The Efficacy of Interlimb-Coordinated Intervention on Gait and Motor Function Recovery in Patients with Acute Stroke: A Multi-Center Randomized Controlled Trial Study Protocol
Abstract
1. Introduction
2. Materials and Methods
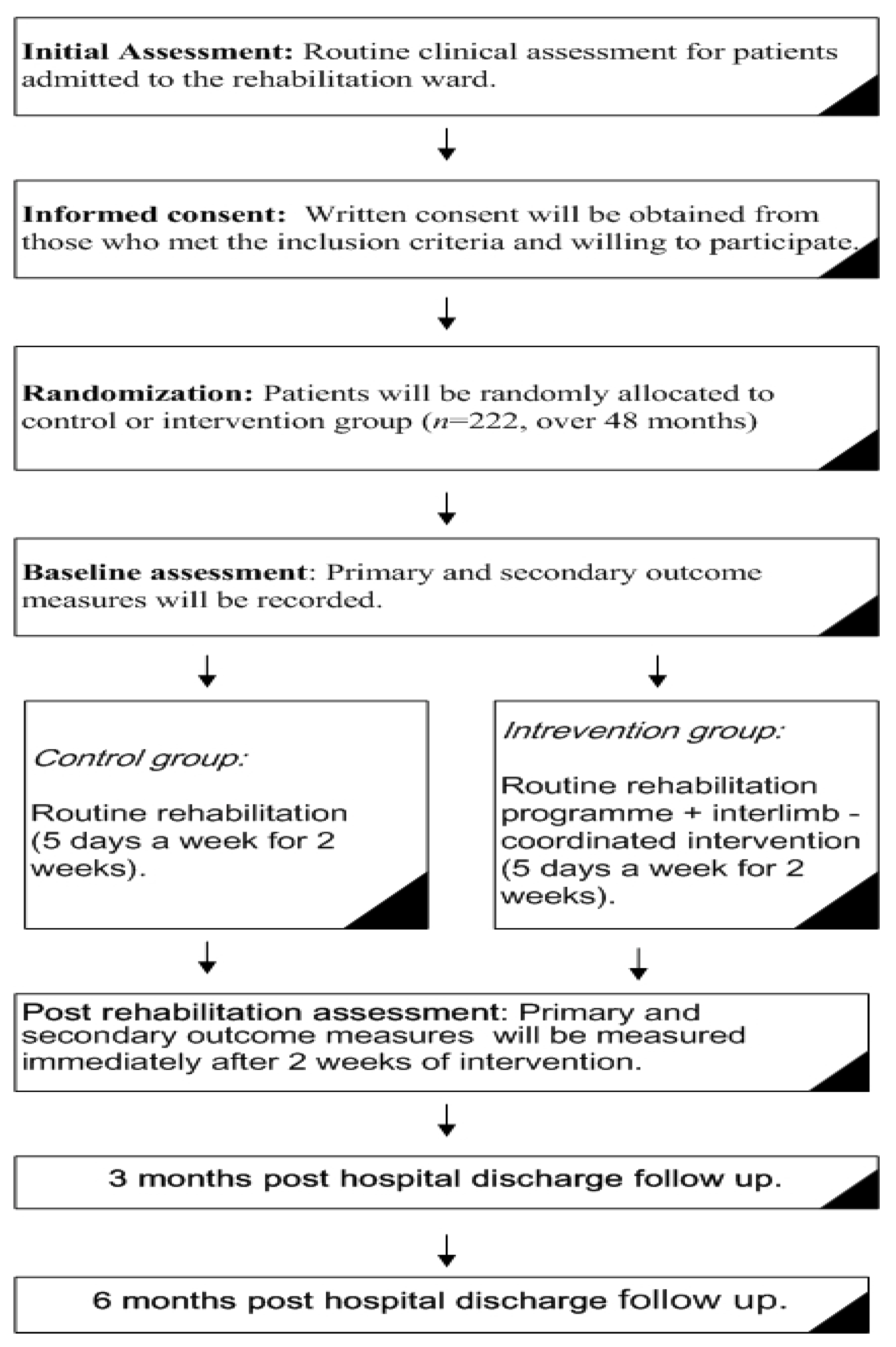
2.1. Study Design
2.2. Study Setting
2.3. Recruitment
2.4. Sample Population
2.5. Randomization
2.6. Ethics
2.7. Outcome Measures
2.7.1. Primary Outcome Measure
2.7.2. Secondary Outcome Measure
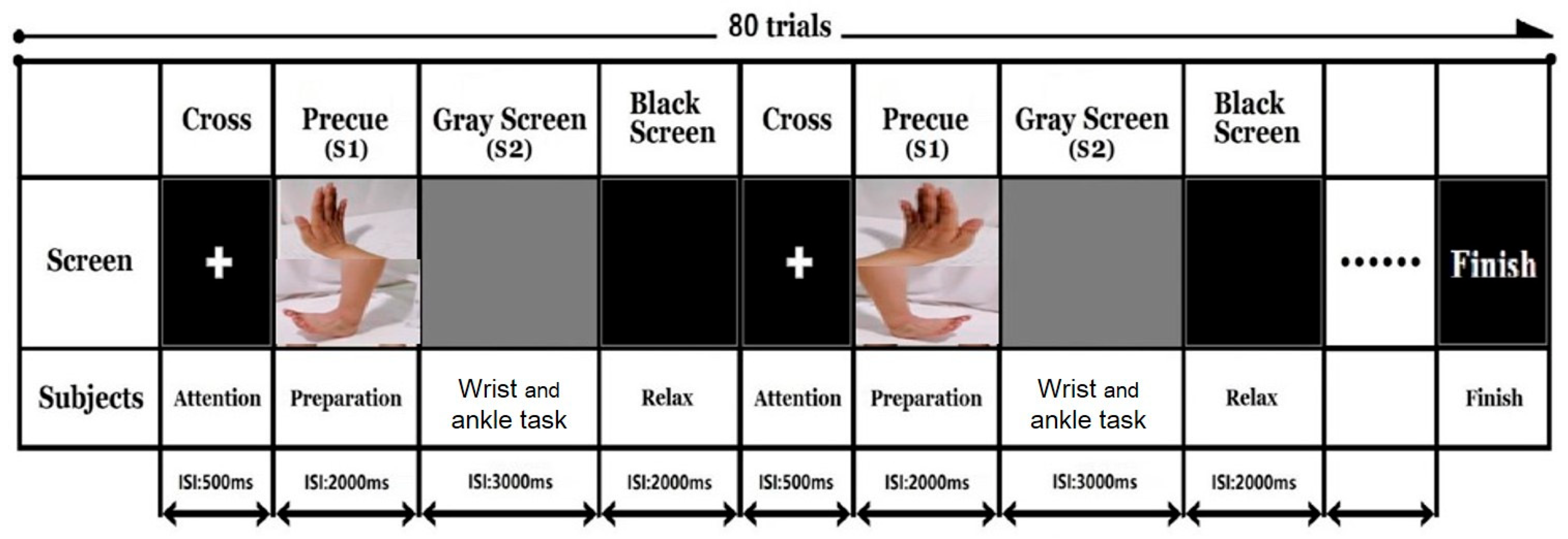
2.8. Event Related Potentials
2.9. fMRI
2.10. fNIRS
2.11. Outcome Assessments
2.12. Sample Size
3. Procedure
3.1. Control Group
3.2. Intervention Group
4. Safety and Adverse Event Reporting
- Serious adverse events (SAEs) are defined as an event that incurs harm to the participants, which may or may not require medical or surgical intervention as a preventative measure to avoid the following outcomes: death, further impairment of body function, damage to a body structure, and prolonged the hospitalization period.
- All SAEs related to the interventions will be recorded on a SAEs event report form in accordance with the procedures of each research centre.
