The Number or Type of Stimuli Used for Somatosensory Stimulation Affected the Modulation of Corticospinal Excitability
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measurement of Corticospinal Excitability
2.3. Conditioning Stimulation Setting
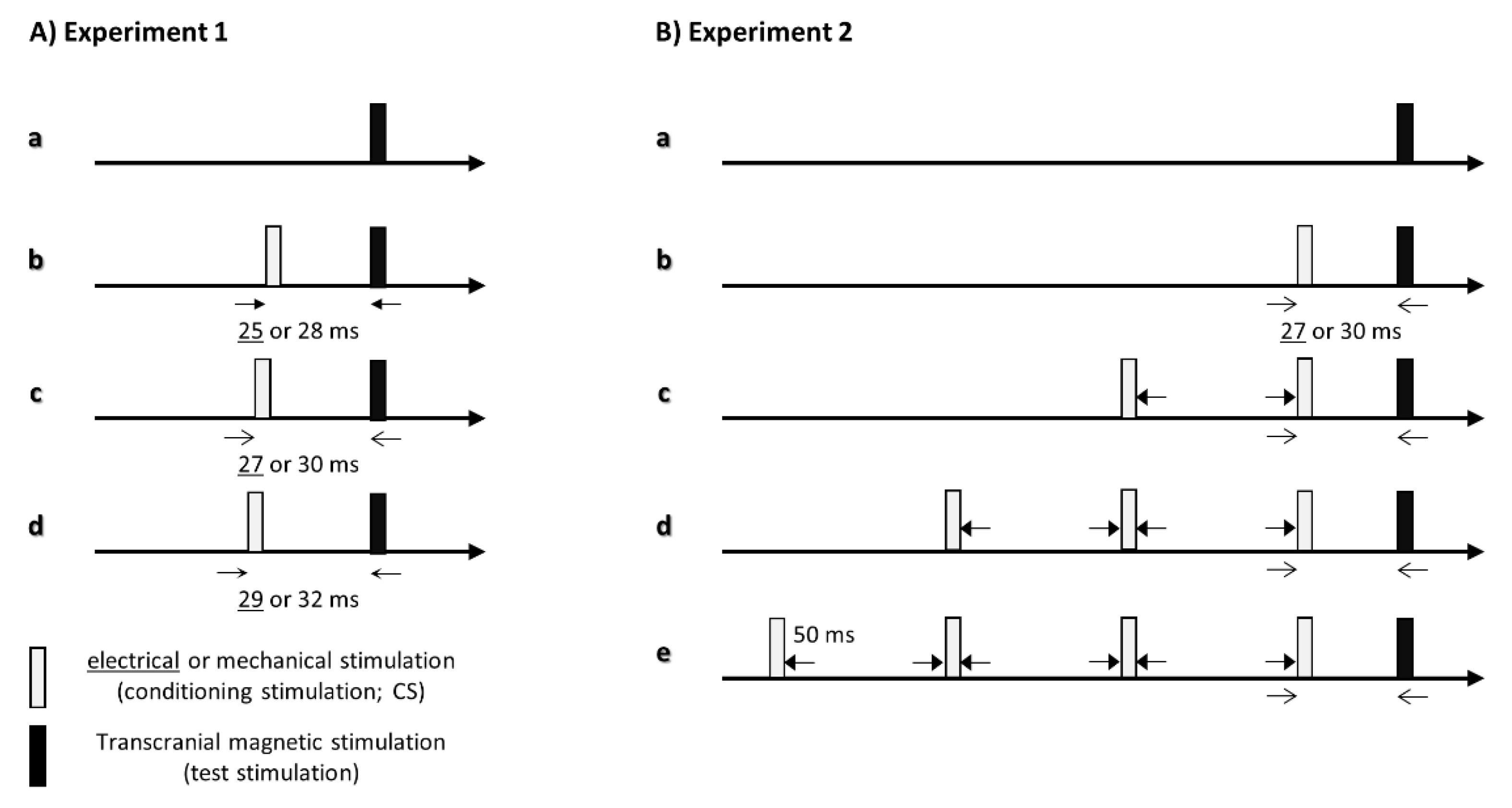
2.4. Experiment 1: Effects of the Interstimulus Interval between the One Conditioning Stimulation and TMS on Corticospinal Excitability
2.5. Experiment 2: Effects of the Number of Conditioning Stimuli on Corticospinal Excitability
2.6. Data and Statistical Analyses
3. Results
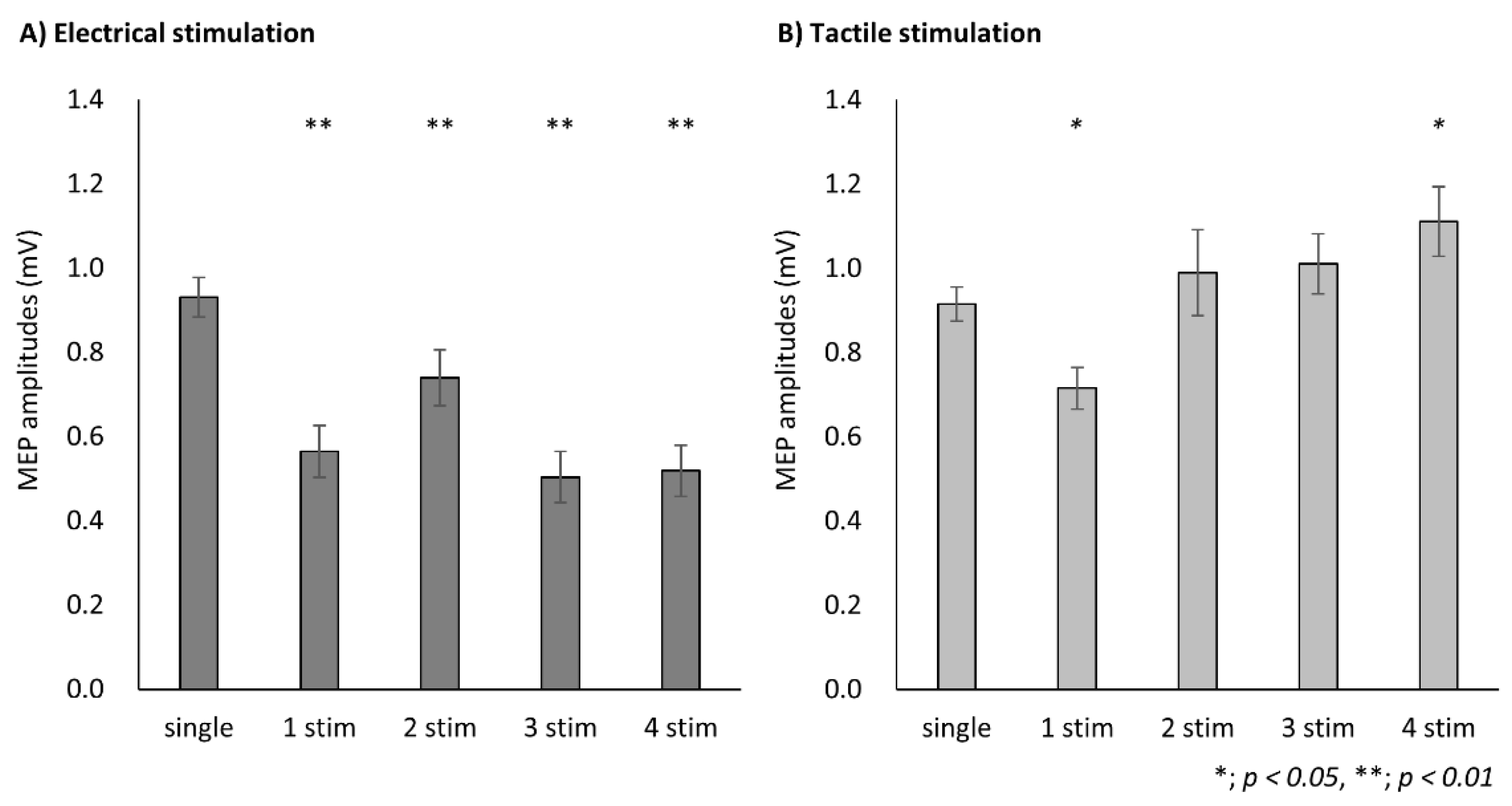
3.1. Experiment 1
3.2. Experiment 2
4. Discussion
4.1. Effects of Different Somatosensory Input Methods on Corticospinal Excitability
4.2. Effects of the Number of Somatosensory Stimulations on Corticospinal Excitability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ridding, M.C.; Brouwer, B.; Miles, T.S.; Pitcher, J.B.; Thompson, P.D. Changes in muscle responses to stimulation of the motor cortex induced by peripheral nerve stimulation in human subjects. Exp. Brain Res. 2000, 131, 135–143. [Google Scholar] [CrossRef]
- Tokimura, H.; Di Lazzaro, V.; Tokimura, Y.; Oliviero, A.; Profice, P.; Insola, A.; Mazzone, P.; Tonali, P.; Rothwell, J. Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J. Physiol. 2000, 523, 503–513. [Google Scholar] [CrossRef]
- MMcDonnell, N.; Ridding, M.C. Afferent stimulation facilitates performance on a novel motor task. Exp. Brain Res. 2006, 170, 109–115. [Google Scholar] [CrossRef]
- Christova, M.; Rafolt, D.; Golaszewski, S.; Nardone, R.; Gallasch, E. Electrical stimulation during skill training with a therapeutic glove enhances the induction of cortical plasticity and has a positive effect on motor memory. Behav. Brain Res. 2014, 270, 171–178. [Google Scholar] [CrossRef]
- Kojima, S.; Onishi, H.; Sugawara, K.; Miyaguchi, S.; Kirimoto, H.; Tamaki, H.; Shirozu, H.; Kameyama, S. No relation between afferent facilitation induced by digital nerve stimulation and the latency of cutaneomuscular reflexes and somatosensory evoked magnetic fields. Front. Hum. Neurosci. 2014, 8, 1023. [Google Scholar] [CrossRef]
- Kotan, S.; Kojima, S.; Miyaguchi, S.; Sugawara, K.; Onishi, H. Depression of corticomotor excitability after muscle fatigue induced by electrical stimulation and voluntary contraction. Front. Hum. Neurosci. 2015, 9, 363. [Google Scholar] [CrossRef]
