A Combined Administration of Testosterone and Arginine Vasopressin Affects Aggressive Behavior in Males
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
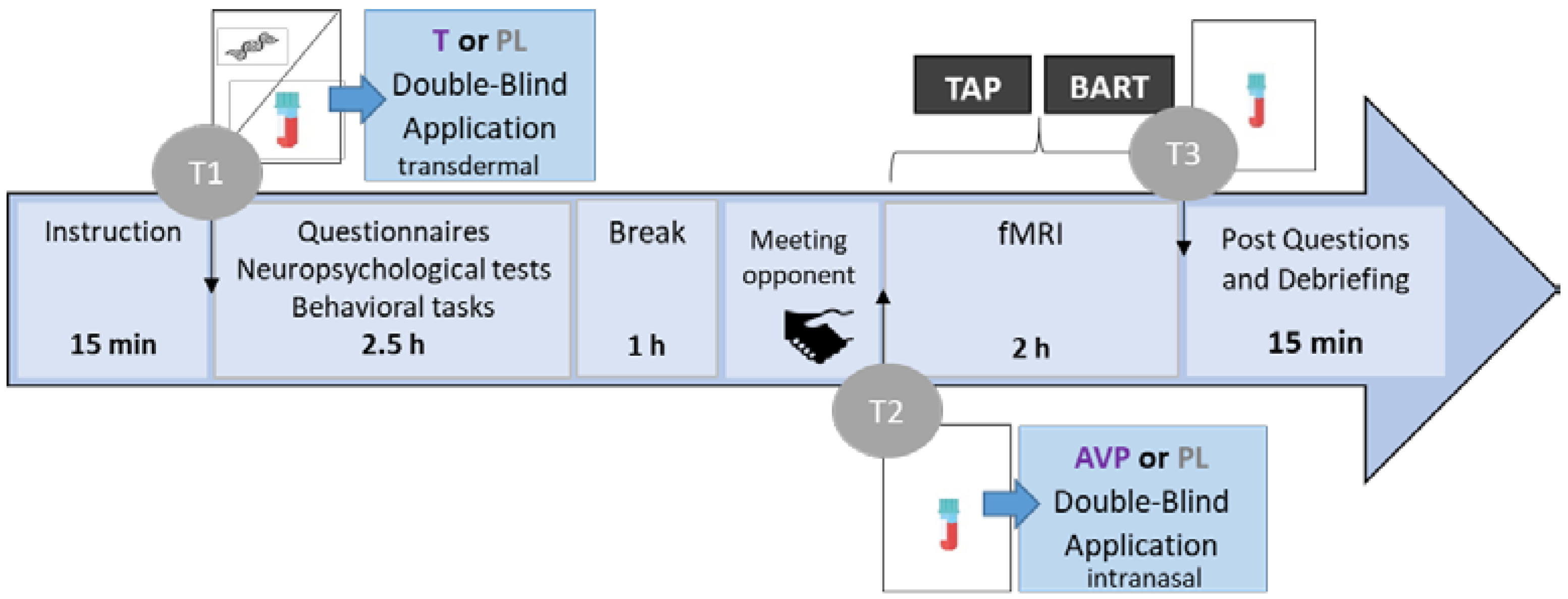
2.2. Procedure
2.3. Hormonal Levels
2.4. Modified Taylor Aggression Paradigm (TAP)
2.5. Questionnaires for Neuropsychological Assessment
2.6. fMRI Data Acquisition
2.7. Data Analysis
2.7.1. Hormonal Levels
2.7.2. Taylor Aggression Paradigm (TAP)
2.7.3. fMRI
2.7.4. Correlation Analyses
3. Results
3.1. Participants
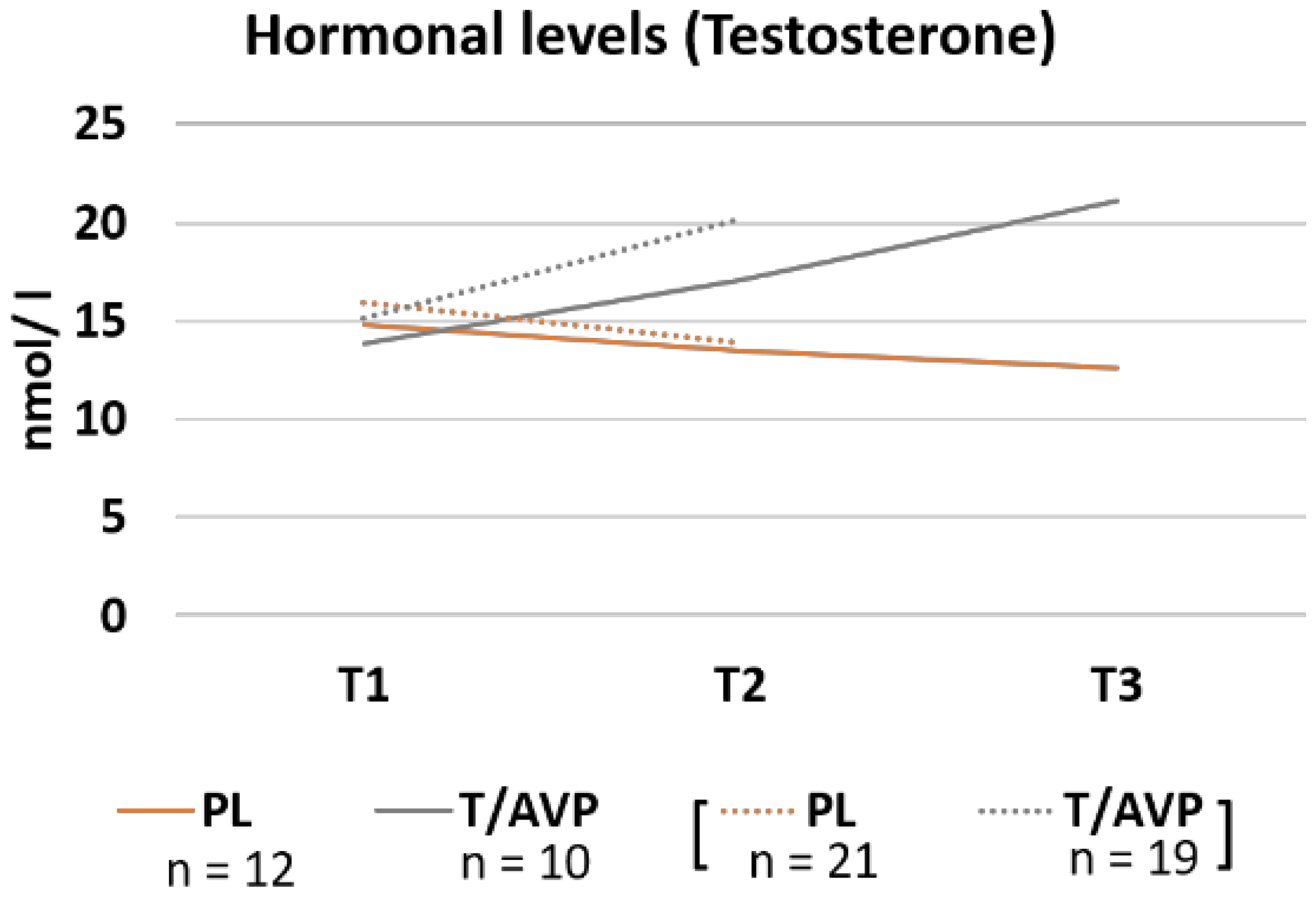
3.2. Hormone Concentration
3.3. Task Behavior
3.4. fMRI Results
4. Discussion
4.1. Hormonal Modulation of Aggression
4.2. Hormonal Modulation of Brain Signals
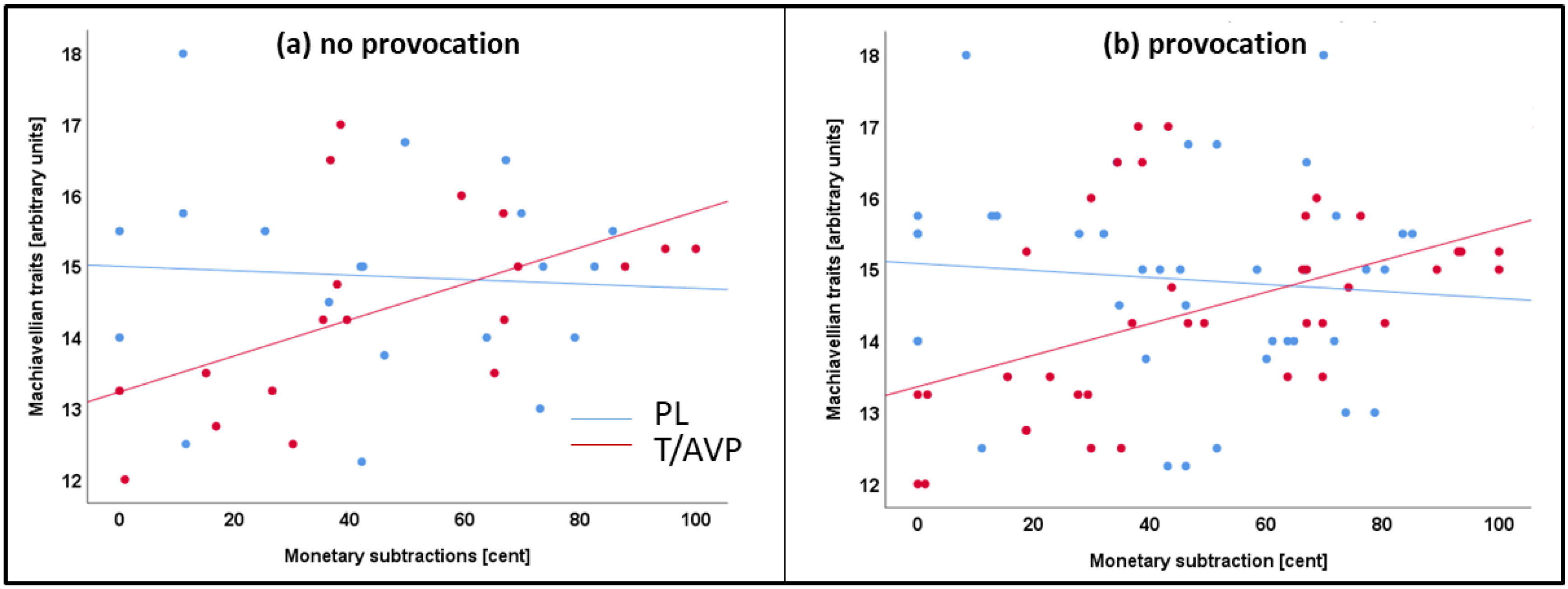
4.3. Machiavellian Traits
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Neal, E.C.; Langley, T. Human Aggression, by Russell G. Geen. Pacific Grove, California, Brooks/Cole, 1990, xiii + 241 pp. Aggress. Behav. 1992, 18, 75–76. [Google Scholar] [CrossRef]
- Blair, R.J.R.; Meffert, H.; Hwang, S.; White, S.F. Psychopathy and brain function: Insights from neuroimaging research. In Handbook of Psychopathy, 2nd ed.; The Guilford Press: New York, NY, USA, 2018; pp. 401–421. ISBN 978-1-4625-3513-2. [Google Scholar]
- Dugré, J.R.; Radua, J.; Carignan-Allard, M.; Dumais, A.; Rubia, K.; Potvin, S. Neurofunctional Abnormalities in Antisocial Spectrum: A Meta-Analysis of FMRI Studies on Five Distinct Neurocognitive Research Domains. Neurosci. Biobehav. Rev. 2020, 119, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Krämer, U.M.; Jansma, H.; Tempelmann, C.; Münte, T.F. Tit-for-Tat: The Neural Basis of Reactive Aggression. NeuroImage 2007, 38, 203–211. [Google Scholar] [CrossRef]
- Lotze, M.; Veit, R.; Anders, S.; Birbaumer, N. Evidence for a Different Role of the Ventral and Dorsal Medial Prefrontal Cortex for Social Reactive Aggression: An Interactive FMRI Study. NeuroImage 2007, 34, 470–478. [Google Scholar] [CrossRef]
- Skibsted, A.P.; Cunha-Bang, S.D.; Carré, J.M.; Hansen, A.E.; Beliveau, V.; Knudsen, G.M.; Fisher, P.M. Aggression-Related Brain Function Assessed with the Point Subtraction Aggression Paradigm in FMRI. Aggress. Behav. 2017, 43, 601–610. [Google Scholar] [CrossRef]
- Aron, A.R.; Fletcher, P.C.; Bullmore, E.T.; Sahakian, B.J.; Robbins, T.W. Stop-Signal Inhibition Disrupted by Damage to Right Inferior Frontal Gyrus in Humans. Nat. Neurosci. 2003, 6, 115–116. [Google Scholar] [CrossRef]
- Puiu, A.A.; Wudarczyk, O.; Kohls, G.; Bzdok, D.; Herpertz-Dahlmann, B.; Konrad, K. Meta-Analytic Evidence for a Joint Neural Mechanism Underlying Response Inhibition and State Anger. Hum. Brain Mapp. 2020, 41, 3147–3160. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.T.; Kornienko, O. The Neurobiology of Human Social Behavior: A Review of How Testosterone and Cortisol Underpin Competition and Affiliation Dynamics. In Salivary Bioscience; Granger, D., Taylor, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Holtfrerich, S.K.C.; Pfister, R.; El Gammal, A.T.; Bellon, E.; Diekhof, E.K. Endogenous Testosterone and Exogenous Oxytocin Influence the Response to Baby Schema in the Female Brain. Sci. Rep. 2018, 8, 7672. [Google Scholar] [CrossRef] [Green Version]
- Kawada, A.; Nagasawa, M.; Murata, A.; Mogi, K.; Watanabe, K.; Kikusui, T.; Kameda, T. Vasopressin Enhances Human Preemptive Strike in Both Males and Females. Sci. Rep. 2019, 9, 9664. [Google Scholar] [CrossRef] [Green Version]
