Is Aducanumab for LMICs? Promises and Challenges
Abstract
:1. Introduction
2. Management of Alzheimer’s Disease
2.1. ChEIs and NMDA Receptor Antagonists
2.2. SSRIs and SNRIs
2.3. Aducanumab—Antibody-Based Immunotherapy
3. Aducanumab in Low- and Middle-Income Countries
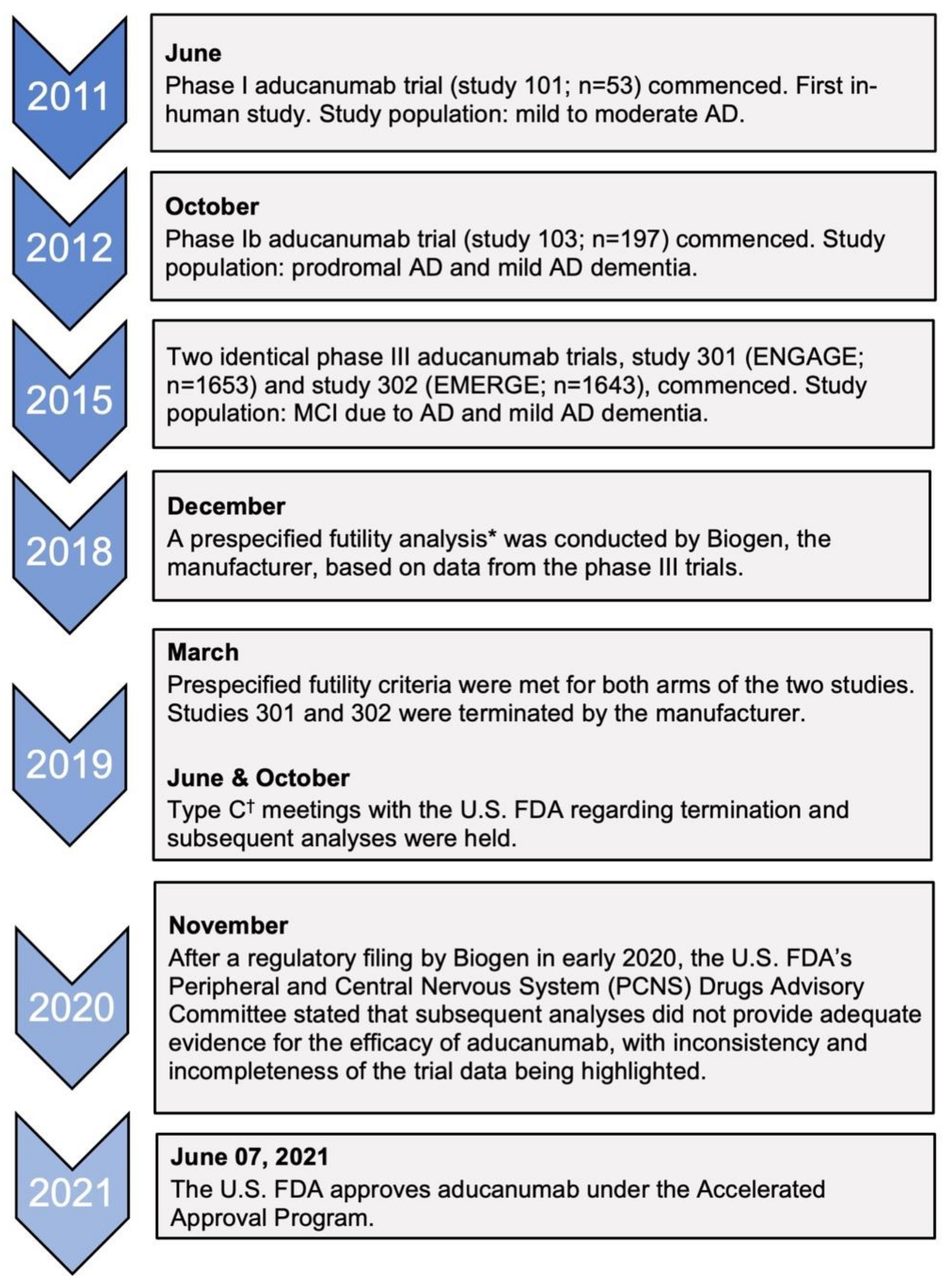
3.1. Accelerated Approval and the Efficacy of Aducanumab
3.2. Treatment Challenges
3.3. Adverse Effects
3.4. Apolipoprotein E and Interethnic Differences
3.5. The Economic Burden
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gleerup, H.S.; Hasselbalch, S.G.; Simonsen, A.H. Biomarkers for Alzheimer’s Disease in Saliva: A Systematic Review. Dis. Markers 2019, 2019, 4761054. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J. A Hundred Years of Alzheimer’s Disease Research. Neuron 2006, 52, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Rajan, K.B.; Wilson, R.S.; Weuve, J.; Barnes, L.L.; Evans, D.A. Cognitive impairment 18 years before clinical diagnosis of Alzheimer disease dementia. Neurology 2015, 85, 898–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, L.; Correia, A.S.A.; Miguel, R.; Alegria, P.; Bugalho, P. Alzheimer’s disease: A clinical practice-oriented review. Front. Neurol. 2012, 3, 63. [Google Scholar] [CrossRef] [Green Version]
- Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2020, 16, 391–460. [Google Scholar] [CrossRef]
- Silva, M.V.; Loures, C.D.; Alves, L.C.; de Souza, L.C.; Borges, K.B.; das Graças Carvalho, M. Alzheimer’s disease: Risk factors and potentially protective measures. J. Biomed. Sci. 2019, 26, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prince, M.; Guerchet, M.; Prina, M. WHO Thematic Briefing: The Epidemiology and Impact of Dementia—Current State and Future Trends; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- World Health Organization. Global Action Plan on the Public Health Response to Dementia 2017–2025; World Health Organization: Geneva, Switzerland, 2017; ISBN 9789241513487. [Google Scholar]
- Nichols, E.; Szoeke, C.E.; Vollset, S.E.; Abbasi, N.; Abd-Allah, F.; Abdela, J.; Aichour, M.T.; Akinyemi, R.O.; Alahdab, F.; Asgedom, S.W.; et al. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 88–106. [Google Scholar] [CrossRef] [Green Version]