- Sasaki, R.; Kotan, S.; Nakagawa, M.; Miyaguchi, S.; Kojima, S.; Saito, K.; Inukai, Y.; Onishi, H. Presence and Absence of Muscle Contraction Elicited by Peripheral Nerve Electrical Stimulation Differentially Modulate Primary Motor Cortex Excitability. Front. Hum. Neurosci. 2017, 11, 146. [Google Scholar] [CrossRef]
- Bailey, A.Z.; Asmussen, M.J.; Nelson, A.J. Short-latency afferent inhibition determined by the sensory afferent volley. J. Neurophysiol. 2016, 116, 637–644. [Google Scholar] [CrossRef]
- Christova, M.; Rafolt, D.; Golaszewski, S.; Gallasch, E. Outlasting corticomotor excitability changes induced by 25 Hz whole-hand mechanical stimulation. Eur. J. Appl. Physiol. 2011, 111, 3051–3059. [Google Scholar] [CrossRef]
- Brinkman, J.; Colebatch, J.G.; Porter, R.; York, D.H. Responses of precentral cells during cooling of post-central cortex in conscious monkeys. J. Physiol. 1985, 368, 611–625. [Google Scholar] [CrossRef]
- Mao, T.; Kusefoglu, D.; Hooks, B.M.; Huber, D.; Petreanu, L.; Svoboda, K. Long-range neuronal circuits underlying the interaction between sensory and motor cortex. Neuron 2011, 72, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.T.; Lundbye-Jensen, J.; Leukel, C.; Nielsen, J.B. Cutaneous mechanisms of isometric ankle force control. Exp. Brain Res. 2013, 228, 377–384. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ohashi, H.; Gribble, P.L.; Ostry, D.J. Somatosensory cortical excitability changes precede those in motor cortex during human motor learning. J. Neurophysiol. 2019, 122, 1397–1405. [Google Scholar] [CrossRef]
- Sawaki, L.; Wu, C.W.; Kaelin-Lang, A.; Cohen, L.G. Effects of somatosensory stimulation on use-dependent plasticity in chronic stroke. Stroke 2006, 37, 246–247. [Google Scholar] [CrossRef] [PubMed]
- Wikström, H.; Huttunen, J.; Korvenoja, A.; Virtanen, J.; Salonen, O.; Aronen, H.; Ilmoniemi, R.J. Effects of interstimulus interval on somatosensory evoked magnetic fields (SEFs): A hypothesis concerning SEF generation at the primary sensorimotor cortex. Electroencephalogr. Clin. Neurophysiol. 1996, 100, 479–487. [Google Scholar] [CrossRef]
- Hari, R.; Forss, N. Magnetoencephalography in the study of human somatosensory cortical processing. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999, 354, 1145–1154. [Google Scholar] [CrossRef]