- Geniole, S.N.; Bird, B.M.; McVittie, J.S.; Purcell, R.B.; Archer, J.; Carré, J.M. Is Testosterone Linked to Human Aggression? A Meta-Analytic Examination of the Relationship between Baseline, Dynamic, and Manipulated Testosterone on Human Aggression. Horm. Behavior. 2020, 123, 104644. [Google Scholar] [CrossRef]
- Carré, J.M.; Archer, J. Testosterone and Human Behavior: The Role of Individual and Contextual Variables. Aggress. Violence 2018, 19, 149–153. [Google Scholar] [CrossRef] [Green Version]
- Carré, J.M.; Geniole, S.N.; Ortiz, T.L.; Bird, B.M.; Videto, A.; Bonin, P.L. Exogenous Testosterone Rapidly Increases Aggressive Behavior in Dominant and Impulsive Men. Biol. Psychiatry 2017, 82, 249–256. [Google Scholar] [CrossRef]
- Wagels, L.; Votinov, M.; Kellermann, T.; Eisert, A.; Beyer, C.; Habel, U. Exogenous Testosterone Enhances the Reactivity to Social Provocation in Males. Front. Behav. Neurosci. 2018, 12, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagels, L.; Votinov, M.; Kellermann, T.; Konzok, J.; Jung, S.; Montag, C.; Schneider, F.; Eisert, A.; Beyer, C.; Habel, U. Exogenous Testosterone and the Monoamine-Oxidase A Polymorphism Influence Anger, Aggression and Neural Responses to Provocation in Males. Curr. Status Neurobiol. Aggress. Impuls. 2019, 156, 107491. [Google Scholar] [CrossRef]
- Goetz, S.M.M.; Tang, L.; Thomason, M.E.; Diamond, M.P.; Hariri, A.R.; Carré, J.M. Testosterone Rapidly Increases Neural Reactivity to Threat in Healthy Men: A Novel Two-Step Pharmacological Challenge Paradigm. Biol. Psychiatry 2014, 76, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Hermans, E.J.; Ramsey, N.F.; van Honk, J. Exogenous Testosterone Enhances Responsiveness to Social Threat in the Neural Circuitry of Social Aggression in Humans. Biol. Psychiatry 2008, 63, 263–270. [Google Scholar] [CrossRef]
- Roney, J.R. Theoretical Frameworks for Human Behavioral Endocrinology. Horm. Behav. 2016, 84, 97–110. [Google Scholar] [CrossRef] [Green Version]
- Caldwell, H.K.; Albers, H.E. Effect of Photoperiod on Vasopressin-Induced Aggression in Syrian Hamsters. Horm. Behav. 2004, 46, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Wersinger, S.R.; Ginns, E.I.; O’Carroll, A.-M.; Lolait, S.J.; Young, W.S., III. Vasopressin V1b Receptor Knockout Reduces Aggressive Behavior in Male Mice. Mol. Psychiatry 2002, 7, 975–984. [Google Scholar] [CrossRef] [Green Version]
- Coccaro, E.F.; Kavoussi, R.J.; Hauger, R.L.; Cooper, T.B.; Ferris, C.F. Cerebrospinal Fluid Vasopressin Levels: Correlates With Aggression and Serotonin Function in Personality-Disordered Subjects. Arch. Gen. Psychiatry 1998, 55, 708–714. [Google Scholar] [CrossRef] [Green Version]
- Fetissov, S.O.; Hallman, J.; Nilsson, I.; Lefvert, A.-K.; Oreland, L.; Hökfelt, T. Aggressive Behavior Linked to Corticotropin-Reactive Autoantibodies. Biol. Psychiatry 2006, 60, 799–802. [Google Scholar] [CrossRef]
- Brunnlieb, C.; Münte, T.F.; Krämer, U.; Tempelmann, C.; Heldmann, M. Vasopressin Modulates Neural Responses during Human Reactive Aggression. Soc. Neurosci. 2013, 8, 148–164. [Google Scholar] [CrossRef] [PubMed]
- Brunnlieb, C.; Münte, T.F.; Tempelmann, C.; Heldmann, M. Vasopressin Modulates Neural Responses Related to Emotional Stimuli in the Right Amygdala. Brain Res. 2013, 1499, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Zink, C.F.; Meyer-Lindenberg, A. Human Neuroimaging of Oxytocin and Vasopressin in Social Cognition. Horm. Behavior. 2012, 61, 400–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zink, C.F.; Kempf, L.; Hakimi, S.; Rainey, C.A.; Stein, J.L.; Meyer-Lindenberg, A. Vasopressin Modulates Social Recognition-Related Activity in the Left Temporoparietal Junction in Humans. Transl. Psychiatry 2011, 1, e3. [Google Scholar] [CrossRef] [Green Version]