- All of the SAEs related to the interventions must be reported to the Principal Investigator within 24 h of learning of the event.
- SAEs that are related to the interventions will be reported to the University Clinical Trial Unit and Research Ethics Committee within 15 days of the occurrence of the SAEs. The following information will be reported to relevant parties: (1) the concerned research protocol; (2) a report on the description of the SAE and subsequent outcome; (3) a proposal of changes in response to the SAE to prevent further occurrence of SAEs
4.1. Data Management
4.2. Data Analysis
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ranzani, R.; Lambercy, O.; Metzger, J.C.; Califfi, A.; Regazzi, S.; Dinacci, D.; Petrillo, C.; Rossi, P.; Conti, F.M.; Gassert, R. Neurocognitive robot-assisted rehabilitation of hand function: A randomized control trial on motor recovery in subacute stroke. J. Neuroeng. Rehabil. 2020, 17, 115. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wu, B.; Liu, M.; Chen, Z.; Wang, W.; Anderson, C.S.; Sandercock, P.; Wang, Y.; Huang, Y.; Cui, L.; et al. Stroke in China: Advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019, 18, 394–405. [Google Scholar] [CrossRef]
- Guan, T.; Ma, J.; Li, M.; Xue, T.; Lan, Z.; Guo, J.; Shen, Y.; Chao, B.; Tian, G.; Zhang, Q.; et al. Rapid transitions in the epidemiology of stroke and its risk factors in China from 2002 to 2013. Neurology 2017, 89, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Longde, W.; Ling, Y.; Yang, H.; Yi, Z.; Yongjun, W.; Xunming, J.; Xiaoyuan, N.; Qiumin, Q.; Li, H.; Yuming, X.; et al. Fixed-dose combination treatment after stroke for secondary prevention in China: A national community-based study. Stroke 2015, 46, 1295–1300. [Google Scholar] [CrossRef]
- Global, Regional, and Country-Specific Lifetime Risks of Stroke, 1990 and 2016. N. Engl. J. Med. 2018, 25, 2429–2437.
- Mayo, N.E.; Wood-Dauphinee, S.; Robert, C.; Durcan, L.; Carlton, J. Activity, participation, and quality of life 6 months poststroke. Arch. Phys. Med. Rehabil. 2002, 83, 1035–1042. [Google Scholar] [CrossRef]
- Mayo, E.; Wood-Dauphinee, S.; Ahmed, S.; Carron, G.; Higgins, J.; Mcewen, S.; Salbach, N. Disablement following stroke. Disabil. Rehabil. 1999, 21, 258–268. [Google Scholar] [CrossRef]
- Feigin, V.L.; Lawes, C.M.; Bennett, D.A.; Anderson, C.S. Stroke epidemiology: A review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003, 2, 43–53. [Google Scholar] [CrossRef]
- Hong, E. Comparison of quality of life according to community walking in stroke patients. J. Phys. Ther. Sci. 2015, 27, 2391–2393. [Google Scholar] [CrossRef]
- Quan, M.; Xun, P.; Wang, R.; He, K.; Chen, P. Walking pace and the risk of stroke: A meta-analysis of prospective cohort studies. J. Sport Health Sci. 2020, 9, 521–529. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Williams Andrews, A. Normal walking speed: A descriptive meta-analysis. Physiotherapy 2011, 97, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.-R.; Lo, W.L.; Lin, Q.; Li, L.; Xiao, X.; Raghavan, P.; Huang, D.-F. The Effect of Body Weight Support Treadmill Training on Gait Recovery, Proximal Lower Limb Motor Pattern, and Balance in Patients with Subacute Stroke. BioMed Res. Int. 2015, 2015, 175719. [Google Scholar] [CrossRef]
