A Mechanism-Based Approach to Anti-Aggression Psychotherapy in Borderline Personality Disorder: Group Treatment Affects Amygdala Activation and Connectivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design and Study Procedure
2.2. Participants
2.3. Psychometric and Clinical Assessments
2.4. Treatments
2.5. Functional MRI Paradigm
2.6. Functional MRI Data Acquisition
3. Data Analysis
3.1. Demographics, Psychometric Data and Clinical Scores
3.2. FMRI Data
4. Results
4.1. Demographics, Psychometric Data and Clinical Scores
4.2. Task-Related Amygdala Activation at Pre-Treatment Timepoint
4.3. Effect of MAAP on Task-Related Amygdala Activation
4.4. Effect of MAAP on the Coupling of the Amygdala with Other Brain Regions
4.5. Correlation of Change in Amygdala Activation and Connectivity with Change in Overt Aggression
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newhill, C.E.; Eack, S.M.; Mulvey, E.P. Violent behavior in borderline personality. J. Personal. Disord. 2009, 23, 541–554. [Google Scholar] [CrossRef]
- Mancke, F.; Herpertz, S.C.; Bertsch, K. Aggression in borderline personality disorder: A multidimensional model. Personal. Disord. 2015, 6, 278–291. [Google Scholar] [CrossRef] [PubMed]
- Bertsch, K.; Florange, J.; Herpertz, S.C. Understanding Brain Mechanisms of Reactive Aggression. Curr. Psychiatry Rep. 2020, 22, 81. [Google Scholar] [CrossRef]
- Coccaro, E.F.; Harvey, P.D.; Kupsaw-Lawrence, E.; Herbert, J.L.; Bernstein, D.J.T.J.o.N.; Neurosciences, C. Development of neuropharmacologically based behavioral assessments of impulsive aggressive behavior. J. Neuropsychiatry Clin. Neurosci. 1991, 3, S44–S51. [Google Scholar]
- Herpertz, S.C.; Matzke, B.; Hillmann, K.; Neukel, C.; Mancke, F.; Jaentsch, B.; Schwenger, U.; Honecker, H.; Bullenkamp, R.; Steinmann, S.; et al. A mechanism-based group-psychotherapy approach to aggressive behaviour in borderline personality disorder: Findings from a cluster-randomised controlled trial. BJPsych Open 2020, 7, e17. [Google Scholar] [CrossRef]
- Kazdin, A.E. Mediators and mechanisms of change in psychotherapy research. Annu. Rev. Clin. Psychol. 2007, 3, 1–27. [Google Scholar] [CrossRef]
- Seeley, W.W. The Salience Network: A Neural System for Perceiving and Responding to Homeostatic Demands. J. Neurosci. 2019, 39, 9878–9882. [Google Scholar] [CrossRef]
- Shackman, A.J.; Stockbridge, M.D.; Tillman, R.M.; Kaplan, C.M.; Tromp, D.P.; Fox, A.S.; Gamer, M. The neurobiology of dispositional negativity and attentional biases to threat: Implications for understanding anxiety disorders in adults and youth. J. Exp. Psychopathol. 2016, 7, 311–342. [Google Scholar] [CrossRef] [PubMed]