- Zink, C.F.; Stein, J.L.; Kempf, L.; Hakimi, S.; Meyer-Lindenberg, A. Vasopressin Modulates Medial Prefrontal Cortex–Amygdala Circuitry during Emotion Processing in Humans. J. Neurosci. 2010, 30, 7017. [Google Scholar] [CrossRef]
- Rilling, J.K.; DeMarco, A.C.; Hackett, P.D.; Thompson, R.; Ditzen, B.; Patel, R.; Pagnoni, G. Effects of Intranasal Oxytocin and Vasopressin on Cooperative Behavior and Associated Brain Activity in Men. Psychoneuroendocrinology 2012, 37, 447–461. [Google Scholar] [CrossRef] [Green Version]
- Delville, Y.; Mansour, K.M.; Ferris, C.F. Testosterone Facilitates Aggression by Modulating Vasopressin Receptors in the Hypothalamus. Physiol. Behav. 1996, 60, 25–29. [Google Scholar] [CrossRef]
- Koolhaas, J.M.; Van Den Brink, T.H.C.; Roozendaal, B.; Boorsma, F. Medial Amygdala and Aggressive Behavior: Interaction between Testosterone and Vasopressin. Aggress. Behav. 1990, 16, 223–229. [Google Scholar] [CrossRef]
- Christie, R.; Geis, F.L. (Eds.) Relationships between machiavellianism and measures of ability, opinion, and personality. In Studies in Machiavellianism; Academic Press: Cambridge, MA, USA, 1970; pp. 35–52. [Google Scholar] [CrossRef]
- Jones, D.N.; Neria, A.L. The Dark Triad and Dispositional Aggression. Personal. Individ. Differ. 2015, 86, 360–364. [Google Scholar] [CrossRef]
- Rauthmann, J.F.; Will, T. Proposing a Multidimensional Machiavellianism Conceptualization. Soc. Behav. Personal. Int. J. 2011, 39, 391–404. [Google Scholar] [CrossRef]
- Jones, D.N.; Paulhus, D.L. Machiavellianism. In Handbook of Individual Differences in Social Behavior; The Guilford Press: New York, NY, USA, 2009; pp. 93–108. ISBN 978-1-59385-647-2. [Google Scholar]
- Knight, N.M.; Dahlen, E.R.; Bullock-Yowell, E.; Madson, M.B. The HEXACO Model of Personality and Dark Triad in Relational Aggression. Personal. Individ. Differ. 2018, 122, 109–114. [Google Scholar] [CrossRef]
- Nocera, T.R.; Dahlen, E.R. Dark Triad Personality Traits in Cyber Aggression Among College Students. Violence Vict. 2020, 35, 524–538. [Google Scholar] [CrossRef]
- Dinić, B.M.; Wertag, A. Effects of Dark Triad and HEXACO Traits on Reactive/Proactive Aggression: Exploring the Gender Differences. Personal. Individ. Differ. 2018, 123, 44–49. [Google Scholar] [CrossRef]
- Wibral, M.; Dohmen, T.; Klingmüller, D.; Weber, B.; Falk, A. Testosterone Administration Reduces Lying in Men. PLoS ONE 2012, 7, e46774. [Google Scholar] [CrossRef] [Green Version]
- Van Honk, J.; Montoya, E.R.; Bos, P.A.; van Vugt, M.; Terburg, D. New Evidence on Testosterone and Cooperation. Nature 2012, 485, E4–E5. [Google Scholar] [CrossRef]
- Van Honk, J.; Will, G.-J.; Terburg, D.; Raub, W.; Eisenegger, C.; Buskens, V. Effects of Testosterone Administration on Strategic Gambling in Poker Play. Sci. Rep. 2016, 6, 18096. [Google Scholar] [CrossRef] [Green Version]
- Pfattheicher, S. Testosterone, Cortisol and the Dark Triad: Narcissism (but Not Machiavellianism or Psychopathy) Is Positively Related to Basal Testosterone and Cortisol. Personal. Individ. Differ. 2016, 97, 115–119. [Google Scholar] [CrossRef]
- Dane, L.K.; Jonason, P.K.; McCaffrey, M. Physiological Tests of the Cheater Hypothesis for the Dark Triad Traits: Testosterone, Cortisol, and a Social Stressor. Personal. Individ. Differ. 2018, 121, 227–231. [Google Scholar] [CrossRef]