- Huttunen, J.; Komssi, S.; Lauronen, L. Spatial dynamics of population activities at S1 after median and ulnar nerve stimulation revisited: An MEG study. Neuroimage 2006, 32, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Onishi, H.; Sugawara, K.; Yamashiro, K.; Sato, D.; Suzuki, M.; Kirimoto, H.; Tamaki, H.; Murakami, H.; Kameyama, S. Effect of the number of pins and inter-pin distance on somatosensory evoked magnetic fields following mechanical tactile stimulation. Brain Res. 2013, 1535, 78–88. [Google Scholar] [CrossRef]
- Tamburin, S.; Fiaschi, A.; Andreoli, A.; Marani, S.; Zanette, G. Sensorimotor integration to cutaneous afferents in humans: The effect of the size of the receptive field. Exp. Brain Res. 2005, 167, 362–369. [Google Scholar] [CrossRef]
- Bikmullina, R.; Baumer, T.; Zittel, S.; Munchau, A. Sensory afferent inhibition within and between limbs in humans. Clin. Neurophysiol. 2009, 120, 610–618. [Google Scholar] [CrossRef]
- Devanne, H.; Degardin, A.; Tyvaert, L.; Bocquillon, P.; Houdayer, E.; Manceaux, A.; Derambure, P.; Cassim, F. Afferent-induced facilitation of primary motor cortex excitability in the region controlling hand muscles in humans. Eur. J. Neurosci. 2009, 30, 439–448. [Google Scholar] [CrossRef]
- Kojima, S.; Onishi, H.; Miyaguchi, S.; Kotan, S.; Sugawara, K.; Kirimoto, H.; Tamaki, H. Effects of cathodal transcranial direct current stimulation to primary somatosensory cortex on short-latency afferent inhibition. Neuroreport 2015, 26, 634–637. [Google Scholar] [CrossRef]
- Scelzo, E.; Giannicola, G.; Rosa, M.; Ciocca, M.; Ardolino, G.; Cogiamanian, F.; Ferrucci, R.; Fumagalli, M.; Mameli, F.; Barbieri, S.; et al. Increased short latency afferent inhibition after anodal transcranial direct current stimulation. Neurosci. Lett. 2011, 498, 167–170. [Google Scholar] [CrossRef]
- Sasaki, R.; Miyaguchi, S.; Kotan, S.; Kojima, S.; Kirimoto, H.; Onishi, H. Modulation of Cortical Inhibitory Circuits after Cathodal Transcranial Direct Current Stimulation over the Primary Motor Cortex. Front. Hum. Neurosci. 2016, 10, 30. [Google Scholar] [CrossRef]
- Guerra, A.; Pogosyan, A.; Nowak, M.; Tan, H.; Ferreri, F.; Di Lazzaro, V.; Brown, P. Phase Dependency of the Human Primary Motor Cortex and Cholinergic Inhibition Cancelation During Beta tACS. Cereb. Cortex. 2016, 26, 3977–3990. [Google Scholar] [CrossRef]