- Mirelman, A.; Bonato, P.; Deutsch, J.E. Effects of training with a robot-virtual reality system compared with a robot alone on the gait of individuals after stroke. Stroke 2009, 40, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, B.; Artemiadis, P. A Review of Robot-Assisted Lower-Limb Stroke Therapy: Unexplored Paths and Future Directions in Gait Rehabilitation. Front. Neurorobot. 2020, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Mehrholz, J.; Thomas, S.; Elsner, B. Treadmill training and body weight support for walking after stroke. Cochrane Database Syst. Rev. 2017, 8, CD002840. [Google Scholar] [CrossRef]
- French, B.; Thomas, L.H.; Coupe, J.; McMahon, N.E.; Connell, L.; Harrison, J.; Sutton, C.J.; Tishkovskaya, S.; Watkins, C.L. Repetitive task training for improving functional ability after stroke. Cochrane Database Syst. Rev. 2016, 11, CD006073. [Google Scholar] [CrossRef]
- Yang, Y.R.; Tsai, M.P.; Chuang, T.Y.; Sung, W.H.; Wang, R.Y. Virtual reality-based training improves community ambulation in individuals with stroke: A randomized controlled trial. Gait Posture 2008, 28, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Givon, N.; Zeilig, G.; Weingarden, H.; Rand, D. Video-games used in a group setting is feasible and effective to improve indicators of physical activity in individuals with chronic stroke: A randomized controlled trial. Clin. Rehabil. 2016, 30, 383–392. [Google Scholar] [CrossRef]
- Fulk, G.D.; Ludwig, M.; Dunning, K.; Golden, S.; Boyne, P.; West, T. Estimating Clinically Important Change in Gait Speed in People With Stroke Undergoing Outpatient Rehabilitation. J. Neurol. Phys. Ther. 2011, 35, 82–89. [Google Scholar] [CrossRef]
- Slater, L.; Gilbertson, N.M.; Hyngstrom, A.S. Improving gait efficiency to increase movement and physical activity—The impact of abnormal gait patterns and strategies to correct. Prog. Cardiovasc. Dis. 2021, 64, 83–87. [Google Scholar] [CrossRef]
- Kim, S.J.; Cho, H.Y.; Kim, Y.L.; Lee, S.M. Effects of stationary cycling exercise on the balance and gait abilities of chronic stroke patients. J. Phys. Ther. Sci. 2015, 27, 3529–3531. [Google Scholar] [CrossRef] [PubMed]
- Raasch, C.C.; Zajac, F.E. Locomotor strategy for pedaling: Muscle groups and biomechanical functions. J. Neurophysiol. 1999, 82, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Frigon, A. The neural control of interlimb coordination during mammalian locomotion. J. Neurophysiol. 2017, 117, 2224–2241. [Google Scholar] [CrossRef]
- Arya, K.N.; Pandian, S.; Sharma, A.; Kumar, V.; Kashyap, V.K. Interlimb coupling in poststroke rehabilitation: A pilot randomized controlled trial. Top. Stroke Rehabil. 2020, 27, 272–289. [Google Scholar] [CrossRef] [PubMed]
- Weersink, J.B.; de Jong, B.M.; Halliday, D.M.; Maurits, N.M. Intermuscular coherence analysis in older adults reveals that gait-related arm swing drives lower limb muscles via subcortical and cortical pathways. J. Physiol. 2021, 599, 2283–2298. [Google Scholar] [CrossRef] [PubMed]
- Kaupp, C.; Pearcey, G.E.P.; Klarner, T.; Sun, Y.; Cullen, H.; Barss, T.S.; Zehr, E.P. Rhythmic arm cycling training improves walking and neurophysiological integrity in chronic stroke: The arms can give legs a helping hand in rehabilitation. J. Neurophysiol. 2018, 119, 1095–1112. [Google Scholar] [CrossRef] [PubMed]
