Reliability of Tibialis Anterior Muscle Voluntary Activation Using the Interpolated Twitch Technique and the Central Activation Ratio in People with Stroke
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
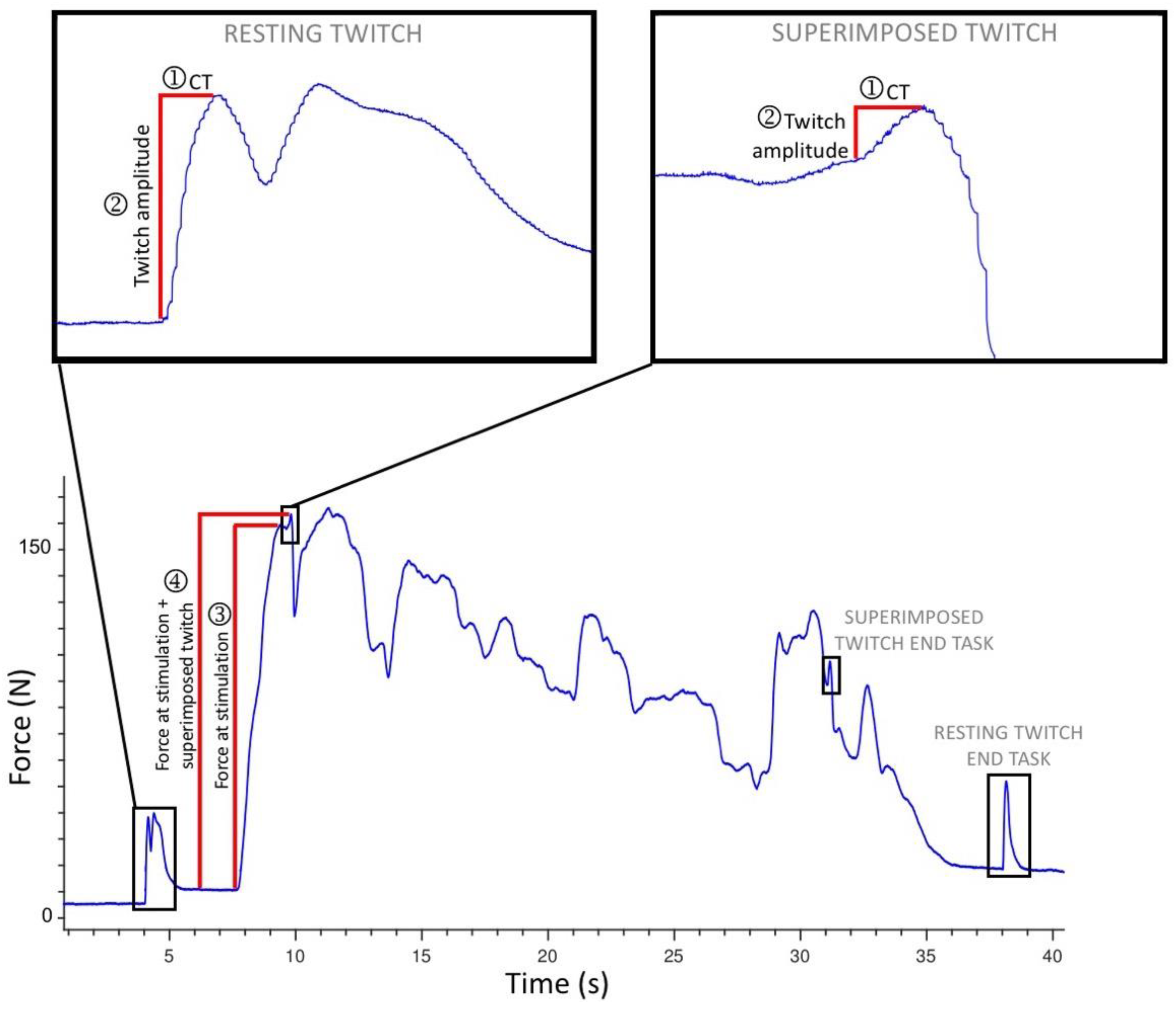
2.3. Procedures
2.4. Data Processing
2.5. Statistical Analysis
3. Results
3.1. Study Participants
3.2. Descriptive Results
3.3. Reliability Results
4. Discussion
4.1. Voluntary Activation
4.1.1. Transformed versus Non-Transformed Data
4.1.2. ITT versus CAR Calculations
4.1.3. Voluntary Activation as an Outcome Measure
4.2. Central Fatigue
4.3. Methodological Considerations
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gandevia:, S.C.; Allen, G.M.; Butler, J.E.; Taylor, J.L. Supraspinal factors in human muscle fatigue: Evidence for suboptimal output from the motor cortex. J. Physiol. 1996, 490, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, J.L.; Taylor, J.L.; Gandevia, S.C. CORP: Measurement of upper and lower limb muscle strength and voluntary activation. J. Appl. Physiol. 2019, 126, 513–543. [Google Scholar] [CrossRef] [PubMed]
- Belanger, A.Y.; McComas, A.J. Extent of motor unit activation during effort. J. Appl. Physiol. 1981, 51, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Gandevia, S.C. Spinal and supraspinal factors in human muscle fatigue. Physiol. Rev. 2001, 81, 1725–1789. [Google Scholar] [CrossRef]
- Bowden, J.L.; Taylor, J.L.; McNulty, P.A. Voluntary activation is reduced in both the more- and less-affected upper limbs after unilateral stroke. Front. Neurol. 2014, 5. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Bhadane, M.; Zhou, P.; Rymer, W.Z. Activation deficit correlates with weakness in chronic stroke: Evidence from evoked and voluntary EMG recordings. Clin. Neurophysiol. 2014, 125, 2413–2417. [Google Scholar] [CrossRef]
- Riley, N.A.; Bilodeau, M. Changes in upper limb joint torque patterns and EMG signals with fatigue following a stroke. Disabil. Rehabil. 2002, 24, 961–969. [Google Scholar] [CrossRef]
- Horstman, A.M.; Beltman, M.J.; Gerrits, K.H.; Koppe, P.; Janssen, T.W.; Elich, P.; de Haan, A. Intrinsic muscle strength and voluntary activation of both lower limbs and functional performance after stroke. Clin. Physiol. Funct. Imaging 2008, 28, 251–261. [Google Scholar] [CrossRef]
- Newham, D.J.; Davies, J.M.; Mayston, M.J. Voluntary force generation and activation in the knee muscles of stroke patients with mild spastic hemiparesis. J. Physiol. 1995, 483, P128. [Google Scholar]
- Newham, D.J.; Hsiao, S.F. Knee muscle isometric strength, voluntary activation and antagonist co-contraction in the first six months after stroke. Disabil. Rehabil. 2001, 23, 379–386. [Google Scholar] [CrossRef]