- Cummings, J.; Aisen, P.; Apostolova, L.G.; Atri, A.; Salloway, S.; Weiner, M. Aducanumab: Appropriate Use Recommendations. J. Prev. Alzheimer’s Dis. 2021, 8, 1–13. [Google Scholar] [CrossRef]
- U.S Food & Drug Administration. Drugs (FDA) FDA-Approved Drugs. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=761178 (accessed on 3 August 2021).
- Prince, M.; Wimo, A.; Guerchet, M.; Ali, G.-C.; Wu, Y.-T.; Prina, M. World Alzheimer Report 2015—The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends. Alzheimer’s Dis. Int. 2015. [Google Scholar]
- Guerchet, M.; Mayston, R.; Lloyd-Sherlock, P.; Prince, M.; Aboderin, I.; Akinyemi, R.; Paddick, S.-M.; Wimo, A.; Amoakoh-Coleman, M.; Uwakwe, R.; et al. Dementia in Sub-Saharan Africa: Challenges and Opportunities; Alzheimer’s Disease International: London, UK, 2017. [Google Scholar]
- Anand, A.; Patience, A.A.; Sharma, N.; Khurana, N. The present and future of pharmacotherapy of Alzheimer’s disease: A comprehensive review. Eur. J. Pharmacol. 2017, 815, 364–375. [Google Scholar] [CrossRef]
- Cummings, J.; Lee, G.; Zhong, K.; Fonseca, J.; Taghva, K. Alzheimer’s disease drug development pipeline: 2021. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2021, 7, e12179. [Google Scholar] [CrossRef]
- Blanco-Silvente, L.; Castells, X.; Saez, M.; Barceló, M.A.; Garre-Olmo, J.; Vilalta-Franch, J.; Capellà, D. Discontinuation, Efficacy, and Safety of Cholinesterase Inhibitors for Alzheimer’s Disease: A Meta-Analysis and Meta-Regression of 43 Randomized Clinical Trials Enrolling 16 106 Patients. Int. J. Neuropsychopharmacol. 2017, 20, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.; McShane, R.; Lindesay, J.; Ritchie, C.; Baldwin, A.; Barber, R.; Burns, A.; Dening, T.; Findlay, D.; Holmes, C.; et al. Donepezil and Memantine for Moderate-to-Severe Alzheimer’s Disease. N. Engl. J. Med. 2012, 366, 893–903. [Google Scholar] [CrossRef] [Green Version]
- McShane, R.; Westby, M.J.; Roberts, E.; Minakaran, N.; Schneider, L.; Farrimond, L.E.; Maayan, N.; Ware, J.; Debarros, J. Memantine for dementia. Cochrane Database Syst. Rev. 2019, 3, CD003154. [Google Scholar] [CrossRef] [PubMed]
- Cerejeira, J.; Lagarto, L.; Mukaetova-Ladinska, E.B. Behavioral and psychological symptoms of dementia. Front. Neurol. 2012, 3, 73. [Google Scholar] [CrossRef] [Green Version]