- Koenigsberg, H.W.; Siever, L.J.; Lee, H.; Pizzarello, S.; New, A.S.; Goodman, M.; Cheng, H.; Flory, J.; Prohovnik, I. Neural correlates of emotion processing in borderline personality disorder. Psychiatry Res. 2009, 172, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Herpertz, S.C.; Dietrich, T.M.; Wenning, B.; Krings, T.; Erberich, S.G.; Willmes, K.; Thron, A.; Sass, H. Evidence of abnormal amygdala functioning in borderline personality disorder: A functional MRI study. Biol. Psychiatry 2001, 50, 292–298. [Google Scholar] [CrossRef]
- Schulze, L.; Schmahl, C.; Niedtfeld, I. Neural Correlates of Disturbed Emotion Processing in Borderline Personality Disorder: A Multimodal Meta-Analysis. Biol. Psychiatry 2016, 79, 97–106. [Google Scholar] [CrossRef]
- Bilek, E.; Itz, M.L.; Stossel, G.; Ma, R.; Berhe, O.; Clement, L.; Zang, Z.; Robnik, L.; Plichta, M.M.; Neukel, C.; et al. Deficient Amygdala Habituation to Threatening Stimuli in Borderline Personality Disorder Relates to Adverse Childhood Experiences. Biol. Psychiatry 2019, 86, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Bertsch, K.; Gamer, M.; Schmidt, B.; Schmidinger, I.; Walther, S.; Kastel, T.; Schnell, K.; Buchel, C.; Domes, G.; Herpertz, S.C. Oxytocin and reduction of social threat hypersensitivity in women with borderline personality disorder. Am. J. Psychiatry 2013, 170, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Carré, J.M.; Fisher, P.M.; Manuck, S.B.; Hariri, A.R. Interaction between trait anxiety and trait anger predict amygdala reactivity to angry facial expressions in men but not women. Soc. Cognit. Affect. Neurosci. 2012, 7, 213–221. [Google Scholar] [CrossRef][Green Version]
- Mancke, F.; Herpertz, S.C.; Kleindienst, N.; Bertsch, K. Emotion Dysregulation and Trait Anger Sequentially Mediate the Association Between Borderline Personality Disorder and Aggression. J. Pers. Disord. 2017, 31, 256–272. [Google Scholar] [CrossRef]
- Bertsch, K.; Krauch, M.; Roelofs, K.; Cackowski, S.; Herpertz, S.C.; Volman, I. Out of control? Acting out anger is associated with deficient prefrontal emotional action control in male patients with borderline personality disorder. Neuropharmacology 2019, 156, 107463. [Google Scholar] [CrossRef]
- Herpertz, S.C.; Nagy, K.; Ueltzhoffer, K.; Schmitt, R.; Mancke, F.; Schmahl, C.; Bertsch, K. Brain Mechanisms Underlying Reactive Aggression in Borderline Personality Disorder-Sex Matters. Biol. Psychiatry 2017, 82, 257–266. [Google Scholar] [CrossRef]
- White, S.F.; VanTieghem, M.; Brislin, S.J.; Sypher, I.; Sinclair, S.; Pine, D.S.; Hwang, S.; Blair, R.J. Neural Correlates of the Propensity for Retaliatory Behavior in Youths With Disruptive Behavior Disorders. Am. J. Psychiatry 2016, 173, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Volman, I.; von Borries, A.K.; Bulten, B.H.; Verkes, R.J.; Toni, I.; Roelofs, K. Testosterone Modulates Altered Prefrontal Control of Emotional Actions in Psychopathic Offenders. eNeuro 2016, 3, e0107. [Google Scholar] [CrossRef]