- Carré, J.M.; McCormick, C.M.; Hariri, A.R. The Social Neuroendocrinology of Human Aggression. Psychoneuroendocrinology 2011, 36, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, R.C. The Assessment and Analysis of Handedness: The Edinburgh Inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Schmidt, K.H.; Metzler, P. Vocabulary Test (Wortschatztest (WST)); Belz Test GmbH: Weinheim, Germany, 1992. [Google Scholar]
- Buss, A.H.; Perry, M. The Aggression Questionnaire. J. Pers. Soc. Psychol. 1992, 63, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Lilienfeld, S.O.; Andrews, B.P. Development and Preliminary Validation of a Self-Report Measure of Psychopathic Personality Traits in Noncriminal Population. J. Pers. Assess. 1996, 66, 488–524. [Google Scholar] [CrossRef] [PubMed]
- Patton, J.H.; Stanford, M.S.; Barratt, E.S. Factor Structure of the Barratt Impulsiveness Scale. J. Clin. Psychol. 1995, 51, 768–774. [Google Scholar] [CrossRef]
- Abler, B.; Kessler, H. ERQ—Emotion Regulation Questionnaire-deutsche Fassung. Emot. Regul. Quest. 2011. [Google Scholar] [CrossRef]
- Christie, R.; Geis, F.L. Studies in Machiavellianism; Academic Press: New York, NY, USA, 1970. [Google Scholar]
- Wagels, L.; Votinov, M.; Radke, S.; Clemens, B.; Montag, C.; Jung, S.; Habel, U. Blunted Insula Activation Reflects Increased Risk and Reward Seeking as an Interaction of Testosterone Administration and the MAOA Polymorphism. Hum. Brain Mapp. 2017, 38, 4574–4593. [Google Scholar] [CrossRef] [Green Version]
- Pietrowsky, R.; Strüben, C.; Mölle, M.; Fehm, H.L.; Born, J. Brain Potential Changes after Intranasal vs. Intravenous Administration of Vasopressin: Evidence for a Direct Nose-Brain Pathway for Peptide Effects in Humans. Biol. Psychiatry 1996, 39, 332–340. [Google Scholar] [CrossRef]
- Uzefovsky, F.; Shalev, I.; Israel, S.; Knafo, A.; Ebstein, R.P. Vasopressin Selectively Impairs Emotion Recognition in Men. Psychoneuroendocrinology 2012, 37, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.R.; George, K.; Walton, J.C.; Orr, S.P.; Benson, J. Sex-Specific Influences of Vasopressin on Human Social Communication. Proc. Natl. Acad. Sci. USA 2006, 103, 7889. [Google Scholar] [CrossRef] [Green Version]
- Price, D.; Burris, D.; Cloutier, A.; Thompson, C.B.; Rilling, J.K.; Thompson, R.R. Dose-Dependent and Lasting Influences of Intranasal Vasopressin on Face Processing in Men. Front. Endocrinol. 2017, 8, 220. [Google Scholar] [CrossRef]
- Chen, X.; Hackett, P.D.; DeMarco, A.C.; Feng, C.; Stair, S.; Haroon, E.; Ditzen, B.; Pagnoni, G.; Rilling, J.K. Effects of Oxytocin and Vasopressin on the Neural Response to Unreciprocated Cooperation within Brain Regions Involved in Stress and Anxiety in Men and Women. Brain Imaging Behav. 2016, 10, 581–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galbusera, A.; De Felice, A.; Girardi, S.; Bassetto, G.; Maschietto, M.; Nishimori, K.; Chini, B.; Papaleo, F.; Vassanelli, S.; Gozzi, A. Intranasal Oxytocin and Vasopressin Modulate Divergent Brainwide Functional Substrates. Neuropsychopharmacology 2017, 42, 1420–1434. [Google Scholar] [CrossRef]
- Taylor, S.P.; Epstein, S. Aggression as a Function of the Interaction of the Sex of the Aggressor and the Sex of the Victim. J. Pers. 1967, 35, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Warburton, W.A.; Bushman, B.J. The Competitive Reaction Time Task: The Development and Scientific Utility of a Flexible Laboratory Aggression Paradigm. Aggress. Behav. 2019, 45, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Weidler, C.; Habel, U.; Hüpen, P.; Akkoc, D.; Schneider, F.; Blendy, J.A.; Wagels, L. On the Complexity of Aggressive Behavior: Contextual and Individual Factors in the Taylor Aggression Paradigm. Front. Psychiatry 2019, 10, 521. [Google Scholar] [CrossRef]