- Yeganeh Doost, M.; Herman, B.; Denis, A.; Sapin, J.; Galinski, D.; Riga, A.; Laloux, P.; Bihin, B.; Vandermeeren, Y. Bimanual motor skill learning and robotic assistance for chronic hemiparetic stroke: A randomized controlled trial. Neural Regen. Res. 2021, 16, 1566–1573. [Google Scholar] [CrossRef]
- Debaere, F.; Swinnen, S.P.; Béatse, E.; Sunaert, S.; Van Hecke, P.; Duysens, J. Brain Areas Involved in Interlimb Coordination: A Distributed Network. NeuroImage 2001, 14, 947–958. [Google Scholar] [CrossRef]
- Van Impe, A.; Coxon, J.P.; Goble, D.J.; Wenderoth, N.; Swinnen, S.P. Ipsilateral coordination at preferred rate: Effects of age, body side and task complexity. NeuroImage 2009, 47, 1854–1862. [Google Scholar] [CrossRef]
- Ockenfeld, C.; Tong, R.K.; Susanto, E.A.; Ho, S.K.; Hu, X.L. Fine finger motor skill training with exoskeleton robotic hand in chronic stroke: Stroke rehabilitation. IEEE Int. Conf. Rehabil. Robot. Proc. 2013, 2013, 6650392. [Google Scholar] [CrossRef]
- Kilbreath, S.L.; Heard, R.C. Frequency of hand use in healthy older persons. Aust. J. Physiother. 2005, 51, 119–122. [Google Scholar] [CrossRef]
- Kilbreath, S.L.; Crosbie, J.; Canning, C.G.; Lee, M.J. Inter-limb coordination in bimanual reach-to-grasp following stroke. Disabil. Rehabil. 2006, 28, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Lodha, N. Functional implications of impaired bimanual force coordination in chronic stroke. Neurosci. Lett. 2020, 738, 135387. [Google Scholar] [CrossRef] [PubMed]
- Pollock, A.; Farmer, S.E.; Brady, M.C.; Langhorne, P.; Mead, G.E.; Mehrholz, J.; van Wijck, F. Interventions for improving upper limb function after stroke. Cochrane Database Syst. Rev. 2014, 11, CD010820. [Google Scholar] [CrossRef]
- Khalid, S.; Alnajjar, F.; Gochoo, M.; Renawi, A.; Shimoda, S. Robotic assistive and rehabilitation devices leading to motor recovery in upper limb: A systematic review. Disabil. Rehabil. Assist. Technol. 2021, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kwakkel, G.; Veerbeek, J.M.; van Wegen, E.E.; Wolf, S.L. Constraint-induced movement therapy after stroke. Lancet Neurol. 2015, 14, 224–234. [Google Scholar] [CrossRef]
- Bao, X.; Mao, Y.; Lin, Q.; Qiu, Y.; Chen, S.; Li, L.; Cates, R.S.; Zhou, S.; Huang, D. Mechanism of Kinect-based virtual reality training for motor functional recovery of upper limbs after subacute stroke. Neural Regen. Res. 2013, 8, 2904–2913. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Bushnell, C.; Bettger, J.P.; Cockroft, K.M.; Cramer, S.C.; Edelen, M.O.; Hanley, D.; Katzan, I.L.; Mattke, S.; Nilsen, D.M.; Piquado, T.; et al. Chronic Stroke Outcome Measures for Motor Function Intervention Trials: Expert Panel Recommendations. Circ. Cardiovasc. Qual. Outcomes 2015, 8 (Suppl. 3), S163–S169. [Google Scholar] [CrossRef]
- Blum, L.; Korner-Bitensky, N. Usefulness of the Berg Balance Scale in Stroke Rehabilitation: A Systematic Review. Phys. Ther. 2008, 88, 559–566. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.P.; Si Tou, J.I.; Tse, M.M.; Ng, S.S. Reliability and Validity of the Timed up and Go Test with a Motor Task in People with Chronic Stroke. Arch. Phys. Med. Rehabil. 2017, 98, 2213–2220. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Urabe, Y.; Murakami, M.; Itotani, K.; Kato, J. Discriminant analysis for predictor of falls in stroke patients by using the Berg Balance Scale. Singap. Med. J. 2015, 56, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Kitcharanant, N.; Vanitcharoenkul, E.; Unnanuntana, A. Validity and reliability of the self-rated fall risk questionnaire in older adults with osteoporosis. BMC Musculoskelet. Disord. 2020, 21, 757. [Google Scholar] [CrossRef]