- Schmitt, R.; Winter, D.; Niedtfeld, I.; Herpertz, S.C.; Schmahl, C. Effects of Psychotherapy on Neuronal Correlates of Reappraisal in Female Patients With Borderline Personality Disorder. Biol. Psychiatry Cognit. Neurosci. Neuroimaging 2016, 1, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Niedtfeld, I.; Schmitt, R.; Winter, D.; Bohus, M.; Schmahl, C.; Herpertz, S.C. Pain-mediated affect regulation is reduced after dialectical behavior therapy in borderline personality disorder: A longitudinal fMRI study. Soc. Cognit. Affect. Neurosci. 2017, 12, 739–747. [Google Scholar] [CrossRef]
- Zaehringer, J.; Ende, G.; Santangelo, P.; Kleindienst, N.; Ruf, M.; Bertsch, K.; Bohus, M.; Schmahl, C.; Paret, C. Improved emotion regulation after neurofeedback: A single-arm trial in patients with borderline personality disorder. Neuroimage Clin. 2019, 24, 102032. [Google Scholar] [CrossRef] [PubMed]
- Honecker, H.; Bertsch, K.; Spiess, K.; Krauch, M.; Kleindienst, N.; Herpertz, S.C.; Neukel, C. Impact of a Mechanism-Based Anti-Aggression Psychotherapy on Behavioral Mechanisms of Aggression in Patients With Borderline Personality Disorder. Front. Psychiatry 2021, 12, 689267. [Google Scholar] [CrossRef]
- First, M.B.; Gibbon, M. The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) and the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II). In Comprehensive Handbook of Psychological Assessment; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004. [Google Scholar]
- Loranger, A.W.; Sartorius, N.; Andreoli, A.; Berger, P.; Buchheim, P.; Channabasavanna, S.; Coid, B.; Dahl, A.; Diekstra, R.F.; Ferguson, B.; et al. The international personality disorder examination: The World Health Organization/Alcohol, Drug Abuse, and Mental Health Administration international pilot study of personality disorders. Arch. Gen. Psychiatry 1994, 51, 215–224. [Google Scholar] [CrossRef]
- Zanarini, M.C. Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD): A continuous measure of DSM-IV borderline psychopathology. J. Personal. Disord. 2003, 17, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Raven, J. The Raven’s progressive matrices: Change and stability over culture and time. Cognit. Psychol. 2000, 41, 1–48. [Google Scholar] [CrossRef]
- Hariri, A.R.; Bookheimer, S.Y.; Mazziotta, J.C. Modulating emotional responses: Effects of a neocortical network on the limbic system. Neuroreport 2000, 11, 43–48. [Google Scholar] [CrossRef]
- Carré, J.M.; Hyde, L.W.; Neumann, C.S.; Viding, E.; Hariri, A.R. The neural signatures of distinct psychopathic traits. Soc. Neurosci. 2013, 8, 122–135. [Google Scholar] [CrossRef]