- Onishi, H.; Sugawara, K.; Yamashiro, K.; Sato, D.; Kirimoto, H.; Tamaki, H.; Shirozu, H.; Kameyama, S. Inhibitory effect of intensity and interstimulus interval of conditioning stimuli on somatosensory evoked magnetic fields. Eur. J. Neurosci. 2016, 44, 2104–2113. [Google Scholar] [CrossRef]
- Huttunen, J.; Pekkonen, E.; Kivisaari, R.; Autti, T.; Kahkonen, S. Modulation of somatosensory evoked fields from SI and SII by acute GABA A-agonism and paired-pulse stimulation. Neuroimage 2008, 40, 427–434. [Google Scholar] [CrossRef]
- Huttunen, J. In search of augmentation at human SI: Somatosensory cortical responses to stimulus trains and their modulation by motor activity. Brain Res. 2010, 1331, 74–79. [Google Scholar] [CrossRef]
- Kojima, S.; Onishi, H.; Miyaguchi, S.; Kotan, S.; Sasaki, R.; Nakagawa, M.; Kirimoto, H.; Tamaki, H. Modulation of corticospinal excitability depends on the pattern of mechanical tactile stimulation. Neural. Plast. 2018, 5383514. [Google Scholar] [CrossRef]
- Onishi, H.; Oyama, M.; Soma, T.; Kubo, M.; Kirimoto, H.; Murakami, H.; Kameyama, S. Neuromagnetic activation of primary and secondary somatosensory cortex following tactile-on and tactile-off stimulation. Clin. Neurophysiol. 2010, 121, 588–593. [Google Scholar] [CrossRef]
- di Lazzaro, V.; Pilato, F.; Dileone, M.; Tonali, P.A.; Ziemann, U. Dissociated effects of diazepam and lorazepam on short-latency afferent inhibition. J. Physiol. 2005, 569, 315–323. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Oliviero, A.; Saturno, E.; Dileone, M.; Pilato, F.; Nardone, R.; Ranieri, F.; Musumeci, G.; Fiorilla, T.; Tonali, P. Effects of lorazepam on short latency afferent inhibition and short latency intracortical inhibition in humans. J. Physiol. 2005, 564, 661–668. [Google Scholar] [CrossRef]
- Udupa, K.; Ni, Z.; Gunraj, C.; Chen, R. Interactions between short latency afferent inhibition and long interval intracortical inhibition. Exp. Brain Res. 2009, 199, 177–183. [Google Scholar] [CrossRef]
- Celebi, O.; Temucin, C.M.; Elibol, B.; Saka, E. Short latency afferent inhibition in Parkinson’s disease patients with dementia. Mov. Disord. 2012, 27, 1052–1055. [Google Scholar] [CrossRef]
- Nardone, R.; Bergmann, J.; Christova, M.; Caleri, F.; Tezzon, F.; Ladurner, G.; Trinka, E.; Golaszewski, S. Short latency afferent inhibition differs among the subtypes of mild cognitive impairment. J. Neural. Transm. 2012, 119, 463–471. [Google Scholar] [CrossRef]