- Tible, O.P.; Riese, F.; Savaskan, E.; von Gunten, A. Best practice in the management of behavioural and psychological symptoms of dementia. Ther. Adv. Neurol. Disord. 2017, 10, 297–309. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Rajasekhar, K.; Govindaraju, T. Current progress, challenges and future prospects of diagnostic and therapeutic interventions in Alzheimer’s disease. RSC Adv. 2018, 8, 23780–23804. [Google Scholar] [CrossRef] [Green Version]
- Sevigny, J.; Chiao, P.; Bussière, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 2016, 537, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, J.; Williams, L.; Stella, H.; Leitermann, K.; Mikulskis, A.; O’Gorman, J.; Sevigny, J. First-in-human, double-blind, placebo-controlled, single-dose escalation study of aducanumab (BIIB037) in mild-to-moderate Alzheimer’s disease. Alzheimer’s Dement. 2016, 2, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Dunstan, R.; Bussiere, T.; Fahrer, D.; Quigley, C.; Zhang, X.; Themeles, M.; Engber, T.; Rhodes, K.; Arastu, M.; Li, M. Quantitation of beta-amyloid in transgenic mice using whole slide digital imaging and image analysis software. Alzheimer’s Dement. 2011, 7, S700. [Google Scholar] [CrossRef]
- Crehan, H.; Lemere, C.A. Chapter 7–Anti-Amyloid-β Immunotherapy for Alzheimer’s Disease. In Developing Therapeutics for Alzheimer’s Disease; Wolfe, M.S., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 193–226. [Google Scholar]
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Alexander, G.C.; Karlawish, J. The Problem of Aducanumab for the Treatment of Alzheimer Disease. Ann. Intern. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Haeberlein, S.B.; von Hehn, C.; Tian, Y.; Chalkias, S.; Muralidharan, K.K.; Chen, T.; Wu, S.; Skordos, L.; Nisenbaum, L.; Rajagovindan, R.; et al. Emerge and Engage topline results: Phase 3 studies of aducanumab in early Alzheimer’s disease. Alzheimer’s Dement. 2020, 16, e047259. [Google Scholar] [CrossRef]
- Lin, G.A.; Whittington, M.D.; Synnott, P.G.; McKenna, A.; Campbell, J.; Pearson, S.D.; Rind, D.M. Aducanumab for Alzheimer’s Disease: Effectiveness and Value; Final Evidence Report and Meeting Summary. Institute for Clinical and Economic Review, August 5, 2021. Available online: https://icer.org/assessment/alzheimers-disease-2021/ (accessed on 5 August 2021).
