Do Pregnancy-Induced Brain Changes Reverse? The Brain of a Mother Six Years after Parturition
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. MRI Data Acquisition
2.3. Image Processing
2.4. Statistical Analyses
2.4.1. Region of Interest Analyses
2.4.2. Whole-Brain Analyses
2.4.3. Pattern Recognition Analyses
2.4.4. Positive Predictive Value (PPV)
2.4.5. Supplementary Analyses
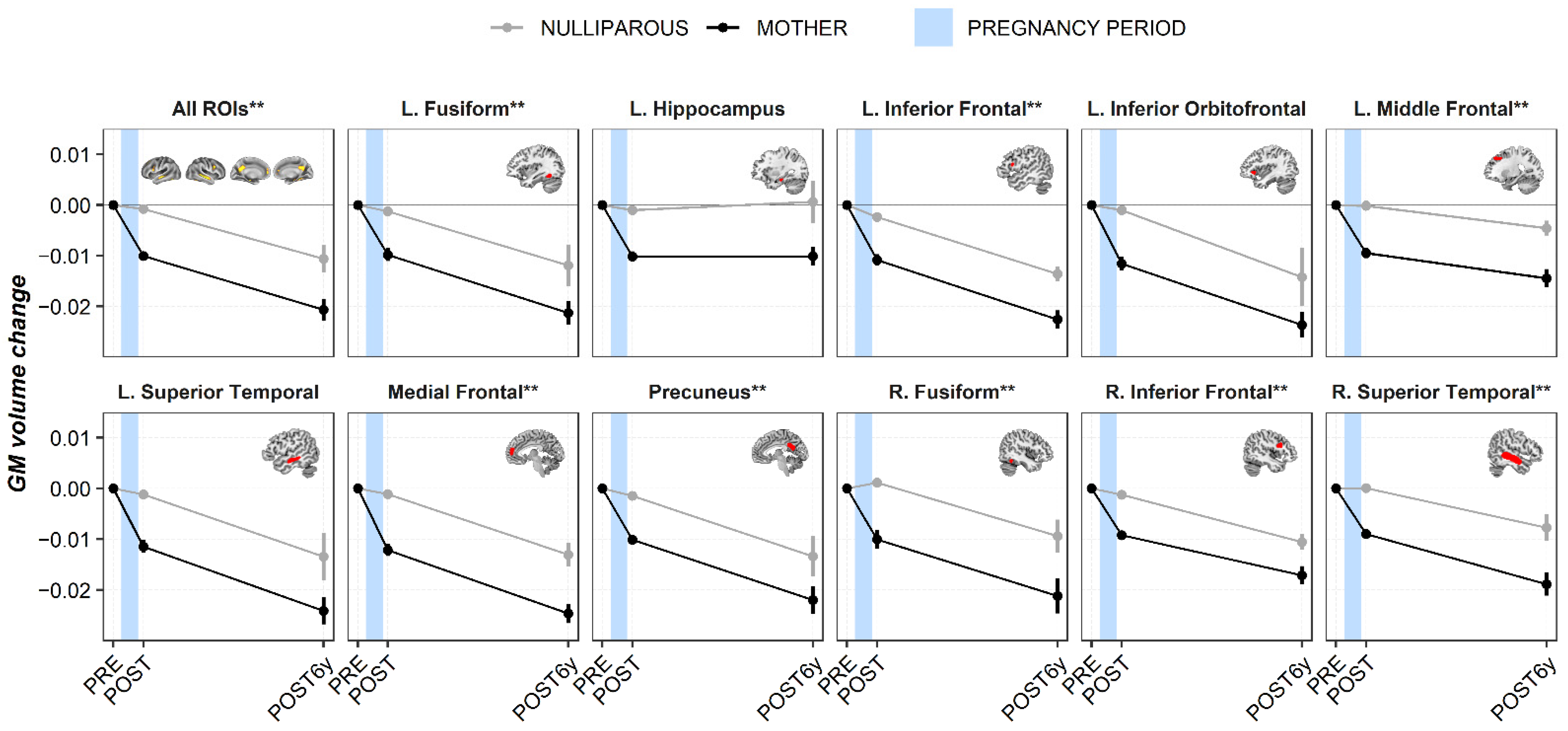
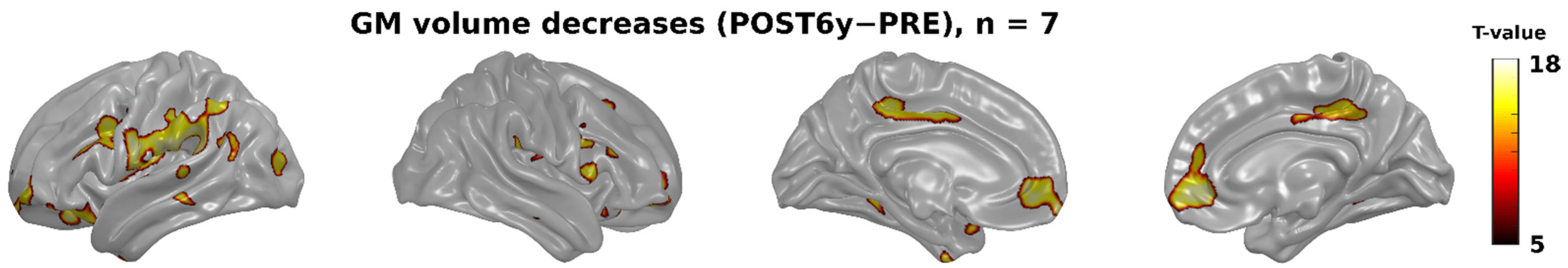
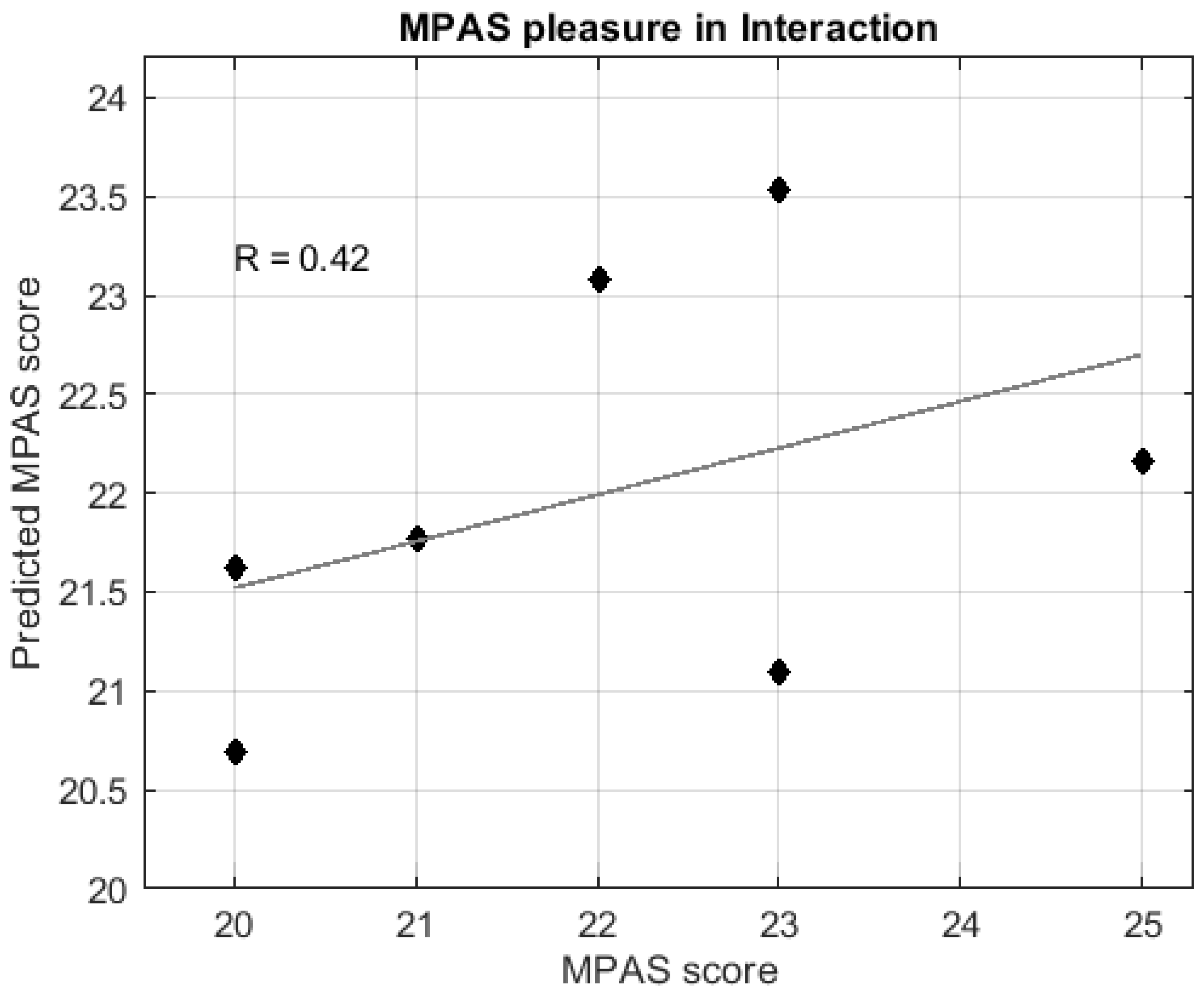
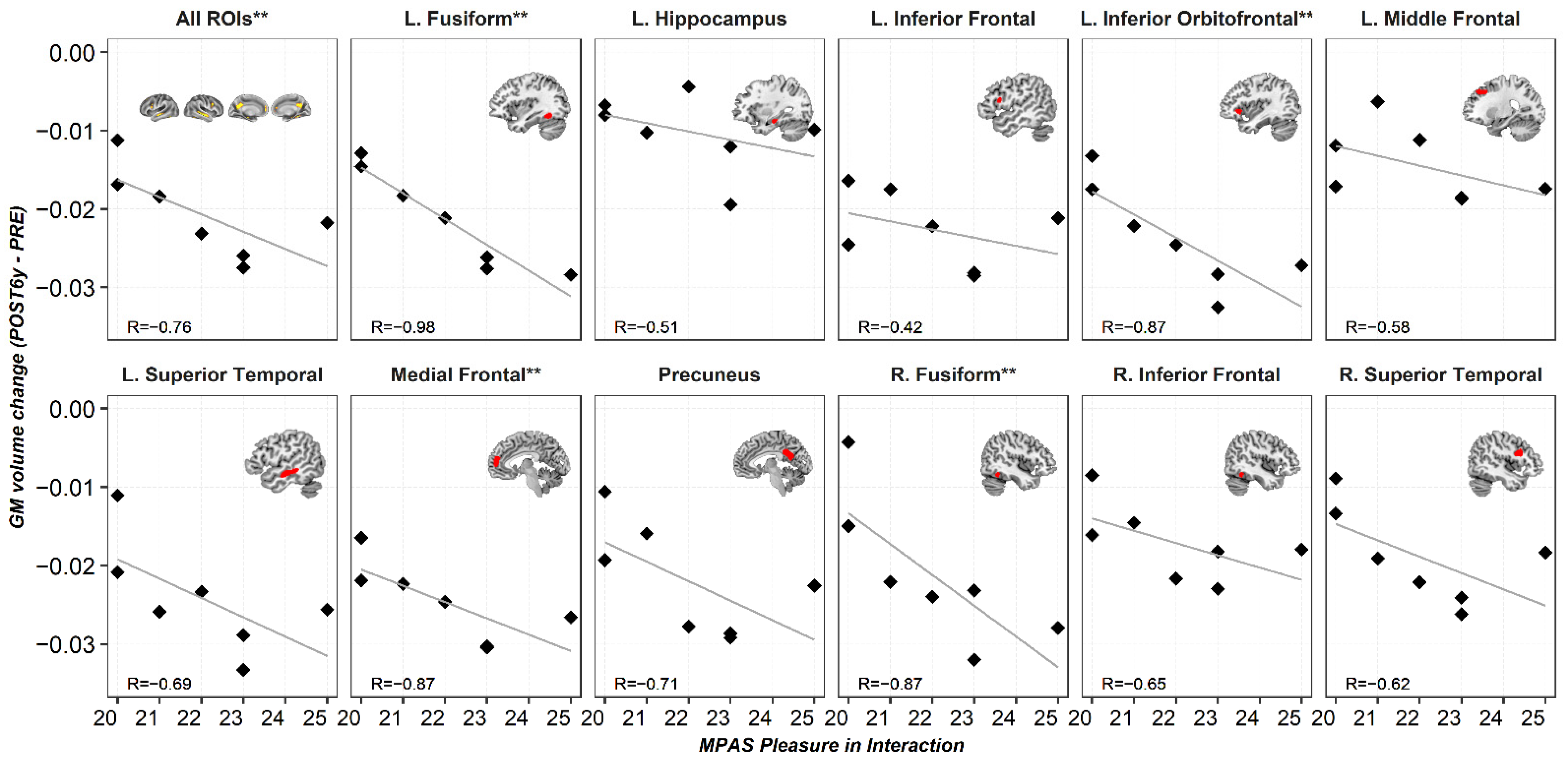
3. Results
3.1. Region of Interest Analyses
3.2. Whole-Brain Analyses
3.3. Pattern Recognition Analyses
3.3.1. Classification
3.3.2. Regression
3.4. Positive Predictive Value (PPV)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lambert, K.G.; Kinsley, C.H. Brain and behavioral modifications that accompany the onset of motherhood. Parenting 2012, 12, 74–88. [Google Scholar] [CrossRef]
- Brunton, P.; Russell, J. Maternal brain adaptations in pregnancy. In Knobil and Neill’s Physiology of Reproduction: Two-Volume Set; Plant, T., Zeleznik, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 2, ISBN 978-0-12-397175-3. [Google Scholar]
- Numan, M.; Young, L.J. Neural mechanisms of mother-infant bonding and pair bonding: Similarities, differences, and broader implications. Horm. Behav. 2016, 77, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Numan, M.; Woodside, B. Maternity: Neural mechanisms, motivational processes, and physiological adaptations. Behav. Neurosci. 2010, 124, 715–741. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Strathearn, L.; Swain, J.E. The maternal brain and its plasticity in humans. Horm. Behav. 2016, 77, 113–123. [Google Scholar] [CrossRef]
- Atzil, S.; Hendler, T.; Zagoory-Sharon, O.; Winetraub, Y.; Feldman, R. Synchrony and specificity in the maternal and the paternal brain: Relations to oxytocin and vasopressin. J. Am. Acad. Child Adolesc. Psychiatry 2012. [Google Scholar] [CrossRef]