- Goetz, S.M.; Tang, L.; Thomason, M.E.; Diamond, M.P.; Hariri, A.R.; Carré, J.M. Testosterone rapidly increases neural reactivity to threat in healthy men: A novel two-step pharmacological challenge paradigm. Biol. Psychiatry 2014, 76, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Prather, A.A.; Bogdan, R.; Hariri, P.A.R. Impact of sleep quality on amygdala reactivity, negative affect, and perceived stress. Psychosom. Med. 2013, 75, 350. [Google Scholar] [CrossRef]
- Ekman, P.; Friesen, W. Pictures of Facial Affect; Consulting Psychologists Press: Palo Alto, CA, USA, 1976. [Google Scholar]
- McLaren, D.G.; Ries, M.L.; Xu, G.; Johnson, S.C. A generalized form of context-dependent psychophysiological interactions (gPPI): A comparison to standard approaches. Neuroimage 2012, 61, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Brett, M.; Anton, J.-L.; Valabregue, R.; Poline, J.-B. Region of interest analysis using an SPM toolbox. In Proceedings of the 8th International Conference on Functional Mapping of The Human Brain, Sendai, Japan, 2–6 June 2002; p. 497. [Google Scholar]
- Dixon, M.L.; Thiruchselvam, R.; Todd, R.; Christoff, K. Emotion and the prefrontal cortex: An integrative review. Psychol. Bull. 2017, 143, 1033–1081. [Google Scholar] [CrossRef]
- Berboth, S.; Morawetz, C. Amygdala-prefrontal connectivity during emotion regulation: A meta-analysis of psychophysiological interactions. Neuropsychologia 2021, 153, 107767. [Google Scholar] [CrossRef]
- Herwig, U.; Lutz, J.; Scherpiet, S.; Scheerer, H.; Kohlberg, J.; Opialla, S.; Preuss, A.; Steiger, V.R.; Sulzer, J.; Weidt, S.; et al. Training emotion regulation through real-time fMRI neurofeedback of amygdala activity. Neuroimage 2019, 184, 687–696. [Google Scholar] [CrossRef]
- Habas, C. Research note: A resting-state, cerebello-amygdaloid intrinsically connected network. Cerebellum Ataxias 2018, 5, 4. [Google Scholar] [CrossRef]
- Sang, L.; Qin, W.; Liu, Y.; Han, W.; Zhang, Y.; Jiang, T.; Yu, C. Resting-state functional connectivity of the vermal and hemispheric subregions of the cerebellum with both the cerebral cortical networks and subcortical structures. Neuroimage 2012, 61, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, C.J.; Schmahmann, J.D. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex 2010, 46, 831–844. [Google Scholar] [CrossRef]
- Etkin, A.; Buchel, C.; Gross, J.J. The neural bases of emotion regulation. Nat. Rev. Neurosci. 2015, 16, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Plichta, M.M.; Schwarz, A.J.; Grimm, O.; Morgen, K.; Mier, D.; Haddad, L.; Gerdes, A.B.; Sauer, C.; Tost, H.; Esslinger, C.; et al. Test-retest reliability of evoked BOLD signals from a cognitive-emotive fMRI test battery. Neuroimage 2012, 60, 1746–1758. [Google Scholar] [CrossRef]
- Johansson, P.; Høglend, P. Identifying mechanisms of change in psychotherapy: Mediators of treatment outcome. Clin. Psychol. Psychother. 2007, 14, 1–9. [Google Scholar] [CrossRef]

| BPD | Group Comparison | HP | Group Comparison | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (MAAP vs. NSSP) | (BPD vs. HP) | ||||||||||