- Wimo, A.; Guerchet, M.; Ali, G.-C.; Wu, Y.-T.; Prina, A.M.; Winblad, B.; Jönsson, L.; Liu, Z.; Prince, M. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimer’s Dement. 2017, 13, 1–7. [Google Scholar] [CrossRef]
- Hung, S.-Y.; Fu, W.-M. Drug candidates in clinical trials for Alzheimer’s disease. J. Biomed. Sci. 2017, 24, 1–12. [Google Scholar] [CrossRef]
- Li, D.-D.; Zhang, Y.-H.; Zhang, W.; Zhao, P. Meta-Analysis of Randomized Controlled Trials on the Efficacy and Safety of Donepezil, Galantamine, Rivastigmine, and Memantine for the Treatment of Alzheimer’s Disease. Front. Neurosci. 2019, 13, 472. [Google Scholar] [CrossRef]
- Birks, J.S.; Harvey, R.J. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst. Rev. 2018, 10, 14651858. [Google Scholar] [CrossRef] [PubMed]
- Birks, J.S.; Chong, L.Y.; Grimley Evans, J. Rivastigmine for Alzheimer’s disease. Cochrane Database Syst. Rev. 2015, 16, 295–315. [Google Scholar] [CrossRef]
- Jiang, D.; Yang, X.; Li, M.; Wang, Y.; Wang, Y. Efficacy and safety of galantamine treatment for patients with Alzheimer’s disease: A meta-analysis of randomized controlled trials. J. Neural Transm. 2015, 122, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Kishi, T.; Matsunaga, S.; Oya, K.; Nomura, I.; Ikuta, T.; Iwata, N. Memantine for Alzheimer’s Disease: An Updated Systematic Review and Meta-analysis. J. Alzheimer’s Dis. 2017, 60, 401–425. [Google Scholar] [CrossRef]
- Birks, J.S. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst. Rev. 2006, 1, CD005593. [Google Scholar] [CrossRef]
- Liu, K.Y.; Howard, R. Can we learn lessons from the FDA’s approval of aducanumab? Nat. Rev. Neurol. 2021, 17, 715–722. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. Briefing Document: Combined FDA and Applicant PCNS Drugs Advisory Committee. Available online: https://www.fda.gov/media/143502/download (accessed on 27 June 2021).
- Retinasamy, T.; Shaikh, M.F. Aducanumab for Alzheimer’s disease: An update. Neurosci. Res. Notes 2021, 4, 17–20. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. Guidance for Industry: Formal Meetings between the FDA and Sponsors or Applicants. Available online: https://www.fda.gov/media/72253/download (accessed on 11 November 2021).
- Yuan, J.; Maserejian, N.; Liu, Y.; Devine, S.; Gillis, C.; Massaro, J.; Au, R.; Bondi, M. Severity Distribution of Alzheimer’s Disease Dementia and Mild Cognitive Impairment in the Framingham Heart Study. J. Alzheimer’s Dis. 2021, 79, 807–817. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration. PCNS Drugs Advisory Committee: Aducanumab for the Treatment of Alzheimer’s Disease. Available online: https://www.fda.gov/media/143506/download (accessed on 2 August 2021).
- Bitton, A.; Fifield, J.; Ratcliffe, H.; Karlage, A.; Wang, H.; Veillard, J.H.; Schwarz, D.; Hirschhorn, L.R. Primary healthcare system performance in low-income and middle-income countries: A scoping review of the evidence from 2010 to 2017. BMJ Glob. Health 2019, 4, e001551. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, D.; Hirschhorn, L.R.; Kim, J.-H.; Ratcliffe, H.L.; Bitton, A. Continuity in primary care: A critical but neglected component for achieving high-quality universal health coverage. BMJ Glob. Health 2019, 4, e001435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Bank Data. Physicians (per 1000 People). Available online: https://data.worldbank.org/indicator/SH.MED.PHYS.ZS (accessed on 27 June 2021).