- Echeverría, A.; Cauas, R.; Díaz, B.; Sáez, C.; Cárcamo, M. Herramientas de evaluación de actividades de la vida diaria instrumentales en población adulta: Revisión sistemática. Rev. Méd. Clín. Condes 2021, 32, 474–490. [Google Scholar] [CrossRef]
- Fugl-Meyer, A.R.; Jaasko, L.; Leyman, I.; Olsson, S.; Steglind, S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar]
- Berg, K.O.; Wood-Dauphinee, S.L.; Williams, J.I.; Maki, B. Measuring balance in the elderly: Validation of an instrument. Can. J. Public Health 1992, 83 (Suppl. 2), S7–S11. [Google Scholar]
- Kossi, O.; Agbetou, M.; Noukpo, S.I.; Triccas, L.T.; Dossou-Yovo, D.-E.; Amanzonwe, E.R.; Adoukonou, T. Factors associated with balance impairments amongst stroke survivors in northern Benin: A cross-sectional study. S. Afr. J. Physiother. 2021, 77, 1559. [Google Scholar] [CrossRef]
- Lyle, R.C. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int. J. Rehabil. Res. 1981, 4, 483–492. [Google Scholar] [CrossRef]
- Lawton, M.P.; Brody, E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef]
- Chen, L.; Mao, Y.; Ding, M.; Li, L.; Leng, Y.; Zhao, J.; Xu, Z.; Huang, D.F.; Lo, W.L.A. Assessing the Relationship Between Motor Anticipation and Cortical Excitability in Subacute Stroke Patients With Movement-Related Potentials. Front. Neurol. 2018, 9, 881. [Google Scholar] [CrossRef]
- Khanmohammadi, R.; Talebian, S.; Hadian, M.R.; Olyaei, G.; Bagheri, H. Preparatory postural adjustments during gait initiation in healthy younger and older adults: Neurophysiological and biomechanical aspects. Brain Res. 2015, 1629, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Lin, Q.; Lo, W.L.; Mao, Y.R.; Shi, X.C.; Cates, R.S.; Zhou, S.F.; Huang, D.F.; Li, L. Cerebral Reorganization in Subacute Stroke Survivors after Virtual Reality-Based Training: A Preliminary Study. Behav. Neurol. 2017, 2017, 6261479. [Google Scholar] [CrossRef] [PubMed]
- Tilson, J.K.; Sullivan, K.J.; Cen, S.Y.; Rose, D.K.; Koradia, C.H.; Azen, S.P.; Duncan, P.W. Meaningful gait speed improvement during the first 60 days poststroke: Minimal clinically important difference. Phys. Ther. 2010, 90, 196–208. [Google Scholar] [CrossRef]
- Fulk, G.D.; Echternach, J.L. Test-retest reliability and minimal detectable change of gait speed in individuals undergoing rehabilitation after stroke. J. Neurol. Phys. Ther. 2008, 32, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Indredavik, B.; Bakke, F.; Solberg, R.; Rokseth, R.; Haaheim, L.L.; Holme, I. Benefit of a stroke unit: A randomized controlled trial. Stroke J. Cereb. Circ. 1991, 22, 1026–1031. [Google Scholar] [CrossRef]
- Wade, D.T. The hemiplegic arm after stroke: Measurement and recovery. J. Neurol. Neurosurg. Psychiatry 1983, 46, 521–524. [Google Scholar] [CrossRef]
- Lawrence, M.; Celestino, F.T., Jr.; Matozinho, H.H.S.; Govan, L.; Booth, J.; Beecher, J. Yoga for stroke rehabilitation. Cochrane Database Syst. Rev. 2017, 12, CD011483. [Google Scholar] [CrossRef]