| MAAP-BPD M (SD)/N (%) | NSSP-BPD M (SD)/N (%) | t (df)/χ | p | Cohen d/φ | M (SD)/N (%) | t (df)/χ | p | Cohen d/φ | |||
| Age | 30.2 (10.0) | 30.5 (10.2) | −0.073 (31) | 0.943 | −0.026 | 30.2 (7.8) | 0.043 (56) | 0.966 | 0.011 | ||
| Gender (female) | 15 (75) | 9 (69.2) | 0.132 | 0.716 | 0.063 | 18 (72) | 0.004 | 0.951 | 0.008 | ||
| IQ | 102.7 (3.4) | 99.7 (5.5) | 0.488 (30) | 0.629 | 0.177 | 115.0 (10.4) | −3.513 (54) | 0.001 | −0.949 | ||
| ZAN (Total) | 14.1 (5.0) | 12.7 (2.9) | 0.900 (30) | 0.375 | 0.325 | 0.1 (0.4) | 17.363 (31.9) | <0.001 | 4.130 | ||
| overt aggression at inclusion | 44.0 (35.3) | 30.5 (20.3) | 1.244 (31) | 0.223 | 0.444 | 0.2 (0.4) | 7.229 (32.0) | <0.001 | 1.664 | ||
| overt aggression at T0 | 41.8 (40.0) | 16.3 (18.3) | 2.479 (28.5) | 0.019 | 0.765 | - | - | - | |||
| overt aggression at T1 | 15.8 (20.6) | 9.4 (7.1) | 1.073 (30) | 0.292 | 0.383 | 0.9 (1.7) | 4.131 (31.9) | <0.001 | 0.960 | ||
| overt aggression at T2 | 12.5 (14.9) | 27 (47) | −0.993 (11.1) | 0.342 | −0.461 | 1.2 (2.1) | 3.007 (30.4) | 0.005 | 0.720 | ||
| Current psychotropic medication | |||||||||||
| Antidepressants | 6 (30) | 4 (30.8) | 0.002 | 0.961 | −0.009 | - | |||||
| Neuroleptics | 3 (15) | 3 (23.1) | 0.269 | 0.604 | 0.092 | - | |||||
| Other | 2 (10) | 1 (7.7) | 0.073 | 0.787 | −0.048 | - | |||||
| Comorbidities | current | lifetime | current | lifetime | |||||||
| Major depression | 5 (25) | 15 (75) | 3 (23.1) | 11 (84.6) | - | - | - | ||||
| Dysthymia | 3 (15) | - | 2 (15.4) | - | - | - | |||||
| Alcohol addiction/abuse | 0 | 3 (15) | 0 | 4 (30.8) | - | - | - | ||||
| Anxiety disorders | 11 (55) | 8 (40) | 4 (30.8) | 6 (46.2) | - | - | - | ||||
| Obsessive-compulsive disorder | 1 (5) | 1 (5) | 0 | 0 | - | - | - | ||||
| Post-traumatic stress disorder | 7 (35) | 6 (30) | 4 (30.8) | 4 (30.8) | - | - | - | ||||
| Somatization Disorder | 1 (5) | - | 0 | - | - | - | - | ||||
| Eating Disorders | 3 (15) | 3 (15) | 1 (7.7) | 1 (7.7) | - | - | - | ||||
| Antisocial Personality Disorder | 3 (15) | 5 (25) | 0 | 1 (7.7) | - | - | - | ||||
| Avoidant Personality Disorder | 3 (15) | 3 (15) | 4 (30.8) | 4 (30.8) | - | - | - | ||||
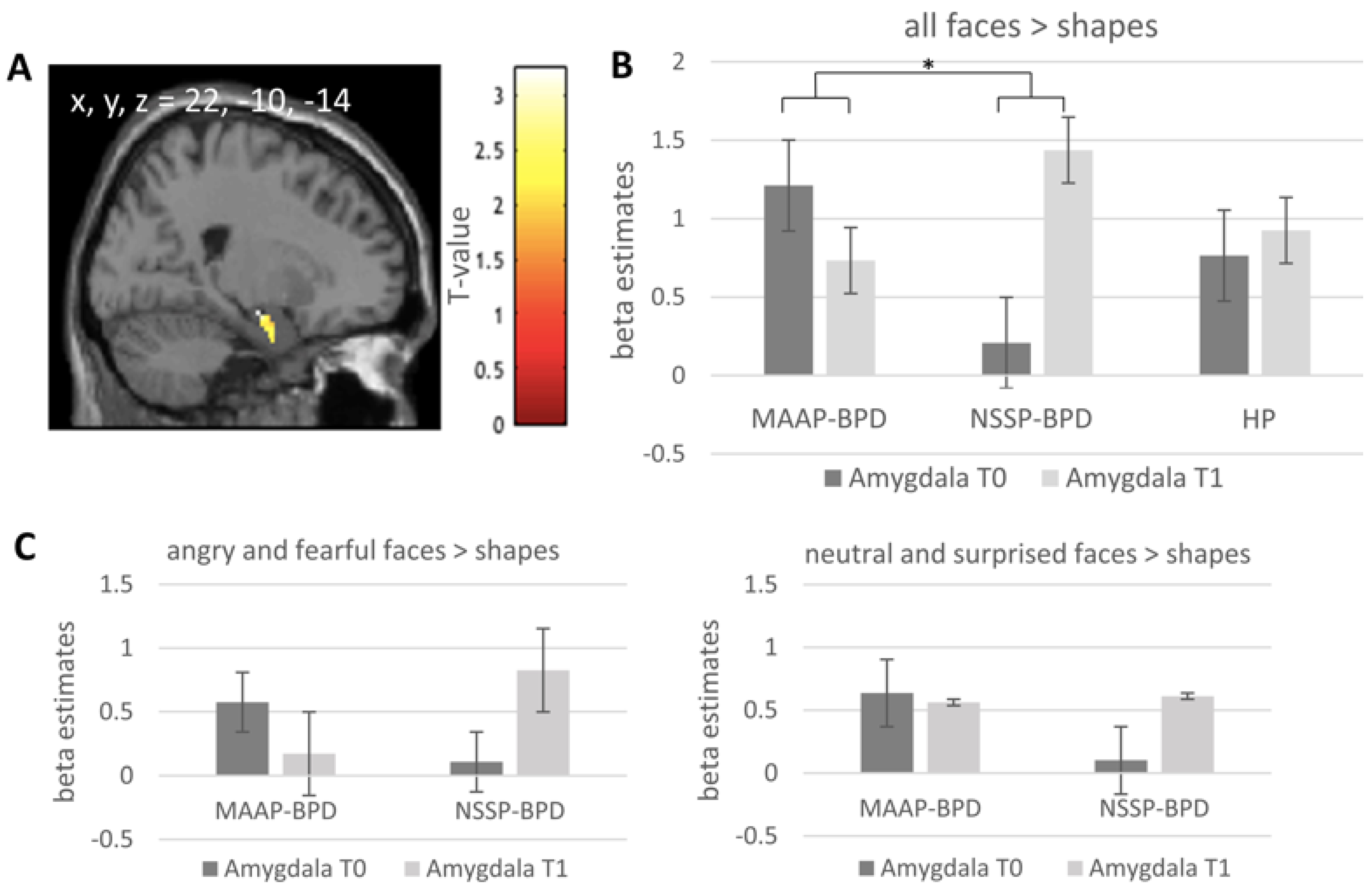
| Timepoint | Contrast | Cluster Size (k) | T Value | pFWE Value | Peak Voxel MNI: x, y, z (mm) |
|---|---|---|---|---|---|
| all faces > shapes | |||||
| Pre-treatment | all | 77 | 4.86 | <0.001 | 24, 0, −22 |
| 51 | 3.68 | 0.026 | −24, −6, 16 | ||
| BPD > HP | 8 | 2.8 | 0.133 | 24, −6, −24 | |
| MAAP-BPD > NSSP-BPD | 11 | 2.98 | 0.118 | −26, −10, −16 | |
| T0 vs. T1 | (MAAP-BPD > NSSP-BPD) × (T0 > T1) | 11 | 3.24 | 0.042 | 22, −10, −14 |
| 27 | 2.99 | 0.078 | −30, −8, −24 | ||
| MAAP-BPD: T0 > T1 | 4 | 2.94 | 0.046 | 24, 0, −22 | |
| NSSP-BPD: T1 > T0 | 8 | 2.80 | 0.065 | 26, −8, −16 | |