- Cummings, J.L.; Tong, G.; Ballard, C. Treatment Combinations for Alzheimer’s Disease: Current and Future Pharmacotherapy Options. J. Alzheimer’s Dis. 2019, 67, 779–794. [Google Scholar] [CrossRef] [Green Version]
- International Atomic Energy Agency (IAEA). IMAGINE—IAEA Medical Imaging and Nuclear Medicine Global Resources Database. Available online: https://humanhealth.iaea.org/HHW/DBStatistics/IMAGINEMaps3.html (accessed on 27 June 2021).
- Zhang, X.; Fu, Z.; Meng, L.; He, M.; Zhang, Z. The Early Events That Initiate β-Amyloid Aggregation in Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Zhou, Y.; Xiao, M.; Yan, L.-J.; He, W. Activation of mTOR: A culprit of Alzheimer’s disease? Neuropsychiatr. Dis. Treat. 2015, 2015, 1015–1030. [Google Scholar] [CrossRef] [Green Version]
- Sarlus, H.; Heneka, M.T. Microglia in Alzheimer’s disease. J. Clin. Investig. 2017, 127, 3240–3249. [Google Scholar] [CrossRef] [PubMed]
- Arimon, M.; Takeda, S.; Post, K.L.; Svirsky, S.; Hyman, B.T.; Berezovska, O. Oxidative stress and lipid peroxidation are upstream of amyloid pathology. Neurobiol. Dis. 2015, 84, 109–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, W.J.; Ossenkoppele, R.; Knol, D.L.; Tijms, B.M.; Scheltens, P.; Verhey, F.R.; Visser, P.J.; Aalten, P.; Aarsland, D.; Alcolea, D.; et al. Prevalence of cerebral amyloid pathology in persons without dementia: A meta-analysis. JAMA 2015, 313, 1924–1938. [Google Scholar] [CrossRef]
- Li, C.; Loewenstein, D.A.; Duara, R.; Cabrerizo, M.; Barker, W.; Adjouadi, M.; Alzheimer’s Disease Neuroimaging, I. The Relationship of Brain Amyloid Load and APOE Status to Regional Cortical Thinning and Cognition in the ADNI Cohort. J. Alzheimer’s Dis. 2017, 59, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- Kern, S.; Mehlig, K.; Kern, J.; Zetterberg, H.; Thelle, D.; Skoog, I.; Lissner, L.; Blennow, K.; Börjesson-Hanson, A. The Distribution of Apolipoprotein E Genotype Over the Adult Lifespan and in Relation to Country of Birth. Am. J. Epidemiol. 2015, 181, 214–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattsson, N.; Groot, C.; Jansen, W.J.; Landau, S.M.; Villemagne, V.L.; Engelborghs, S.; Mintun, M.M.; Lleo, A.; Molinuevo, J.L.; Jagust, W.J.; et al. Prevalence of the apolipoprotein E ε4 allele in amyloid β positive subjects across the spectrum of Alzheimer’s disease. Alzheimer’s Dement 2018, 14, 913–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- VandeVrede, L.; Gibbs, D.M.; Koestler, M.; La Joie, R.; Ljubenkov, P.A.; Provost, K.; Soleimani-Meigooni, D.; Strom, A.; Tsoy, E.; Rabinovici, G.D.; et al. Symptomatic amyloid-related imaging abnormalities in an APOE ε4/ε4 patient treated with aducanumab. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2020, 12, e12101. [Google Scholar] [CrossRef] [PubMed]