- Yarnall, A.J.; Ho, B.S.W.; Eshun, E.; David, R.; Rochester, L.; Burn, D.J.; Baker, M.R. Short latency afferent inhibition: Effects of ageing. Clin. Neurophysiol. 2016, 127, 2410–2413. [Google Scholar] [CrossRef][Green Version]
- Fischer, M.; Orth, M. Short-latency sensory afferent inhibition: Conditioning stimulus intensity, recording site, and effects of 1 Hz repetitive TMS. Brain Stimul. 2011, 4, 202–209. [Google Scholar] [CrossRef]
- Dubbioso, R.; Manganelli, F.; Siebner, H.R.; di Lazzaro, V. Fast intracortical sensory-motor integration: A window into the pathophysiology of Parkinson’s disease. Front. Hum. Neurosci. 2019, 13, 111. [Google Scholar] [CrossRef]
- Classen, J.; Steinfelder, B.; Liepert, J.; Stefan, K.; Celnik, P.; Cohen, L.G.; Hess, A.; Kunesch, E.; Chen, R.; Benecke, R.; et al. Cutaneomotor integration in humans is somatotopically organized at various levels of the nervous system and is task dependent. Exp. Brain Res. 2000, 130, 48–59. [Google Scholar] [CrossRef]
- Asmussen, M.J.; Zapallow, C.M.; Jacobs, M.F.; Lee, K.G.; Tsang, P.; Nelson, A.J. Modulation of short-latency afferent inhibition depends on digit and task-relevance. PLoS ONE 2014, 9, e104807. [Google Scholar] [CrossRef]
- Dubbioso, R.; Raffin, E.; Karabanov, A.; Thielscher, A.; Siebner, H.R. Centre-surround organization of fast sensorimotor integration in human motor hand area. Neuroimage 2017, 158, 37–47. [Google Scholar] [CrossRef]
- Tamburin, S.; Manganotti, P.; Zanette, G.; Fiaschi, A. Cutaneomotor integration in human hand motor areas: Somatotopic effect and interaction of afferents. Exp. Brain Res. 2001, 141, 232–241. [Google Scholar] [CrossRef]
- Forss, N.; Jousmäki, V.; Hari, R. Interaction between afferent input from fingers in human somatosensory cortex. Brain Res. 1995, 685, 68–76. [Google Scholar] [CrossRef]
- Inui, K.; Wang, X.; Tamura, Y.; Kaneoke, Y.; Kakigi, R. Serial processing in the human somatosensory system. Cereb. Cortex. 2004, 14, 851–857. [Google Scholar] [CrossRef]
- Johnson, K.O.; Hsiao, S.S. Neural mechanisms of tactual form and texture perception. Annu. Rev. Neurosci. 1992, 15, 227–250. [Google Scholar] [CrossRef]
- Ruddy, K.L.; Jaspers, E.; Keller, M.; Wenderoth, N. Interhemispheric sensorimotor integration; an upper limb phenomenon? Neuroscience 2016, 333, 104–113. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Komori, T.; Watson, B.V.; Brown, W.F. Influence of peripheral afferents on cortical and spinal motoneuron excitability. Muscle. Nerve. 1992, 15, 48–51. [Google Scholar] [CrossRef]