- Weidler, C.; Wagels, L.; Regenbogen, C.; Hofhansel, L.; Blendy, J.A.; Clemens, B.; Montag, C.; Habel, U. The Influence of the OPRM1 (A118G) Polymorphism on Behavioral and Neural Correlates of Aggression in Healthy Males. Curr. Status Neurobiol. Aggress. Impuls. 2019, 156, 107467. [Google Scholar] [CrossRef]
- Ritter, D.; Eslea, M. Hot Sauce, Toy Guns, and Graffiti: A Critical Account of Current Laboratory Aggression Paradigms. Aggress. Behav. 2005, 31, 407–419. [Google Scholar] [CrossRef]
- Repple, J.; Habel, U.; Wagels, L.; Pawliczek, C.M.; Schneider, F.; Kohn, N. Sex differences in the neural correlates of aggression. Brain Struct. Funct. 2018, 223, 4115–4124. [Google Scholar] [CrossRef]
- Pope, H.G., Jr.; Kouri, E.M.; Hudson, J.I. Effects of Supraphysiologic Doses of Testosterone on Mood and Aggression in Normal Men: A Randomized Controlled Trial. Arch. Gen. Psychiatry 2000, 57, 133–140. [Google Scholar] [CrossRef]
- Dreher, J.-C.; Dunne, S.; Pazderska, A.; Frodl, T.; Nolan, J.J.; O’Doherty, J.P. Testosterone Causes Both Prosocial and Antisocial Status-Enhancing Behaviors in Human Males. Proc. Natl. Acad. Sci. USA 2016, 113, 11633. [Google Scholar] [CrossRef] [Green Version]
- Panagiotidis, D.; Clemens, B.; Habel, U.; Schneider, F.; Schneider, I.; Wagels, L.; Votinov, M. Exogenous Testosterone in a Non-Social Provocation Paradigm Potentiates Anger but Not Behavioral Aggression. Eur. Neuropsychopharmacol. 2017, 27, 1172–1184. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, D.B.; Archer, J.; Wu, F.C.W. Effects of Testosterone on Mood, Aggression, and Sexual Behavior in Young Men: A Double-Blind, Placebo-Controlled, Cross-over Study. J. Clin. Endocrinol. Metab. 2004, 89, 2837–2845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polezzi, D.; Daum, I.; Rubaltelli, E.; Lotto, L.; Civai, C.; Sartori, G.; Rumiati, R. Mentalizing in Economic Decision-Making. Behav. Brain Res. 2008, 190, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Zheng, L.; Li, L.; Guo, X.; Zhang, W.; Zheng, Y. The Neural Responses to Social Cooperation in Gain and Loss Context. PLoS ONE 2016, 11, e0160503. [Google Scholar] [CrossRef]
- Bzdok, D.; Hartwigsen, G.; Reid, A.; Laird, A.R.; Fox, P.T.; Eickhoff, S.B. Left Inferior Parietal Lobe Engagement in Social Cognition and Language. Neurosci. Biobehav. Rev. 2016, 68, 319–334. [Google Scholar] [CrossRef] [Green Version]
- Igelström, K.M.; Graziano, M.S.A. The Inferior Parietal Lobule and Temporoparietal Junction: A Network Perspective. Neuropsychologia 2017, 105, 70–83. [Google Scholar] [CrossRef]
- Grecucci, A.; Giorgetta, C.; Bonini, N.; Sanfey, A. Reappraising Social Emotions: The Role of Inferior Frontal Gyrus, Temporo-Parietal Junction and Insula in Interpersonal Emotion Regulation. Front. Hum. Neurosci. 2013, 7, 523. [Google Scholar] [CrossRef] [Green Version]
- Liakakis, G.; Nickel, J.; Seitz, R.J. Diversity of the Inferior Frontal Gyrus—A Meta-Analysis of Neuroimaging Studies. Behav. Brain Res. 2011, 225, 341–347. [Google Scholar] [CrossRef]
- Johnston, P.; Mayes, A.; Hughes, M.; Young, A.W. Brain Networks Subserving the Evaluation of Static and Dynamic Facial Expressions. Cortex J. Devoted Study Nerv. Syst. Behav. 2013, 49, 2462–2472. [Google Scholar] [CrossRef]