- Brickman, A.M.; Manly, J.J.; Honig, L.S.; Sanchez, D.; Reyes-Dumeyer, D.; Lantigua, R.A.; Lao, P.J.; Stern, Y.; Vonsattel, J.P.; Teich, A.F.; et al. Plasma p-tau181, p-tau217, and other blood-based Alzheimer’s disease biomarkers in a multi-ethnic, community study. Alzheimer’s Dement. 2021, 17, 1353–1364. [Google Scholar] [CrossRef]
- Howell, J.C.; Watts, K.D.; Parker, M.W.; Wu, J.; Kollhoff, A.; Wingo, T.S.; Dorbin, C.D.; Qiu, D.; Hu, W.T. Race modifies the relationship between cognition and Alzheimer’s disease cerebrospinal fluid biomarkers. Alzheimer’s Res. Ther. 2017, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.A.; Knight, R.; Roughead, E.E.; Brooks, G.; Mant, A. Policy options for pharmaceutical pricing and purchasing: Issues for low- and middle-income countries. Health Policy Plan. 2015, 30, 267–280. [Google Scholar] [CrossRef] [Green Version]
- Winblad, B.; Grossberg, G.; Frölich, L.; Farlow, M.; Zechner, S.; Nagel, J.; Lane, R. IDEAL: A 6-month, double-blind, placebo-controlled study of the first skin patch for Alzheimer disease. Neurology 2007, 69, S14–S22. [Google Scholar] [CrossRef] [PubMed]
- Courtney, C.; Farrell, D.; Gray, R.; Hills, R.; Lynch, L.; Sellwood, E.; Edwards, S.; Hardyman, W.; Raftery, J.; Crome, P.; et al. Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): Randomised double-blind trial. Lancet 2004, 363, 2105–2115. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.; McShane, R.; Lindesay, J.; Ritchie, C.; Baldwin, A.; Barber, R.; Burns, A.; Dening, T.; Findlay, D.; Holmes, C.; et al. Nursing home placement in the Donepezil and Memantine in Moderate to Severe Alzheimer’s Disease (DOMINO-AD) trial: Secondary and post-hoc analyses. Lancet Neurol. 2015, 14, 1171–1181. [Google Scholar] [CrossRef] [Green Version]
- Consumers Union of U.S. Consumer Reports Best Buy Drugs. Evaluating Prescription Drugs Used to Treat: Alzheimer’s Disease. Available online: https://article.images.consumerreports.org/prod/content/dam/cro/news_articles/health/PDFs/Alzheimer’sDisease_fullreport.pdf (accessed on 1 August 2021).
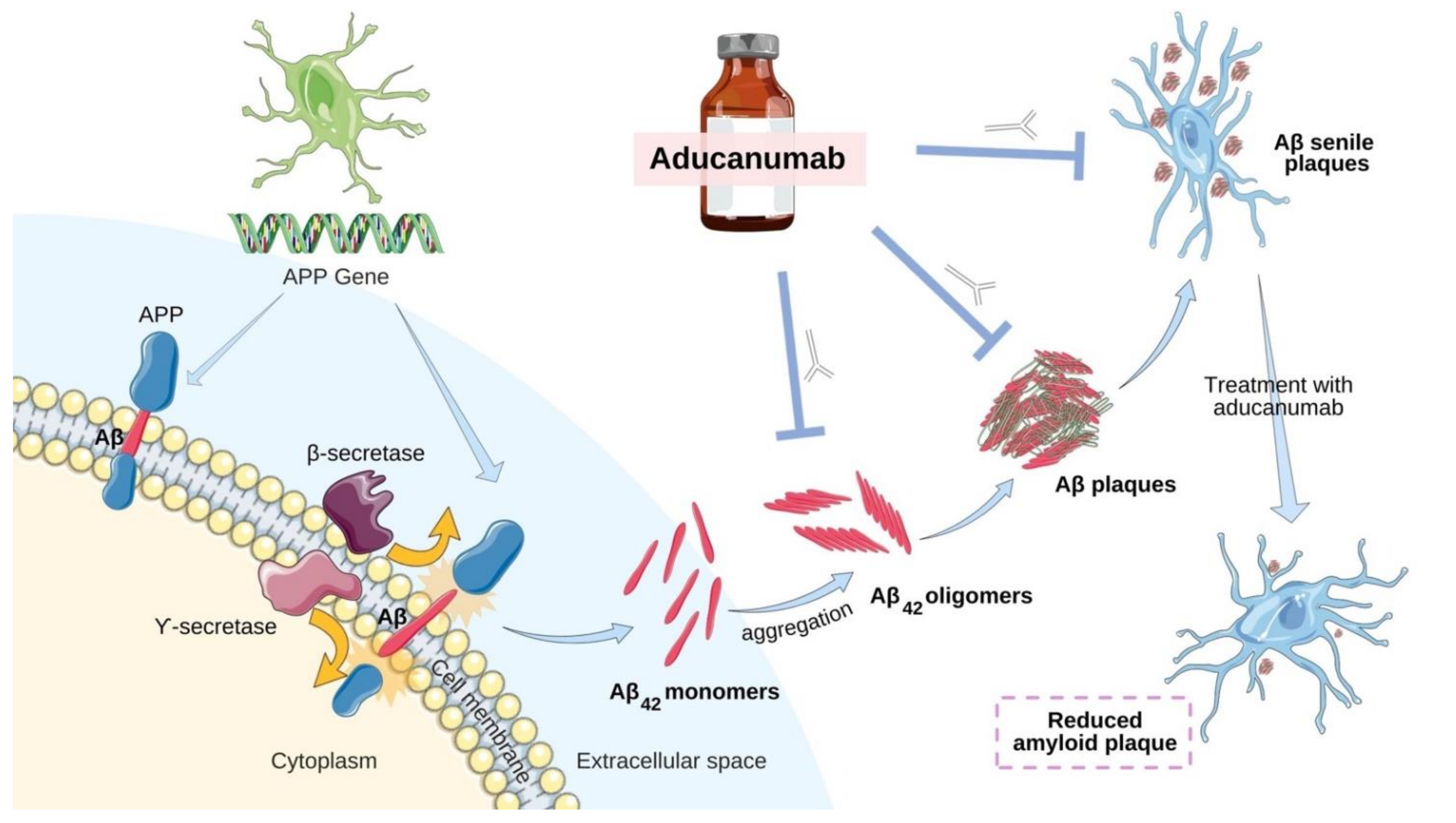