- Yokota, T.; Saito, Y.; Shimizu, Y. Increased corticomotoneuronal excitability after peripheral nerve stimulation in dopa-nonresponsive hemiparkinsonism. J. Neurol. Sci. 1995, 129, 34–39. [Google Scholar] [CrossRef]
- Chen, R.; Corwell, B.; Hallett, M. Modulation of motor cortex excitability by median nerve and digit stimulation. Exp. Brain Res. 1999, 129, 77–86. [Google Scholar] [CrossRef]
- Sailer, A.; Molnar, G.F.; Paradiso, G.; Gunraj, C.A.; Lang, A.E.; Chen, R. Short and long latency afferent inhibition in Parkinson’s disease. Brain 2003, 126, 1883–1894. [Google Scholar] [CrossRef]
- Terao, Y.; Ugawa, Y.; Uesaka, Y.; Hanajima, R.; Gemba-Shimizu, K.; Ohki, Y.; Kanazawa, I. Input-output organization in the hand area of the human motor cortex. Electroencephalogr. Clin. Neurophysiol. 1995, 97, 375–381. [Google Scholar] [CrossRef]
- Ilić, T.V.; Meintzschel, F.; Cleff, U.; Ruge, D.; Kessler, K.R.; Ziemann, U. Short-interval paired-pulse inhibition and facilitation of human motor cortex: The dimension of stimulus intensity. J. Physiol. 2002, 545, 153–167. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kojima, S.; Miyaguchi, S.; Yokota, H.; Saito, K.; Inukai, Y.; Otsuru, N.; Onishi, H. The Number or Type of Stimuli Used for Somatosensory Stimulation Affected the Modulation of Corticospinal Excitability. Brain Sci. 2021, 11, 1494. https://doi.org/10.3390/brainsci11111494
Kojima S, Miyaguchi S, Yokota H, Saito K, Inukai Y, Otsuru N, Onishi H. The Number or Type of Stimuli Used for Somatosensory Stimulation Affected the Modulation of Corticospinal Excitability. Brain Sciences. 2021; 11(11):1494. https://doi.org/10.3390/brainsci11111494
Chicago/Turabian StyleKojima, Sho, Shota Miyaguchi, Hirotake Yokota, Kei Saito, Yasuto Inukai, Naofumi Otsuru, and Hideaki Onishi. 2021. "The Number or Type of Stimuli Used for Somatosensory Stimulation Affected the Modulation of Corticospinal Excitability" Brain Sciences 11, no. 11: 1494. https://doi.org/10.3390/brainsci11111494
APA StyleKojima, S., Miyaguchi, S., Yokota, H., Saito, K., Inukai, Y., Otsuru, N., & Onishi, H. (2021). The Number or Type of Stimuli Used for Somatosensory Stimulation Affected the Modulation of Corticospinal Excitability. Brain Sciences, 11(11), 1494. https://doi.org/10.3390/brainsci11111494