- Klein, C.S.; Brooks, D.; Richardson, D.; McIlroy, W.E.; Bayley, M.T. Voluntary activation failure contributes more to plantar flexor weakness than antagonist coactivation and muscle atrophy in chronic stroke survivors. J. Appl. Physiol. 2010, 109, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.S.; Power, G.A.; Brooks, D.; Rice, C.L. Neural and muscular determinants of dorsiflexors weakness in chronic stroke survivors. Motor Control 2013, 17, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Knorr, S.; Ivanova, T.D.; Doherty, T.J.; Campbell, J.A.; Garland, S.J. The origins of neuromuscular fatigue post-stroke. Exp. Brain Res. 2011, 214, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Stinear, C.M.; Petoe, M.A.; Byblow, W.D. Primary motor cortex excitability during recovery after stroke: Implications for neuromodulation. Brain Stimul. 2015, 8, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Traversa, R.; Cicinelli, P.; Oliveri, M.; Pamieri, M.G.; Filippi, M.M.; Pasqualetti, P.; Rossini, P.M. Neurophysiological follow-up of motor cortical output in stroke patients. Clin. Neurophysiol. 2000, 111, 1695–1703. [Google Scholar] [CrossRef]
- Rozand, V.; Sundberg, C.W.; Hunter, S.K.; Smith, A.E. Age-Related deficits in voluntary activation: A systematic review and meta-analysis. Med. Sci. Sports Exerc. 2020, 52, 549–560. [Google Scholar] [CrossRef]
- Kent-Braun, J.A.; Le Blanc, R. Quantification of central activation failure during maximal voluntary contractions in humans. Muscle Nerve 1996, 19, 861–869. [Google Scholar] [CrossRef]
- Grindstaff, T.L.; Threlkeld, A.J. Optimal stimulation parameters to detect deficits in quadriceps voluntary activation. J. Strength Cond. Res. 2014, 28, 381–389. [Google Scholar] [CrossRef]
- Klass, M.; Baudry, S.; Duchateau, J. Aging does not affect voluntary activation of the ankle dorsiflexors during isometric, concentric, and eccentric contractions. J. Appl. Physiol. 2005, 99, 31–38. [Google Scholar] [CrossRef]
- Place, N.; Maffiuletti, N.A.; Martin, A.; Lepers, R. Assessment of the reliability of central and peripheral fatigue after sustained maximal voluntary contraction of the quadriceps muscle. Muscle Nerve 2007, 35, 486–495. [Google Scholar] [CrossRef]
- Todd, G.; Taylor, J.L.; Gandevia, S.C. Measurement of voluntary activation of fresh and fatigued human muscles using transcranial magnetic stimulation. J. Physiol. 2003, 551, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Bremner, C.B.; Holcomb, W.R.; Brown, C.D.; Perreault, M.E. The effectiveness of neuromuscular electrical stimulation in improving voluntary activation of the quadriceps: A critically appraised topic. J. Sport Rehabil. 2017, 26, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Elboim-Gabyzon, M.; Rozen, N.; Laufer, Y. Does neuromuscular electrical stimulation enhance the effectiveness of an exercise programme in subjects with knee osteoarthritis? A randomized controlled trial. Clin. Rehabil. 