- Wan, M.W.; Downey, D.; Strachan, H.; Elliott, R.; Williams, S.R.; Abel, K.M. The neural basis of maternal bonding. PLoS ONE 2014, 9, e88436. [Google Scholar] [CrossRef]
- Feldman, R. The adaptive human parental brain: Implications for children’s social development. Trends Neurosci. 2015, 38, 387–399. [Google Scholar] [CrossRef]
- Saxbe, D.; Rossin-Slater, M.; Goldenberg, D. The transition to parenthood as a critical window for adult health. Am. Psychol. 2018, 73, 1190–1200. [Google Scholar] [CrossRef]
- Barba-Müller, E.; Craddock, S.; Carmona, S.; Hoekzema, E. Brain plasticity in pregnancy and the postpartum period: Links to maternal caregiving and mental health. Arch. Women’s Ment. Health 2019, 22, 289–299. [Google Scholar] [CrossRef]
- Cárdenas, E.F.; Kujawa, A.; Humphreys, K.L. Neurobiological changes during the peripartum period: Implications for health and behavior. Soc. Cogn. Affect. Neurosci. 2019, 1–14. [Google Scholar] [CrossRef]
- Duarte-Guterman, P.; Leuner, B.; Galea, L.A.M. The long and short term effects of motherhood on the brain. Front. Neuroendocrinol. 2019, 53, 100740. [Google Scholar] [CrossRef] [PubMed]
- Oatridge, A.; Holdcroft, A.; Saeed, N.; Hajnal, J.V.; Puri, B.K.; Fusi, L.; Bydder, G.M. Change in brain size during and after pregnancy: Study in healthy women and women with preeclampsia. AJNR Am. J. Neuroradiol. 2002, 23, 19–26. [Google Scholar] [PubMed]
- Hoekzema, E.; Barba-Müller, E.; Pozzobon, C.; Picado, M.; Lucco, F.; García-García, D.; Soliva, J.C.; Tobeña, A.; Desco, M.; Crone, E.A.; et al. Pregnancy leads to long-lasting changes in human brain structure. Nat. Neurosci. 2017, 20, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Carmona, S.; Martínez-García, M.; Paternina-Die, M.; Barba-Müller, E.; Wierenga, L.M.; Alemán-Gómez, Y.; Pretus, C.; Marcos-Vidal, L.; Beumala, L.; Cortizo, R.; et al. Pregnancy and adolescence entail similar neuroanatomical adaptations: A comparative analysis of cerebral morphometric changes. Hum. Brain Mapp. 2019, 40, 2143–2152. [Google Scholar] [CrossRef]