- Wilson, R.P.; Colizzi, M.; Bossong, M.G.; Allen, P.; Kempton, M.; Abe, N.; Barros-Loscertales, A.R.; Bayer, J.; Beck, A.; Bjork, J.; et al. Correction to: The Neural Substrate of Reward Anticipation in Health: A Meta-Analysis of FMRI Findings in the Monetary Incentive Delay Task. Neuropsychol. Rev. 2018, 28, 507–508. [Google Scholar] [CrossRef] [Green Version]
- Swick, D.; Ashley, V.; Turken, A.U. Left Inferior Frontal Gyrus Is Critical for Response Inhibition. BMC Neurosci. 2008, 9, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aron, A.R. From Reactive to Proactive and Selective Control: Developing a Richer Model for Stopping Inappropriate Responses. Biol. Psychiatry 2011, 69, e55–e68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aron, A.R.; Robbins, T.W.; Poldrack, R.A. Inhibition and the Right Inferior Frontal Cortex: One Decade On. Trends Cogn. Sci. 2014, 18, 177–185. [Google Scholar] [CrossRef]
- Schurz, M.; Radua, J.; Aichhorn, M.; Richlan, F.; Perner, J. Fractionating Theory of Mind: A Meta-Analysis of Functional Brain Imaging Studies. Neurosci. Biobehav. Rev. 2014, 42, 9–34. [Google Scholar] [CrossRef] [Green Version]
- Vartanian, O.; Beatty, E.L.; Smith, I.; Blackler, K.; Lam, Q.; Forbes, S. One-Way Traffic: The Inferior Frontal Gyrus Controls Brain Activation in the Middle Temporal Gyrus and Inferior Parietal Lobule during Divergent Thinking. Neural Bases Creat. Intell. Common Grounds Differ. 2018, 118, 68–78. [Google Scholar] [CrossRef]
- Caldwell, H.K.; Aulino, E.A.; Rodriguez, K.M.; Witchey, S.K.; Yaw, A.M. Social Context, Stress, Neuropsychiatric Disorders, and the Vasopressin 1b Receptor. Front. Neurosci. 2017, 11, 567. [Google Scholar] [CrossRef] [PubMed]
- Ferris, C.F.; Stolberg, T.; Kulkarni, P.; Murugavel, M.; Blanchard, R.; Blanchard, D.C.; Febo, M.; Brevard, M.; Simon, N.G. Imaging the Neural Circuitry and Chemical Control of Aggressive Motivation. BMC Neurosci. 2008, 9, 111. [Google Scholar] [CrossRef] [Green Version]
- Repple, J.; Pawliczek, C.M.; Voss, B.; Siegel, S.; Schneider, F.; Kohn, N.; Habel, U. From Provocation to Aggression: The Neural Network. BMC Neurosci. 2017, 18, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassell, J.E.; Collins, V.E.; Li, H.; Rogers, J.T.; Austin, R.C.; Visceau, C.; Nguyen, K.T.; Orchinik, M.; Lowry, C.A.; Renner, K.J. Local Inhibition of Uptake2 Transporters Augments Stress-Induced Increases in Serotonin in the Rat Central Amygdala. Neurosci. Lett. 2019, 701, 119–124. [Google Scholar] [CrossRef]
- Caldwell, H.K.; Lee, H.-J.; Macbeth, A.H.; Young, W.S., 3rd. Vasopressin: Behavioral Roles of an “Original” Neuropeptide. Prog. Neurobiol. 2008, 84, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Schultheiss, O.C.; Campbell, K.L.; McClelland, D.C. Implicit Power Motivation Moderates Men’s Testosterone Responses to Imagined and Real Dominance Success. Horm. Behav. 1999, 36, 234–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welker, K.M.; Norman, R.E.; Goetz, S.; Moreau, B.J.P.; Kitayama, S.; Carré, J.M. Preliminary Evidence That Testosterone’s Association with Aggression Depends on Self-Construal. Horm. Hum. Compet. 2017, 92, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Lobbestael, J.; Baumeister, R.F.; Fiebig, T.; Eckel, L.A. The Role of Grandiose and Vulnerable Narcissism in Self-Reported and Laboratory Aggression and Testosterone Reactivity. Personal. Individ. Differ. 2014, 69, 22–27. [Google Scholar] [CrossRef]
- Bobadilla, L.; Wampler, M.; Taylor, J. Proactive and Reactive Aggression Are Associated with Different Physiological and Personality Profiles. J. Soc. Clin. Psychol. 2012, 31, 458–487. [Google Scholar] [CrossRef]