2013, 27, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhou, S.; Ao, M.; Zhao, M.; Zhang, L.; Cao, L. Unilateral intramuscular needling can improve ankle dorsiflexor strength and muscle activation in both legs. J. Exerc. Sci. Fit. 2015, 13, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Mount, J.; Dacko, S. Effects of dorsiflexor endurance exercises on foot drop secondary to multiple sclerosis: A pilot study. Neurorehabilitation 2006, 21, 43–50. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, J.M.; Donges, S.C.; Taylor, J.L. Paired corticospinal-motoneuronal stimulation increases maximal voluntary activation of human adductor pollicis. J. Neurophysiol. 2018, 119, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Frazer, A.; Williams, J.; Spittles, M.; Rantalainen, T.; Kidgell, D. Anodal transcranial direct current stimulation of the motor cortex increases cortical voluntary activation and neural plasticity. Muscle Nerve 2016, 54, 903–913. [Google Scholar] [CrossRef]
- Kumpulainen, S.; Peltonen, J.; Gruber, M.; Cresswell, A.; Peurala, S.; Linnamo, V.; Avela, J. The effect of paired associative stimulation on fatigue resistance. Neurosci. Res. 2015, 95, 59–65. [Google Scholar] [CrossRef][Green Version]
- Urbach, D.; Awiszus, F. Stimulus strength related effect of transcranial magnetic stimulation on maximal voluntary contraction force of human quadriceps femoris muscle. Exp. Brain Res. 2002, 142, 25–31. [Google Scholar] [CrossRef]
- Urback, D.; Friedemann, A. Effects of transcranial magnetic stimulation on results of the twitch interpolation technique. Muscle Nerve 2000, 23, 1125–1128. [Google Scholar] [CrossRef]
- Geiger, M.; Roche, N.; Vlachos, E.; Cattagni, T.; Zory, R. Acute effects of bi-hemispheric transcranial direct current stimulation on the neuromuscular function of patients with chronic stroke: A randomized controlled study. Clin. Biomech. 2019, 70, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.; Signal, N.; Niazi, I.K.; Rashid, U.; Alder, G.; Mawston, G.; Nedergaard, R.W.; Jochumsen, M.; Taylor, D. Peripheral electrical stimulation paired with movement-related cortical potentials improves isometric muscle strength and voluntary activation following stroke. Front. Hum. Neurosci. 2020, 14, 156. [Google Scholar] [CrossRef] [PubMed]
- Coso, J.D.; Hamouti, N.; Estevez, E.; Mora-Rodriguez, R. Reproducibility of two electrical stimulation techniques to assess neuromuscular fatigue. Eur. J. Sport Sci. 2011, 11, 95–103. [Google Scholar] [CrossRef]
- Morton, J.P.; Atkinson, G.; MacLaren, D.P.; Cable, N.T.; Gilbert, G.; Broome, C.; McArdle, A.; Drust, B. Reliability of maximal muscle force and voluntary activation as markers of exercise-induced muscle damage. Eur. J. Appl. Physiol. 2005, 94, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Norte, G.E.; Kuenze, C.; Hart, J.M. Closed chain assessment of quadriceps activation using the superimposed burst technique. J. Electromyogr. Kinesiol. 2014, 24, 341–347. [Google Scholar] [CrossRef]