- Kim, P.; Leckman, J.F.; Mayes, L.C.; Feldman, R.; Wang, X.; Swain, J.E. The plasticity of human maternal brain: Longitudinal changes in brain anatomy during the early postpartum period. Behav. Neurosci. 2010, 124, 695–700. [Google Scholar] [CrossRef]
- Lisofsky, N.; Gallinat, J.; Lindenberger, U.; Kühn, S. Postpartal neural plasticity of the maternal brain: Early renormalization of pregnancy-related decreases? Neurosignals 2019, 27, 12–24. [Google Scholar] [CrossRef]
- Victora, C.G.; de Onis, M.; Hallal, P.C.; Blossner, M.; Shrimpton, R. Worldwide timing of growth faltering: Revisiting implications for interventions. Pediatrics 2010, 125, e473–e480. [Google Scholar] [CrossRef]
- Kim, P.; Dufford, A.J.; Tribble, R.C. Cortical thickness variation of the maternal brain in the first 6 months postpartum: Associations with parental self-efficacy. Brain Struct. Funct. 2018, 223, 3267–3277. [Google Scholar] [CrossRef]
- Ashburner, J. Symmetric diffeomorphic modeling of longitudinal structural MRI. Front. Neurosci. 2013, 6. [Google Scholar] [CrossRef]
- Ashburner, J.; Friston, K.J. Unified segmentation. NeuroImage 2005, 26, 839–851. [Google Scholar] [CrossRef]
- Ashburner, J. A fast diffeomorphic image registration algorithm. NeuroImage 2007, 38, 95–113. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A. Why most published research findings are false. PLoS Med. 2005, 2, e124. [Google Scholar] [CrossRef] [PubMed]
- Button, K.S.; Ioannidis, J.P.A.; Mokrysz, C.; Nosek, B.A.; Flint, J.; Robinson, E.S.J.; Munafò, M.R. Power failure: Why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 2013, 14, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Luders, E.; Gingnell, M.; Poromaa, I.S.; Engman, J.; Kurth, F.; Gaser, C. Potential brain age reversal after pregnancy: Younger brains at 4–6 weeks postpartum. Neuroscience 2018, 386, 309–314. [Google Scholar] [CrossRef]
