
- De Medeiros, L.M.; De Bastiani, M.A.; Rico, E.P.; Schonhofen, P.; Pfaffenseller, B.; Wollenhaupt-Aguiar, B.; Grun, L.; Barbé-Tuana, F.; Zimmer, E.R.; Castro, M.A.A.; et al. Cholinergic Differentiation of Human Neuroblastoma SH-SY5Y Cell Line and Its Potential Use as an In vitro Model for Alzheimer’s Disease Studies. Mol. Neurobiol. 2019, 56, 7355–7367. [Google Scholar] [CrossRef] [PubMed]
- Kröger, E.; Mouls, M.; Wilchesky, M.; Berkers, M.; Carmichael, P.H.; van Marum, R.; Souverein, P.; Egberts, T.; Laroche, M.L. Adverse drug reactions reported with cholinesterase inhibitors: An analysis of 16 Years of individual case safety reports from VigiBase. Ann. Pharmacother. 2015, 49, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Green, C.; Picot, J.; Loveman, E.; Takeda, A.; Kirby, J.; Clegg, A. Modelling the cost effectiveness of cholinesterase inhibitors in the management of mild to moderately severe Alzheimer’s disease. Pharmacoeconomics 2005, 23, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- Watkins, P.B.; Zimmerman, H.J.; Knapp, M.J.; Gracon, S.I.; Lewis, K.W. Hepatotoxic effects of tacrine administration in patients with Alzheimer’s disease. JAMA 1994, 271, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Snape, M.F.; Misra, A.; Murray, T.K.; De Souza, R.J.; Williams, J.L.; Cross, A.J.; Green, A.R. A comparative study in rats of the in vitro and in vivo pharmacology of the acetylcholinesterase inhibitors tacrine, donepezil and NXX-066. Neuropharmacology 1999, 38, 181–193. [Google Scholar] [CrossRef]
- Bajda, M.; Więckowska, A.; Hebda, M.; Guzior, N.; Sotriffer, C.A.; Malawska, B. Structure-based search for new inhibitors of cholinesterases. Int. J. Mol. Sci. 2013, 14, 5608–5632. [Google Scholar] [CrossRef]
- Rydberg, E.H.; Brumshtein, B.; Greenblatt, H.M.; Wong, D.M.; Shaya, D.; Williams, L.D.; Carlier, P.R.; Pang, Y.-P.; Silman, I.; Sussman, J.L. Complexes of Alkylene-linked Tacrine dimers with Torpedo californica acetylcholinesterase: Binding of Bis (5)-tacrine produces a dramatic rearrangement in the active-site gorge. J. Med. Chem. 2006, 49, 5491–5500. [Google Scholar] [CrossRef]
- Shan, W.-J.; Huang, L.; Zhou, Q.; Meng, F.-C.; Li, X.-S. Synthesis, biological evaluation of 9-N-substituted berberine derivatives as multi-functional agents of antioxidant, inhibitors of acetylcholinesterase, butyrylcholinesterase and amyloid-b aggregation. Eur. J. Med. Chem. 2011, 46, 5885–5893. [Google Scholar] [CrossRef]
- Stavrakov, G.; Philipova, I.; Zheleva, D.; Atanasova, M.; Konstantinov, S.; Doytchinova, I. Docking-based design of galantamine derivatives with dual-site binding to acetylcholinesterase. Mol. Inf. 2016, 35, 278–285. [Google Scholar] [CrossRef]
- Silva, M.A.; Kiametis, A.S.; Treptow, W. Donepezil inhibits acetylcholinesterase via multiple binding modes at room temperature. J. Chem. Inf. Modeling 2020, 60, 3463–3471. [Google Scholar] [CrossRef] [PubMed]
- Mak, S.; Luk, W.W.; Cui, W.; Hu, S.; Tsim, K.W.; Han, Y. Synergistic inhibition on acetylcholinesterase by the combination of berberine and palmatine originally isolated from Chinese medicinal herbs. J. Mol. Neurosci. 2014, 53, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.P.; Liu, E.Y.L.; Chen, Z.C.; Xu, M.L.; Yu, A.X.D.; Wu, Q.Y.; Xia, Y.-J.; Duan, R.; Dong, T.T.X.; Tsim, K.W.K. Synergistic Inhibition of Acetylcholinesterase by Alkaloids Derived from Stephaniae Tetrandrae Radix, Coptidis Rhizoma and Phellodendri Chinensis Cortex. Molecules 2019, 24, 4567. [Google Scholar] [CrossRef] [PubMed]