- Park, J.; Hopkins, J.T. Within- and between-session reliability of the maximal voluntary knee extension torque and activation. Int. J. Neurosci. 2013, 123, 55–59. [Google Scholar] [CrossRef]
- Zech, A.; Witte, K.; Pfeifer, K. Reliability and performance-dependent variations of muscle function variables during isometric knee extension. J. Electromyogr. Kinesiol. 2008, 18, 262–269. [Google Scholar] [CrossRef]
- Behrens, M.; Husmann, F.; Gube, M.; Felser, S.; Weippert, M.; Bruhn, S.; Zschorlich, V.; Mau-Moeller, A. Intersession reliability of the interpolated twitch technique applied during isometric, concentric, and eccentric actions of the human knee extensor muscles. Muscle Nerve 2017, 56, 324–327. [Google Scholar] [CrossRef]
- Stutzig, N.; Siebert, T. Reproducibility of electromyographic and mechanical parameters of the triceps surae during submaximal and maximal plantar flexions. Muscle Nerve 2016, 53, 464–470. [Google Scholar] [CrossRef]
- Bernecke, V.; Pukenas, K.; Imbrasiene, D.; Mickeviciene, D.; Baranauskiene, N.; Eimantas, N.; Brazaitis, M. Test-Retest cross-reliability of tests to assess neuromuscular function as a multidimensional concept. J. Strength Cond. Res. 2015, 29, 1972–1984. [Google Scholar] [CrossRef]
- Cooper, M.; Herda, T.J.; Walter-Herda, A.A.; Costa, P.B.; Ryan, E.D.; Cramer, J.T. The reliability of the interpolated twitch technique during submaximal and maximal isometric muscle actions. J. Strength Cond. Res. 2013, 27, 2909–2913. [Google Scholar] [CrossRef] [PubMed]
- Hight, R.E.; Quarshie, A.T.; Black, C.D. Voluntary muscle activation and evoked volitional-wave responses as a function of torque. J. Electromyogr. Kinesiol. 2018, 41, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Merlet, A.N.; Cattagni, T.; Cornu, C.; Jubeau, M. Effect of knee angle on neuromuscular assessment of plantar flexor muscles: A reliability study. PLoS ONE 2018, 13, e0195220. [Google Scholar] [CrossRef] [PubMed]
- Todd, G.; Gorman, R.B.; Gandevia, S.C. Measurement and reproducibility of strength and voluntary activation of lower-limb muscles. Muscle Nerve 2004, 29, 834–842. [Google Scholar] [CrossRef]
- Blacker, S.D.; Fallowfield, J.L.; Willems, M.E. Intra- and interday reliability of voluntary and electrically stimulated isometric contractions of the quadriceps femoris. J. Electromyogr. Kinesiol. 2013, 23, 886–891. [Google Scholar] [CrossRef]
- Signal, N.E. Neuromuscular fatigue in people with chronic stroke. Master’s Thesis, Auckland University of Technology, Auckland, New Zealand, 2007. [Google Scholar]
- Lin, P.Y.; Yang, Y.R.; Cheng, S.J.; Wang, R.Y. The relation between ankle impairments and gait velocity and symmetry in people with stroke. Arch. Phys. Med. Rehabil. 2006, 87, 562–568. [Google Scholar] [CrossRef]