- Maupin, A.N.; Roginiel, A.C.; Rutherford, H.J.V.; Mayes, L.C. A Preliminary review of whether prior reproductive experience influences caregiving. New Dir. Child Adolesc. Dev. 2016, 2016, 73–86. [Google Scholar] [CrossRef]
- Scanlan, V.F.; Byrnes, E.M.; Bridges, R.S. Reproductive experience and activation of maternal memory. Behav. Neurosci. 2006, 120, 676–686. [Google Scholar] [CrossRef]
- Wartella, J.; Amory, E.; Macbeth, A.; McNamara, I.; Stevens, L.; Lambert, K.G.; Kinsley, C.H. Single or multiple reproductive experiences attenuate neurobehavioral stress and fear responses in the female rat. Physiol. Behav. 2003, 79, 373–381. [Google Scholar] [CrossRef]
- Kinsley, C.H.; Madonia, L.; Gifford, G.W.; Tureski, K.; Griffin, G.R.; Lowry, C.; Williams, J.; Collins, J.; McLearie, H.; Lambert, K.G. Motherhood improves learning and memory. Nature 1999, 402, 137–138. [Google Scholar] [CrossRef]
- Gatewood, J.D.; Morgan, M.D.; Eaton, M.; McNamara, I.M.; Stevens, L.F.; Macbeth, A.H.; Meyer, E.A.A.; Lomas, L.M.; Kozub, F.J.; Lambert, K.G.; et al. Motherhood mitigates aging-related decrements in learning and memory and positively affects brain aging in the rat. Brain Res. Bull. 2005, 66, 91–98. [Google Scholar] [CrossRef]
- Love, G.; Torrey, N.; McNamara, I.; Morgan, M.; Banks, M.; Hester, N.W.; Glasper, E.R.; DeVries, A.C.; Kinsley, C.H.; Lambert, K.G. Maternal experience produces long-lasting behavioral modifications in the rat. Behav. Neurosci. 2005, 119, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, V.; Billard, J.M.; Dutar, P.; George, O.; Piazza, P.V.; Epelbaum, J.; Moal, M.L.; Mayo, W. Motherhood-induced memory improvement persists across lifespan in rats but is abolished by a gestational stress. Eur. J. Neurosci. 2006, 23, 3368–3374. [Google Scholar] [CrossRef] [PubMed]