- Pollock, A.; Baer, G.; Campbell, P.; Choo, P.L.; Forster, A.; Morris, J.; Pomeroy, V.M.; Langhorne, P. Physical rehabilitation approaches for the recovery of function and mobility following stroke. Cochrane Database Syst. Rev. 2014, 4, CD001920. [Google Scholar] [CrossRef]
- Laver, K.E.; Lange, B.; George, S.; Deutsch, J.E.; Saposnik, G.; Crotty, M. Virtual reality for stroke rehabilitation. Cochrane Database Syst. Rev. 2017, 11, CD008349. [Google Scholar] [CrossRef]
- van Dokkum, L.; Hauret, I.; Mottet, D.; Froger, J.; Métrot, J.; Laffont, I. The contribution of kinematics in the assessment of upper limb motor recovery early after stroke. Neurorehabilit. Neural Repair 2014, 28, 4–12. [Google Scholar] [CrossRef]
- Toole, J.F.; Flowers, D.L.; Burdette, J.H.; Absher, J.R. A Pianist’s Recovery From Stroke. Arch. Neurol. 2007, 64, 1184–1188. [Google Scholar] [CrossRef][Green Version]
- Chettouf, S.; Rueda-Delgado, L.M.; de Vries, R.; Ritter, P.; Daffertshofer, A. Are unimanual movements bilateral? Neurosci. Biobehav. Rev. 2020, 113, 39–50. [Google Scholar] [CrossRef]
- Scarpina, F.; Tagini, S.; Rabuffetti, M.; Albani, G.; Garbarini, F.; Mauro, A. Is bimanual interference affected in the case of a central proprioceptive loss? New insight from a left-brain-damaged single-case study. Neuropsychology 2020, 34, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Li, H.; Mao, Y.R.; Lo, W.L.; Zhao, J.L.; Chen, L.; Leng, Y.; Huang, D.F.; Li, L. The Difference of Neural Networks between Bimanual Antiphase and In-Phase Upper Limb Movements: A Preliminary Functional Magnetic Resonance Imaging Study. Behav. Neurol. 2017, 2017, 8041962. [Google Scholar] [CrossRef] [PubMed]
- Grillner, S. Action: The role of motor cortex challenged. Curr. Biol. 2015, 25, R508–R511. [Google Scholar] [CrossRef] [PubMed][Green Version]

| Fugl-Meyer Assessment of Motor Recovery (FMA) [46] | It is a validated tool to evaluate motor function, balance, and joint function in stroke-related hemiplegic patients. The interpretation of FMA is as follows: <50 = Severe; 50–84 = Marked, 85–94 = Moderate; 95–99 = Slight. |
| Berg Balance scale (BBS) [47] | The BBS consists of 14 balance-related tasks that include sit to stand, stand to sit, and standing on one foot. It was developed to assess the ability to maintain dynamic and static balance. |
| Timed up and go test (TUG) | The test requires the person to rise from a chair and walk 3 m at a comfortable pace, turn around at the 3 m mark, and walk back to the starting point. The test’s score is the time it takes the person to complete the test. A cut off of 14 s was proposed to be “normal” [48]. |
| Action Research Arm Test (ARAT) [49] | ARAT is a 19-item test that is divided into four sub-tests, which consist of grasp, grip, pinch, and gross arm movement. A score of between 0 to 4 is given to each task: 0—can perform none of the test; 1—performs part of the test; 2—takes abnormally long or has great difficulty in completing the test; 3—able to perform the test normally. |
| Instrumental Activities of Daily Living (IADL) [50] | The IADL [26] is a functional disability scale that assesses the functional level by asking whether a person receives personal help with daily living activities, such as using the telephone, getting to places outside the house, grocery shopping, preparing meals, doing housework or handyman work, laundry, taking medications, and managing finances. |