| Monoclonal Antibodies | Cholinesterase Inhibitors (ChEIs) | N-methyl-D-aspartate (NMDA) Receptor Antagonists | |
|---|---|---|---|
| Drug | Aducanumab | Donepezil, rivastigmine, and galantamine | Memantine |
| Pharmacotherapy goal(s) | Disease-modifying treatment to reduce cognitive decline. | Symptomatic management of cognition and global functioning. | Symptomatic management of cognition and global functioning. |
| Mechanism of action | Selectively targets and clears Aβ aggregates, Aβ fibrils, and soluble oligomers. A reduction in Aβ build up in the brain is expected to demonstrate a reduction in cognitive decline in patients [23]. | Increases cholinergic transmission by inhibiting cholinesterase at the synaptic cleft, thereby improving cortical cholinergic function [33]. | Exerts neuroprotective effects by blocking pathological stimulation of glutamate NMDA receptors, thereby decreasing excitotoxicity [33]. |
| Indication | Mild cognitive impairment (MCI), mild AD [10] | Mild to moderate AD, advanced disease [35] | Moderate to severe AD, mild AD (off-label) [18] |
| Route of administration | Intravenous infusion | Oral, transdermal patch (rivastigmine only) | Oral |
| Efficacy in terms of cognitive function | A statistically significant improvement in various scales of cognitive function was observed in the high-dose arm of EMERGE [29]. | A meta-analysis of ChEIs revealed modest improvements [38]. | A reduction in deterioration on different scales of clinical efficacy compared to placebo was observed in patients with moderate to severe AD [18]. |
| Difference vs. placebo: −0.39 (95% CI 0.69 to −0.09) [29] Score: CDR-SB * | Donepezil | MD −1.02, (95% CI −1.66 to −0.39, p = 0.002) [37] Scale: ADAS-Cog † | |
| MD −2.67, (95% CI −3.31 to −2.02) [34] Scale: ADAS-Cog † | |||
| MD 1.05, (95% CI 0.73 to 1.37) [34] Score: MMSE ‡ | |||
| Difference vs. placebo: −1.400 (p = 0.00967) [29] Scale: ADAS-Cog ** | Galantamine | ||
| MD −2.95, (95% CI −3.32, −2.57) [36] Scale: ADAS-Cog † | |||
| MD 2.50, (95% CI 0.86 to 4.15) [36] Score: MMSE ‡ | |||
| Difference vs. placebo: 0.6 (p = 0.05) [29] Score: MMSE ‡ | Rivastigmine | ||
| MD −1.79, (95% CI −2.21 to −1.37) [35] Scale: ADAS-Cog † | |||
| MD 0.74, (95% CI 0.52 to 0.97) [35] Score: MMSE ‡ |
| Monoclonal Antibodies | Cholinesterase Inhibitors (ChEIs) | N-methyl-D-aspartate (NMDA) Receptor Antagonists | |
|---|---|---|---|
| Drug | Aducanumab | Donepezil, rivastigmine, and galantamine | Memantine |