- Lopatina, O.; Inzhutova, A.; Pichugina, Y.A.; Okamoto, H.; Salmina, A.B.; Higashida, H. Reproductive experience affects parental retrieval behaviour associated with increased plasma oxytocin levels in wild-type and cd38-knockout mice. J. Neuroendocrinol. 2011, 23, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Kinsley, C.H.; Trainer, R.; Stafisso-Sandoz, G.; Quadros, P.; Marcus, L.K.; Hearon, C.; Meyer, E.A.A.; Hester, N.; Morgan, M.; Kozub, F.J.; et al. Motherhood and the hormones of pregnancy modify concentrations of hippocampal neuronal dendritic spines. Horm. Behav. 2006, 49, 131–142. [Google Scholar] [CrossRef]
- Pawluski, J.L.; Galea, L.A.M. Hippocampal morphology is differentially affected by reproductive experience in the mother. J. Neurobiol. 2006, 66, 71–81. [Google Scholar] [CrossRef]
- Barha, C.K.; Galea, L.A.M. Motherhood alters the cellular response to estrogens in the hippocampus later in life. Neurobiol. Aging 2011, 32, 2091–2095. [Google Scholar] [CrossRef]
- Arbeitman, M.N. Maternal experience leads to lasting gene expression changes in some regions of the mouse brain. G3 Genes Genomes Genet. 2019, 9, 2623–2628. [Google Scholar] [CrossRef]
- De Lange, A.-M.G.; Kaufmann, T.; Van de Meer, D.; Maglanoc, L.; Alnæs, D.; Moberget, T.; Douaud, G.; Andreassen, O.A.; Westlye, L.T. Population-based neuroimaging reveals traces of childbirth in the maternal brain. bioRxiv 2019, 116, 22341–22346. [Google Scholar] [CrossRef]
- Orchard, E.R.; Ward, P.G.; Sforazzini, F.; Storey, E.; Egan, G.F.; Jamadar, S.D. Cortical changes associated with parenthood are present in late life. bioRxiv 2019. [Google Scholar] [CrossRef]
- Sisk, C.L.; Zehr, J.L. Pubertal hormones organize the adolescent brain and behavior. Front. Neuroendocrinol. 2005, 26, 163–174. [Google Scholar] [CrossRef]
- Zubiaurre-Elorza, L.; Junque, C.; Gómez-Gil, E.; Guillamon, A. Effects of cross-sex hormone treatment on cortical thickness in transsexual individuals. J. Sex. Med. 2014, 11, 1248–1261. [Google Scholar] [CrossRef] [PubMed]