- Dorsch, S.; Ada, L.; Canning, C.G.; Al-Zharani, M.; Dean, C. The strength of the ankle dorsiflexors has a significant contribution to walking speed in people who can walk independently after stroke: An observational study. Arch. Phys. Med. Rehabil. 2012, 93, 1072–1076. [Google Scholar] [CrossRef]
- Ng, S.S.; Hui-Chan, C.W. Contribution of ankle dorsiflexor strength to walking endurance in people with spastic hemiplegia after stroke. Arch. Phys. Med. Rehabil. 2012, 93, 1046–1051. [Google Scholar] [CrossRef]
- Ng, S.S.; Hui-Chan, C.W. Ankle dorsiflexor, not plantarflexor strength, predicts the functional mobility of people with spastic hemiplegia. J. Rehabil. Med. 2013, 45, 541–545. [Google Scholar] [CrossRef]
- Gorst, T.; Lyddon, A.; Marsden, J.; Paton, J.; Morrison, S.C.; Cramp, M.; Freeman, J. Foot and ankle impairments affect balance and mobility in stroke (FAiMiS): The views and experiences of people with stroke. Disabil. Rehabil. 2016, 38, 589–596. [Google Scholar] [CrossRef]
- Hermens, H.; Freriks, B.; Merletti, R.; Stegeman, D.; Blok, J.; Rau, G.; Klug, C.; Hägg, G. European Recommendations for Surface Electromyography: Results of the Seniam Project; Roessingh Research and Development: Enschede, The Netherlands, 1999. [Google Scholar]
- Klarner, T.; Barss, T.S.; Sun, Y.; Kaupp, C.; Beattie, S.; Zehr, E.P. Reliability of multiple baseline measures for locomotor retraining after stroke. In Replace, Repair, Restore, Relieve: Bridging Clinical and Engineering Solutions in Neurorehabilitation; Jensen, W., Andersen, O.K., Akay, M., Eds.; Springer: New York, NY, US, 2014; Volume 7, pp. 479–486. [Google Scholar] [CrossRef]
- Baudry, S.; Klass, M.; Pasquet, B.; Duchateau, J. Age-Related fatigability of the ankle dorsiflexor muscles during concentric and eccentric contractions. Eur. J. Appl. Physiol. 2007, 100, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Strojnik, V.; Komi, P.V. Neuromuscular fatigue after maximal stretch-shortening cycle exercise. J. Appl. Physiol. 1998, 84, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, M.A.; Nakagawa, S.; Schielzeth, H.; Goslee, S. rptR: Repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol. Evol. 2017, 8, 1639–1644. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Lek, K.M.; Van De Schoot, R. A comparison of the single, conditional and person-specific standard error of measurement: What do they measure and when to use them? Front. Appl. Math. Stat. 2018, 4, 40. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models usinglme4. J. Stat. Softw. 2015, 67. [Google Scholar] [CrossRef]
- Nakagawa, S.; Johnson, P.C.D.; Schielzeth, H. The coefficient of determination R(2) and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J. R. Soc. Interface 2017, 14. [Google Scholar] [CrossRef]
- Steichen, T.J.; Cox, N.J. A note on the concordance correlation coefficient. Stata J. 2002, 2, 183–189. [Google Scholar] [CrossRef]
- Lawrence, I.; Lin, K. A concordance correlation coefficient to evaluate reproducibility. Biometrics 1989, 45, 255–268. [Google Scholar]