- Sakalaki, M.; Richardson, C.; Thépaut, Y. Machiavellianism and Economic Opportunism. J. Appl. Soc. Psychol. 2007, 37, 1181–1190. [Google Scholar] [CrossRef]
- Sakalaki, M.; Kanellaki, S.; Richardson, C. Is a Manipulator’s Externality Paradoxical? The Relationship Between Machiavellianism, Economic Opportunism, and Economic Locus of Control. J. Appl. Soc. Psychol. 2009, 39, 2591–2603. [Google Scholar] [CrossRef]
- Born, J.; Lange, T.; Kern, W.; McGregor, G.P.; Bickel, U.; Fehm, H.L. Sniffing Neuropeptides: A Transnasal Approach to the Human Brain. Nat. Neurosci. 2002, 5, 514–516. [Google Scholar] [CrossRef]
- Kacheva, S.; Kolk, K.; Morgenthaler, N.G.; Brabant, G.; Karges, W. Gender-Specific Co-Activation of Arginine Vasopressin and the Hypothalamic–Pituitary–Adrenal Axis during Stress. Clin. Endocrinol. 2015, 82, 570–576. [Google Scholar] [CrossRef]



| T/AVP | PL | ||||
|---|---|---|---|---|---|
| M | SEM | M | SEM | p | |
| Age | 24.80 | 1.25 | 23.95 | 0.75 | 0.570 |
| WST | 33.05 | 0.64 | 33.05 | 0.63 | 0.996 |
| BPAQ | 62.84 | 3.04 | 66.29 | 3.39 | 0.455 |
| PPI | 343.68 | 3.46 | 338.85 | 2.33 | 0.249 |
| BIS-11 | 78.62 | 1.75 | 78.87 | 1.77 | 0.923 |
| ERQ—reappraisal | 25.53 | 1.17 | 27.62 | 1.29 | 0.241 |
| ERQ—suppression | 13.42 | 0.88 | 16.33 | 1.05 | 0.175 |
| MACH IV | 14.42 | 0.32 | 14.86 | 0.32 | 0.332 |
| Effects | F | p | η2p |
|---|---|---|---|
| Provocation | 2.81 | 0.102 | 0.072 |
| Provocation × administration group | 6.44 | 0.016 * | 0.152 |
| Provocation × belief | 1.05 | 0.312 | 0.028 |
| Provocation x administration group × belief | 0.10 | 0.417 | 0.018 |
| Administration group | 0.31 | 0.581 | 0.009 |
| Belief | 2.34 | 0.135 | 0.061 |
| Administration group × belief | 0.47 | 0.496 | 0.013 |
| Contrast | Region | k | T | x | y | z |
|---|---|---|---|---|---|---|
| L Insula Lobe | 12,210 | 8.29 | −40 | 0 | 8 | |
| R Caudate Nucleus | 8.15 | 8 | 12 | −12 | ||
| L Putamen | 8.13 | −14 | 12 | −10 | ||
| L Insula Lobe | 7.83 | −38 | 4 | 2 | ||
| L IFG (p. Opercularis) | 7.74 | −52 | 4 | 6 | ||
| R Caudate Nucleus | 7.63 | 16 | 12 | −12 | ||
| Win > loss | L Middle Temporal Gyrus | 7.62 | −44 | −64 | 10 | |
| R Putamen | 7.42 | 22 | 10 | 2 | ||
| R Middle Temporal Gyrus | 869 | 8.84 | 42 | −72 | 8 | |
| R Cuneus | 424 | 6.89 | 18 | −78 | 40 | |
| R Superior Occipital Gyrus | 6.37 | 20 | −90 | 34 | ||
| R Cuneus | 5.66 | 14 | −92 | 22 | ||
| R SupraMarginal Gyrus | 382 | 6.84 | 58 | −38 | 24 | |
| R Superior Temporal Gyrus | 6.73 | 62 | −36 | 16 | ||
| L SupraMarginal Gyrus | 376 | 6.54 | −60 | −26 | 34 | |
| L Lingual Gyrus | 2632 | 9.34 | −24 | −98 | −14 | |
| L Inferior Occipital Gyrus | 9.19 | −36 | −90 | −12 | ||
| L Fusiform Gyrus | 8.70 | −42 | −82 | −14 | ||
| L Middle Occipital Gyrus | 7.25 | −32 | −96 | 0 | ||
| R Lingual Gyrus | 2117 | 8.21 | 20 | −96 | −12 | |
| R Calcarine Gyrus | 8.20 | 18 | −102 | −6 | ||
| R Inferior Occipital Gyrus | 8.11 | 38 | −88 | −14 | ||
| R Middle Temporal Gyrus | 3.92 | 64 | −40 | −12 | ||
| R IFG (p. Orbitalis) | 1058 | 5.15 | 48 | 26 | −14 | |
| Loss > win | R Inferior Temporal Gyrus | 4.67 | 48 | 6 | −36 | |
| R Medial Temporal Pole | 4.05 | 44 | 12 | −28 | ||
| R Middle Temporal Gyrus | 4.02 | 54 | −26 | −12 | ||
| R IFG (p. Orbitalis) | 4.01 | 40 | 38 | −18 | ||
| R Medial Temporal Pole | 3.97 | 52 | 6 | −24 | ||
| R Superior Medial Gyrus | 849 | 4.92 | 10 | 28 | 62 | |
| L Superior Medial Gyrus | 4.26 | −6 | 52 | 22 | ||
| R Superior Parietal Lobule | 508 | 6.02 | 36 | −76 | 50 | |
| R Angular Gyrus | 4.54 | 50 | −64 | 24 | ||
| L Inferior Parietal Lobule | 488 | 4.93 | −36 | −68 | 48 | |