- Parsons, C.E.; Young, K.S.; Petersen, M.V.; Jegindoe Elmholdt, E.-M.; Vuust, P.; Stein, A.; Kringelbach, M.L. Duration of motherhood has incremental effects on mothers’ neural processing of infant vocal cues: A neuroimaging study of women. Sci. Rep. 2017, 7, 1727. [Google Scholar] [CrossRef] [PubMed]
- Abraham, E.; Hendler, T.; Shapira-Lichter, I.; Kanat-Maymon, Y.; Zagoory-Sharon, O.; Feldman, R. Father’s brain is sensitive to childcare experiences. Proc. Natl. Acad. Sci. USA 2014. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Rigo, P.; Mayes, L.C.; Feldman, R.; Leckman, J.F.; Swain, J.E. Neural plasticity in fathers of human infants. Soc. Neurosci. 2014. [Google Scholar] [CrossRef] [PubMed]
- Grasso, D.J.; Moser, J.S.; Dozier, M.; Simons, R. ERP correlates of attention allocation in mothers processing faces of their children. Biol. Psychol. 2009, 81, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Pawluski, J.L.; Galea, L.A.M. Reproductive experience alters hippocampal neurogenesis during the postpartum period in the dam. Neuroscience 2007, 149, 53–67. [Google Scholar] [CrossRef]
- Yeo, B.T.T.; Krienen, F.M.; Sepulcre, J.; Sabuncu, M.R.; Lashkari, D.; Hollinshead, M.; Roffman, J.L.; Smoller, J.W.; Zollei, L.; Polimeni, J.R.; et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011, 106, 1125–1165. [Google Scholar] [CrossRef]
- Schurz, M.; Radua, J.; Aichhorn, M.; Richlan, F.; Perner, J. Fractionating theory of mind: A meta-analysis of functional brain imaging studies. Neurosci. Biobehav. Rev. 2014, 42, 9–34. [Google Scholar] [CrossRef]

| Group | MOTHERS | NULLIPAROUS | ||||
|---|---|---|---|---|---|---|
| Session | PRE | POST | POST6y | PRE | POST | POST6y |
| n of participants | 25 | 25 | 7 | 22 | 21 | 5 |
| Age (mean ± s.d.) (years) | 33.87 ± 3.89 | 35.14 ± 3.87 | 40.55 ± 3.14 | 31.17 ± 5.77 | 32.12 ± 6.03 | 38.83± 6.37 |
| Education (n of subjects) | 2 | 2 | 0 | 2 | 2 | 1 |
| -School | 4 | 4 | 2 | 4 | 3 | 1 |