| Functional outcomes | MD 1.70 (95% CI 0.71 to 2.69) (high-dose arm of EMERGE) [29] Scale: ADCS-AD a | Donepezil: SMD 0.22 (95% CI 0.12–0.33) [34] | MD 0.95 (95% CI 0.22 to 1.76) [18] Scale: ADCS-AD c |
| Galantamine: SMD 0.19 (95% CI 0.01–0.37) [33] | |||
| Rivastigmine: MD 1.80 (95% CI 0.20 to 3.40) [62] | |||
| Scale: ADCS-AD b | |||
| Entry to institutional or nursing care | Not assessed | No significant benefit in terms of delay of entry to institutional care [63]. | No effect on the rate of nursing home placement [64]. |
| Adverse effects | ARIA-E and ARIA-H Symptoms: headaches, confusion, dizziness, falls, vision changes, and nausea [40]. | Donepezil: gastrointestinal symptoms (upset stomach, nausea, diarrhoea, and anorexia) | Dizziness, confusion, weight gain, hallucinations [18]. |
| Galantamine: gastrointestinal symptoms | |||
| Rivastigmine (patch): nausea, vomiting, anorexia, headaches, dizziness | |||
| General vagotonic symptoms: bradycardia and hypotension [38]. | |||
| APOE genotyping | Not a requirement. However, genetic screening may help ascertain ARIA risk. | Not required | Not required |
| Pre-treatment amyloid status | Amyloid-PET or CSF analysis may be conducted [10]. | Not applicable | Not applicable |
| Baseline MRI scan of the brain | Required (a recent scan within one year prior to initiating therapy) [10]. | For clinical diagnosis | For clinical diagnosis |
| Follow-up MRI scans of the brain | Two scans prior to the seventh and twelfth doses. Additional monitoring of ARIAs with MRI if symptomatic [10]. | Not required | Not required |
| Average annual cost (U.S. $) | 56,000 * | 2796 † | 4096 ‡ |
| Generics or biosimilars | None | Available | Available |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunawardena, I.P.C.; Retinasamy, T.; Shaikh, M.F. Is Aducanumab for LMICs? Promises and Challenges. Brain Sci. 2021, 11, 1547. https://doi.org/10.3390/brainsci11111547
Gunawardena IPC, Retinasamy T, Shaikh MF. Is Aducanumab for LMICs? Promises and Challenges. Brain Sciences. 2021; 11(11):1547. https://doi.org/10.3390/brainsci11111547
Chicago/Turabian StyleGunawardena, Illangage P. C., Thaarvena Retinasamy, and Mohd. Farooq Shaikh. 2021. "Is Aducanumab for LMICs? Promises and Challenges" Brain Sciences 11, no. 11: 1547. https://doi.org/10.3390/brainsci11111547
APA StyleGunawardena, I. P. C., Retinasamy, T., & Shaikh, M. F. (2021). Is Aducanumab for LMICs? Promises and Challenges. Brain Sciences, 11(11), 1547. https://doi.org/10.3390/brainsci11111547