| Electroencephalography (EEG)/Event related potential (ERP) | Parameters of contingent negative variation (CNV), alpha, beta, gamma, and theta wave oscillation. |
| Functional magnetic resonance imaging (fMRI) | The voxel count T1-weighted data set of the entire brain will be acquired for each participant. The scan parameters for blood oxygen level-dependent weighted scans are as follows: TR = 200 ms, TE = 25 ms, field of view 200 × 200, matrix 64 × 64, and a slice thickness of 3 mm. |
| Functional Near-Infared Spectroscopy (fNIRS) | The optical system used in the study will be a multi-channel frequency domain NIRS system (BRITE, Artinis Medical Systems, Gelderland, The Netherlands). The particular regions of interest is motor cortex (M1), supplementary motor cortex (SMC), and premotor cortex (PMC). |
| Enrolment | Allocation | Post-Allocation | ||||
|---|---|---|---|---|---|---|
| Timepoint | 0 | 0 | Baseline | 3 Weeks Intervention | 3 Months | 6 Months |
| Enrolment: | ||||||
| Eligibility screen | X | |||||
| Informed consent | X | |||||
| Allocation | X | |||||
| Interventions: | ||||||
| Routine with Interlimb coordinated |  | |||||
| Routine rehabilitation |  | |||||
| Assessments: | ||||||
| Gait speed | X | X | X | X | ||
| EEG | X | X | X | X | ||
| fNIRS | X | X | X | X | ||
| MRI | X | X | X | X | ||
| BBS | X | X | X | X | ||
| ARAT | X | X | X | X | ||
| FMA-UL | X | X | X | X | ||
| IADL | X | X | X | X | ||
| Time (Minutes) | ||
|---|---|---|
| Treatment Type | Control | Intervention |
| Muscle strengthening | 20 | 17 |
| Treadmill gait training | 20 | 17 |
| Balance training | 20 | 17 |
| Passive exercise | 10 | 7 |
| Upper limb training | 20 | 17 |
| Functional practice | 20 | 17 |
| Neuromuscular electrical stimulation | 10 | 8 |
| Interlimb coordinated | 0 | 20 |
| Total | 120 | 120 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo, W.-L.A.; Chen, D.; Zhao, J.; Leng, Y.; Bian, R.; Huang, W.; Liang, Y.; Mao, Y.-R.; Huang, D.-F. The Efficacy of Interlimb-Coordinated Intervention on Gait and Motor Function Recovery in Patients with Acute Stroke: A Multi-Center Randomized Controlled Trial Study Protocol. Brain Sci. 2021, 11, 1495. https://doi.org/10.3390/brainsci11111495
Lo W-LA, Chen D, Zhao J, Leng Y, Bian R, Huang W, Liang Y, Mao Y-R, Huang D-F. The Efficacy of Interlimb-Coordinated Intervention on Gait and Motor Function Recovery in Patients with Acute Stroke: A Multi-Center Randomized Controlled Trial Study Protocol. Brain Sciences. 2021; 11(11):1495. https://doi.org/10.3390/brainsci11111495
Chicago/Turabian StyleLo, Wai-Leung Ambrose, Dandan Chen, Jiangli Zhao, Yan Leng, Ruihao Bian, Wenzhu Huang, Yahui Liang, Yu-Rong Mao, and Dong-Feng Huang. 2021. "The Efficacy of Interlimb-Coordinated Intervention on Gait and Motor Function Recovery in Patients with Acute Stroke: A Multi-Center Randomized Controlled Trial Study Protocol" Brain Sciences 11, no. 11: 1495. https://doi.org/10.3390/brainsci11111495
APA StyleLo, W.-L. A., Chen, D., Zhao, J., Leng, Y., Bian, R., Huang, W., Liang, Y., Mao, Y.-R., & Huang, D.-F. (2021). The Efficacy of Interlimb-Coordinated Intervention on Gait and Motor Function Recovery in Patients with Acute Stroke: A Multi-Center Randomized Controlled Trial Study Protocol. Brain Sciences, 11(11), 1495. https://doi.org/10.3390/brainsci11111495