- Benek, O.; Korabecny, J.; Soukup, O. A Perspective on Multi-target Drugs for Alzheimer’s Disease. Trends Pharmacol. Sci. 2020, 41, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.T.; Uddin, M.S.; Mamun, A.A.; Jeandet, P.; Aleya, L.; Mansouri, R.A.; Ashraf, G.M.; Mathew, B.; Bin-Jumah, M.N.; Abdel-Daim, M.M. Combination Drug Therapy for the Management of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, D.R.; Wilcock, G.K.; Morgan, R.A.; Truman, C.A.; Ford, J.M.; Roberts, C.J. Pharmacokinetics of tacrine hydrochloride in Alzheimer’s disease. Clin. Pharmacol. Ther. 1989, 46, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Lou, G.; Montgomery, P.R.; Sitar, D.S. Bioavailability and pharmacokinetic disposition of tacrine in elderly patients with Alzheimer’s disease. J. Psychiatry Neurosci. 1996, 21, 334–339. [Google Scholar]
- Ye, M.; Fu, S.; Pi, R.; He, F. Neuropharmacological and pharmacokinetic properties of berberine: A review of recent research. J. Pharm. Pharmacol. 2009, 61, 831–837. [Google Scholar] [CrossRef]



| Drug or Drug Combination | CI Value | Acetycholinesterase Inhibition (IC50) (nM) | |||
|---|---|---|---|---|---|
| Donepezil | Tacrine | Berberine | Galantamine | ||
| Don | 57 (h) | ||||
| 91 (tc) | |||||
| Tac | 726 (h) | ||||
| 271 (tc) | |||||
| Ber | 3131 (h) | ||||
| 3234 (tc) | |||||
| Gal | 4183 (h) | ||||
| 4173 (tc) | |||||
| BerTac | 0.880 (h) | 302 (h) | 1451 (h) | ||
| 0.826 (tc) | 99 (tc) | 1486 (tc) | |||
| BerGal | 0.615 (h) | 1101 (h) | 1101 (h) | ||
| 0.744 (tc) | 1355 (tc) | 1355 (tc) | |||
| TacDon | 0.887 (h) | 27 (h) | 299 (h) | ||
| 0.697 (tc) | 29 (tc) | 103 (tc) | |||
| DonGal | 0.876 (h) | 29 (h) | 1538 (h) | ||
| 1.216 (tc) | 51 (tc) | 2729 (tc) | |||
| DonBer | 1.099 (h) | 32 (h) | 1690 (h) | ||
| 1.233 (tc) | 45 (tc) | 2393 (tc) | |||
| TacGal | 1.235 (h) | 489 (h) | 2349 (h) | ||
| 1.139 (tc) | 156 (tc) | 2344 (tc) | |||
| Drug or Drug Combination | CI Value | Acetylcholinesterase Inhibition (IC50) (nM) | |||
|---|---|---|---|---|---|
| Donepezil | Tacrine | Berberine | Galantamine | ||
| Don | 81 | ||||
| Tac | 1019 | ||||
| Ber | 4844 | ||||
| Gal | 7009 | ||||
| BerTac | 0.686 | 348 | 1670 | ||
| BerGal | 0.575 | 1648 | 1648 | ||
| TacDon | 0.963 | 41 | 461 | ||
| DonGal | 0.804 | 40 | 2152 | ||
| DonBer | 0.834 | 36 | 1907 | ||
| TacGal | 1.100 | 660 | 3169 | ||
| Drug or Drug Combination | Cell Cytotoxicity Log IC50 (µg/mL) |
|---|---|
| Don | 2.31 ± 0.057 |
| Tac | 2.53 ± 0.091 |
| Ber | 2.98 ± 0.010 |
| Gal | 4.10 ± 0.041 |
| DonBer | 2.01 ± 0.043 |
| BerTac | 2.16 ± 0.031 |
| BerGal | 3.01 ± 0.046 |
| TacDon | 2.08 ± 0.038 |
| TacGal | 2.16 ± 0.052 |
| DonGal | 2.08 ± 0.059 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amat-ur-Rasool, H.; Ahmed, M.; Hasnain, S.; Carter, W.G. Anti-Cholinesterase Combination Drug Therapy as a Potential Treatment for Alzheimer’s Disease. Brain Sci. 2021, 11, 184. https://doi.org/10.3390/brainsci11020184
Amat-ur-Rasool H, Ahmed M, Hasnain S, Carter WG. Anti-Cholinesterase Combination Drug Therapy as a Potential Treatment for Alzheimer’s Disease. Brain Sciences. 2021; 11(2):184. https://doi.org/10.3390/brainsci11020184
Chicago/Turabian StyleAmat-ur-Rasool, Hafsa, Mehboob Ahmed, Shahida Hasnain, and Wayne G. Carter. 2021. "Anti-Cholinesterase Combination Drug Therapy as a Potential Treatment for Alzheimer’s Disease" Brain Sciences 11, no. 2: 184. https://doi.org/10.3390/brainsci11020184
APA StyleAmat-ur-Rasool, H., Ahmed, M., Hasnain, S., & Carter, W. G. (2021). Anti-Cholinesterase Combination Drug Therapy as a Potential Treatment for Alzheimer’s Disease. Brain Sciences, 11(2), 184. https://doi.org/10.3390/brainsci11020184