| (MAAP-BPD > HP) × (T0 > T1) | 12 | 2.89 | 0.099 | 24, 0, −22 | |
| HP: T1 > T0 | - | - | - | - | |
| angry and fearful faces > shapes | |||||
| Pre-treatment | all | 53 | 4.24 | 0.003 | 20, −6, −14 |
| 50 | 3.81 | 0.012 | −26, −6, −16 | ||
| BPD > HP | - | - | - | - | |
| MAAP-BPD > NSSP-BPD | - | - | - | - | |
| T0 vs. T1 | (MAAP-BPD > NSSP-BPD) × (T0 > T1) | 29 | 3.01 | 0.080 | −26, −6, −15 |
| MAAP-BPD: T0 > T1 | - | - | - | - | |
| NSSP-BPD: T1 > T0 | - | - | - | - | |
| (MAAP-BPD > HP) × (T0 > T1) | 9 | 2.66 | 0.175 | −22, −4, −16 | |
| Contrast | Cluster Size (k) | T Value | pFWE Value | Peak Voxel MNI: x, y, z (mm) | Anatomical Location of Peak Voxel |
|---|---|---|---|---|---|
| all faces > shapes | |||||
| (MAAP-BPD > NSSP-BPD) × (T1 > T0) | 224 | 3.96 | 0.010 | 8, 28, 44 | dorsomedial prefrontal cortex |
| (MAAP-BPD > HP) × (T1 > T0) | 236 | 4.47 | 0.008 | 8, −46, −40 | Cerebellum |
| angry and fearful faces > shapes | |||||
| (MAAP-BPD > HP) × (T1 > T0) | - | - | - | - | - |
| (MAAP-BPD > NSSP-BPD) × (T1 > T0) | - | - | - | - | - |
| Treatment Change in Overt Aggression | ||||||
|---|---|---|---|---|---|---|
| MAAP-BPD | MAAP-NSSP | HP | ||||
| r, p | N | r, p | N | r, p | N | |
| Treatment change in amygdala activity | −0.238, 0.341 | 18 | −0.019, 0.951 | 13 | 0.033, 0.881 | 23 |
| Treatment change in connectivity amygdala-dmPFC | 0.261, 0.295 | 18 | −0.511, 0.074 | 13 | 0.017, 0.939 | 23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neukel, C.; Bertsch, K.; Wenigmann, M.; Spieß, K.; Krauch, M.; Steinmann, S.; Herpertz, S.C. A Mechanism-Based Approach to Anti-Aggression Psychotherapy in Borderline Personality Disorder: Group Treatment Affects Amygdala Activation and Connectivity. Brain Sci. 2021, 11, 1627. https://doi.org/10.3390/brainsci11121627
Neukel C, Bertsch K, Wenigmann M, Spieß K, Krauch M, Steinmann S, Herpertz SC. A Mechanism-Based Approach to Anti-Aggression Psychotherapy in Borderline Personality Disorder: Group Treatment Affects Amygdala Activation and Connectivity. Brain Sciences. 2021; 11(12):1627. https://doi.org/10.3390/brainsci11121627
Chicago/Turabian StyleNeukel, Corinne, Katja Bertsch, Marc Wenigmann, Karen Spieß, Marlene Krauch, Sylvia Steinmann, and Sabine C. Herpertz. 2021. "A Mechanism-Based Approach to Anti-Aggression Psychotherapy in Borderline Personality Disorder: Group Treatment Affects Amygdala Activation and Connectivity" Brain Sciences 11, no. 12: 1627. https://doi.org/10.3390/brainsci11121627
APA StyleNeukel, C., Bertsch, K., Wenigmann, M., Spieß, K., Krauch, M., Steinmann, S., & Herpertz, S. C. (2021). A Mechanism-Based Approach to Anti-Aggression Psychotherapy in Borderline Personality Disorder: Group Treatment Affects Amygdala Activation and Connectivity. Brain Sciences, 11(12), 1627. https://doi.org/10.3390/brainsci11121627