- McBride, G.B. A Proposal for Strength-of-Agreement Criteria for Lin’s Concordance Correlation Coefficient; National Institute of Water & Atmospheric Research Ltd: Auckland, New Zealand, 2005. [Google Scholar]
- Warton, D.I.; Hui, F.K.C. The arcsine is asinine: The analysis of proportions in ecology. Ecology 2011, 92, 3–10. [Google Scholar] [CrossRef]
- Oskouei, M.A.; Van Mazijk, B.C.; Schuiling, M.H.; Herzog, W. Variability in the interpolated twitch torque for maximal and submaximal voluntary contractions. J. Appl. Physiol. 2003, 95, 1648–1655. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Suresh, A.K.; Rymer, W.Z.; Suresh, N.L. Assessing altered motor unit recruitment patterns in paretic muscles of stroke survivors using surface electromyography. J. Neural. Eng. 2015, 12, 066001. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.W.; Stokic, D.S. Force control of quadriceps muscle is bilaterally impaired in subacute stroke. J. Appl. Physiol. 2011, 111, 1290–1295. [Google Scholar] [CrossRef] [PubMed]
- Allen, G.M.; Gandevia, S.C.; McKenzie, D.K. Reliability of measurements of muscle strength and voluntary activation using twitch interpolation. Muscle Nerve 1995, 18, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Shield, A.; Zhou, S. Assessing voluntary muscle activation with the twitch interpolation technique. Sports Med. 2004, 34, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Behm, D.G.; St-Pierre, D.M.M.; Perez, D. Muscle inactivation: Assessment of interpolated twitch technique. J. Appl. Physiol. 1996, 81, 2267–2273. [Google Scholar] [CrossRef]
- Marsh, E.; Sale, D.; MComas, A.J.; Quinlan, J. Influence of joint position on ankle dorsiflexion in humans. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1981, 51, 160–167. [Google Scholar] [CrossRef]
- Reebye, O. Anatomical and clinical study of the common fibular nerve. Part 1: Anatomical study. Surg. Radiol. Anat. 2004, 26, 365–370. [Google Scholar] [CrossRef]
- Norte, G.E.; Frye, J.L.; Hart, J.M. Reliability of the Superimposed-burst technique in patients with patellofemoral pain: A technical report. J. Athl. Train 2015, 50, 1207–1211. [Google Scholar] [CrossRef]

| Test 1 Mean (SD) | Test 2 Mean (SD) | Change in Means (SD) | Analysis: Original Units Model ICC or CCC (95% CI) SEM Where Applicable | Analysis: Arcsin Scale Units Model ICC (95% CI) | |||
|---|---|---|---|---|---|---|---|
| Twitch contraction time (CT) | |||||||
| Resting twitch CT (s) | 0.156 (0.036) | 0.147 (0.026) | 0.010 (0.026) | ||||
| Superimposed twitch CT (s) | 0.063 (0.039) | 0.057 (0.041) | 0.000 (0.020) | ||||
| Superimposed twitch CT end task | 0.087 (0.025) | 0.074 (0.039) | 0.013 (0.025) | ||||
| Resting twitch CT end task | 0.131 (0.023) | 0.132 (0.021) | −0.001 (0.015) | ||||
| Twitch amplitudes | |||||||
| Resting twitch amplitude (N) | 26.87 (9.63) | 27.64 (10.53) | −0.77 (9.34) | Gaussian ICC = 0.57 (0.07, 0.86) SEM = 6.6 | |||