| L Angular Gyrus | 3.95 | −52 | −56 | 28 | ||
| L Middle Temporal Gyrus | 4.68 | −58 | −24 | −16 | ||
| L Precuneus | 371 | 5.78 | 0 | −68 | 36 | |
| R MCC | 3.62 | 8 | −52 | 34 | ||
| L IFG (p. Orbitalis) | 371 | 5.57 | −30 | 22 | −24 | |
| L Temporal Pole | 5.02 | −42 | 20 | −24 |
| Contrast | Region | k | T | x | y | z |
|---|---|---|---|---|---|---|
| L Inferior Occipital Gyrus | 10,572 | 9.84 | −34 | −90 | −12 | |
| L Lingual Gyrus | 9.28 | −22 | −100 | −14 | ||
| R Inferior Occipital Gyrus | 9.11 | 28 | −96 | −6 | ||
| L Middle Occipital Gyrus | 8.26 | −34 | −94 | 0 | ||
| L Inferior Occipital Gyrus | 8.06 | −48 | −68 | −16 | ||
| R Middle Occipital Gyrus | 7.89 | 28 | −90 | 6 | ||
| Loss > win | L Calcarine Gyrus | 1514 | 4.74 | −22 | −66 | 4 |
| R Calcarine Gyrus | 4.63 | 4 | −72 | 16 | ||
| R Lingual Gyrus | 4.29 | 10 | −64 | 2 | ||
| L Cuneus | 3.70 | −10 | −80 | 16 | ||
| R Angular Gyrus | 4.91 | 34 | −70 | 46 | ||
| R Middle Occipital Gyrus | 4.69 | 38 | −66 | 34 | ||
| L Inferior Parietal Lobule | 573 | 5.20 | −34 | −60 | 50 | |
| L Angular Gyrus | 3.33 | −44 | −54 | 32 | ||
| R Inferior Temporal Gyrus | 380 | 5.46 | 50 | −4 | −36 | |
| R Medial Temporal Pole | 4.76 | 48 | 16 | −28 | ||
| R Temporal Pole | 4.70 | 50 | 16 | −24 | ||
| L Paracentral Lobule | 331 | 4.69 | −2 | −36 | 80 | |
| L Precuneus | 4.44 | −4 | −40 | 80 | ||
| L IFG (p. Triangularis) | 274 | 4.13 | −46 | 14 | 28 | |
| L Middle Frontal Gyrus | 3.46 | −46 | 20 | 36 | ||
| R Superior Medial Gyrus | 272 | 5.17 | 14 | 60 | 32 | |
| L Precentral Gyrus | 12,344 | 10.05 | −38 | −12 | 50 | |
| L Posterior−Medial Frontal | 8.27 | −4 | −10 | 64 | ||
| R Posterior−Medial Frontal | 8.14 | 8 | −6 | 66 | ||
| L MCC | 7.54 | −14 | −26 | 44 | ||
| R Middle Frontal Gyrus | 7.31 | 42 | −4 | 52 | ||
| Win > loss | R Precentral Gyrus | 7.08 | 52 | 0 | 48 | |
| L MCC | 6.86 | −6 | 4 | 38 | ||
| L Insula Lobe | 835 | 5.97 | −44 | 6 | 6 | |
| L Rolandic Operculum | 5.76 | −50 | 4 | 4 | ||
| L IFG (p. Triangularis) | 4.51 | −36 | 22 | 8 | ||
| R IFG (p. Opercularis) | 726 | 5.55 | 38 | 12 | 12 | |
| R Insula Lobe | 4.85 | 32 | 26 | 4 | ||
| R Rolandic Operculum | 3.24 | 58 | 0 | 12 | ||
| R Superior Temporal Gyrus | 497 | 5.21 | 50 | −38 | 22 | |
| R Rolandic Operculum | 3.61 | 40 | −30 | 20 | ||
| R Middle Temporal Gyrus | 299 | 6.37 | 42 | −68 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akkoc Altinok, D.C.; Votinov, M.; Henzelmann, F.; Jo, H.; Eisert, A.; Habel, U.; Wagels, L. A Combined Administration of Testosterone and Arginine Vasopressin Affects Aggressive Behavior in Males. Brain Sci. 2021, 11, 1623. https://doi.org/10.3390/brainsci11121623
Akkoc Altinok DC, Votinov M, Henzelmann F, Jo H, Eisert A, Habel U, Wagels L. A Combined Administration of Testosterone and Arginine Vasopressin Affects Aggressive Behavior in Males. Brain Sciences. 2021; 11(12):1623. https://doi.org/10.3390/brainsci11121623
Chicago/Turabian StyleAkkoc Altinok, Dilsa Cemre, Mikhail Votinov, Friederike Henzelmann, HanGue Jo, Albrecht Eisert, Ute Habel, and Lisa Wagels. 2021. "A Combined Administration of Testosterone and Arginine Vasopressin Affects Aggressive Behavior in Males" Brain Sciences 11, no. 12: 1623. https://doi.org/10.3390/brainsci11121623
APA StyleAkkoc Altinok, D. C., Votinov, M., Henzelmann, F., Jo, H., Eisert, A., Habel, U., & Wagels, L. (2021). A Combined Administration of Testosterone and Arginine Vasopressin Affects Aggressive Behavior in Males. Brain Sciences, 11(12), 1623. https://doi.org/10.3390/brainsci11121623