| -College | 19 | 19 | 5 | 16 | 16 | 3 |
| -University | ||||||
| Means of conception (n of subjects) | 9 | 9 | 3 | - | - | - |
| -Natural | 16 | 16 | 4 | |||
| -Fertility assisted | ||||||
| Time since the PRE session (mean ± s.d.) (years) | - | 1.27 ± 0.30 | 7.22 ± 0.50 | - | 1.11 ± 0.31 | 7.57 ± 0.56 |
| Time since parturition date (mean ± s.d.) (months) | - | 2.45 ± 1.59 | 75.80 ± 7.07 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-García, M.; Paternina-Die, M.; Barba-Müller, E.; Martín de Blas, D.; Beumala, L.; Cortizo, R.; Pozzobon, C.; Marcos-Vidal, L.; Fernández-Pena, A.; Picado, M.; et al. Do Pregnancy-Induced Brain Changes Reverse? The Brain of a Mother Six Years after Parturition. Brain Sci. 2021, 11, 168. https://doi.org/10.3390/brainsci11020168
Martínez-García M, Paternina-Die M, Barba-Müller E, Martín de Blas D, Beumala L, Cortizo R, Pozzobon C, Marcos-Vidal L, Fernández-Pena A, Picado M, et al. Do Pregnancy-Induced Brain Changes Reverse? The Brain of a Mother Six Years after Parturition. Brain Sciences. 2021; 11(2):168. https://doi.org/10.3390/brainsci11020168
Chicago/Turabian StyleMartínez-García, Magdalena, María Paternina-Die, Erika Barba-Müller, Daniel Martín de Blas, Laura Beumala, Romina Cortizo, Cristina Pozzobon, Luis Marcos-Vidal, Alberto Fernández-Pena, Marisol Picado, and et al. 2021. "Do Pregnancy-Induced Brain Changes Reverse? The Brain of a Mother Six Years after Parturition" Brain Sciences 11, no. 2: 168. https://doi.org/10.3390/brainsci11020168
APA StyleMartínez-García, M., Paternina-Die, M., Barba-Müller, E., Martín de Blas, D., Beumala, L., Cortizo, R., Pozzobon, C., Marcos-Vidal, L., Fernández-Pena, A., Picado, M., Belmonte-Padilla, E., Massó-Rodriguez, A., Ballesteros, A., Desco, M., Vilarroya, Ó., Hoekzema, E., & Carmona, S. (2021). Do Pregnancy-Induced Brain Changes Reverse? The Brain of a Mother Six Years after Parturition. Brain Sciences, 11(2), 168. https://doi.org/10.3390/brainsci11020168