| Superimposed twitch amplitude (N) | 4.21 (5.18) | 3.94 (4.68) | 0.27 (3.36) | Non-parametric CCC = 0.77 (0.38, 0.93) | Gaussian ICC = 0.83 (0.55, 0.95) | ||
| Superimposed twitch amplitude end task (N) | 7.93 (6.65) | 7.59 (7.18) | 0.35 (7.03) | Gamma ICC = 0.35 (0.05, 0.45) | Gaussian ICC = 0.65 (0.19, 0.88) | ||
| Resting twitch amplitude end task (N) | 25.28 (9.64) | 26.47 (10.22) | −1.19 (7.30) | Gaussian ICC = 0.73 (0.31, 0.92) SEM = 5.16 | |||
| Voluntary activation (VA) | |||||||
| VAITT (%) | 84.6 (19.4) | 85.1 (20.1) | −0.50 (11.21) | Gamma ICC = 0.62 (0.25, 0.74) | Gaussian ICC = 0.82 (0.53, 0.94) | ||
| VAAdj_ITT (%) | 86.2 (18.1) | 87.5 (15.9) | −1.32 (9.60) | Gamma ICC = 0.62 (0.30, 0.74) | Gaussian ICC = 0.82 (0.51, 0.94) | ||
| VACAR (%) | 96.2 (6.1) | 96.1 (6.5) | 0.16 (1.88) | Gamma ICC = 0.82 (0.63, 0.91) | Gaussian ICC = 0.92 (0.77, 0.98) | ||
| VAITT end task (%) | 70.9 (18.8) | 71.3 (32.1) | −0.34 (28.98) | Non-parametric CCC = 0.39 (−0.11, 0.74) | Arcsin transformation not possible | ||
| VACAR end task (%) | 90.5 (11.6) | 88.3 (16.1) | 2.21 (16.71) | Gamma ICC = 0.33 (−0.23, 0.51) | Gaussian ICC = 0.48 (0.00, 0.82) | ||
| Central fatigue (CF) | |||||||
| CFITT (%) | 13.7 (22.4) | 13.8 (20.0) | −0.16 (33.05) | Non-parametric CCC = −0.22 (−0.68, 0.38) | Arcsin transformation not possible | ||
| CFCAR (%) | 5.7 (12.3) | 7.8 (10.7) | −2.05 (16.60) | Non-parametric CCC = −4.1 (−0.57, 0.51) | Arcsin transformation not possible | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olsen, S.; Signal, N.; Niazi, I.K.; Alder, G.; Rashid, U.; Nedergaard, R.B.; Taylor, D. Reliability of Tibialis Anterior Muscle Voluntary Activation Using the Interpolated Twitch Technique and the Central Activation Ratio in People with Stroke. Brain Sci. 2021, 11, 176. https://doi.org/10.3390/brainsci11020176
Olsen S, Signal N, Niazi IK, Alder G, Rashid U, Nedergaard RB, Taylor D. Reliability of Tibialis Anterior Muscle Voluntary Activation Using the Interpolated Twitch Technique and the Central Activation Ratio in People with Stroke. Brain Sciences. 2021; 11(2):176. https://doi.org/10.3390/brainsci11020176
Chicago/Turabian StyleOlsen, Sharon, Nada Signal, Imran Khan Niazi, Gemma Alder, Usman Rashid, Rasmus Bach Nedergaard, and Denise Taylor. 2021. "Reliability of Tibialis Anterior Muscle Voluntary Activation Using the Interpolated Twitch Technique and the Central Activation Ratio in People with Stroke" Brain Sciences 11, no. 2: 176. https://doi.org/10.3390/brainsci11020176
APA StyleOlsen, S., Signal, N., Niazi, I. K., Alder, G., Rashid, U., Nedergaard, R. B., & Taylor, D. (2021). Reliability of Tibialis Anterior Muscle Voluntary Activation Using the Interpolated Twitch Technique and the Central Activation Ratio in People with Stroke. Brain Sciences, 11(2), 176. https://doi.org/10.3390/brainsci11020176









