AngIV-Analog Dihexa Rescues Cognitive Impairment and Recovers Memory in the APP/PS1 Mouse via the PI3K/AKT Signaling Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drugs and Reagents
2.2. Animals and Treatment
2.3. Morris Water Maze (MWM)
2.4. ELISA
2.5. Nissl Staining
2.6. Western Blot
2.7. Statistical Analysis
3. Results
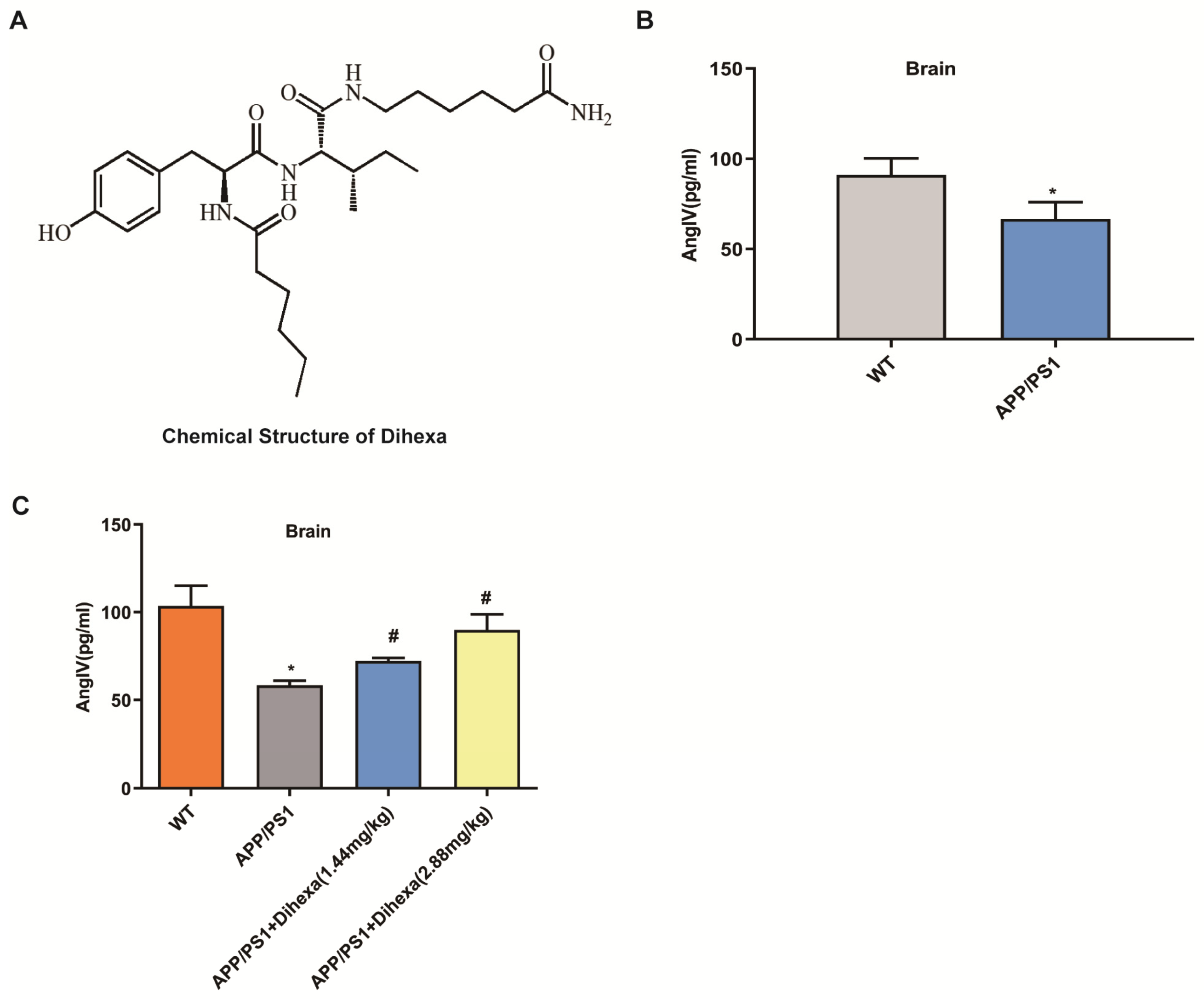
3.1. Dihexa Restored the Decrease in AngIV in APP/PS1 Mice
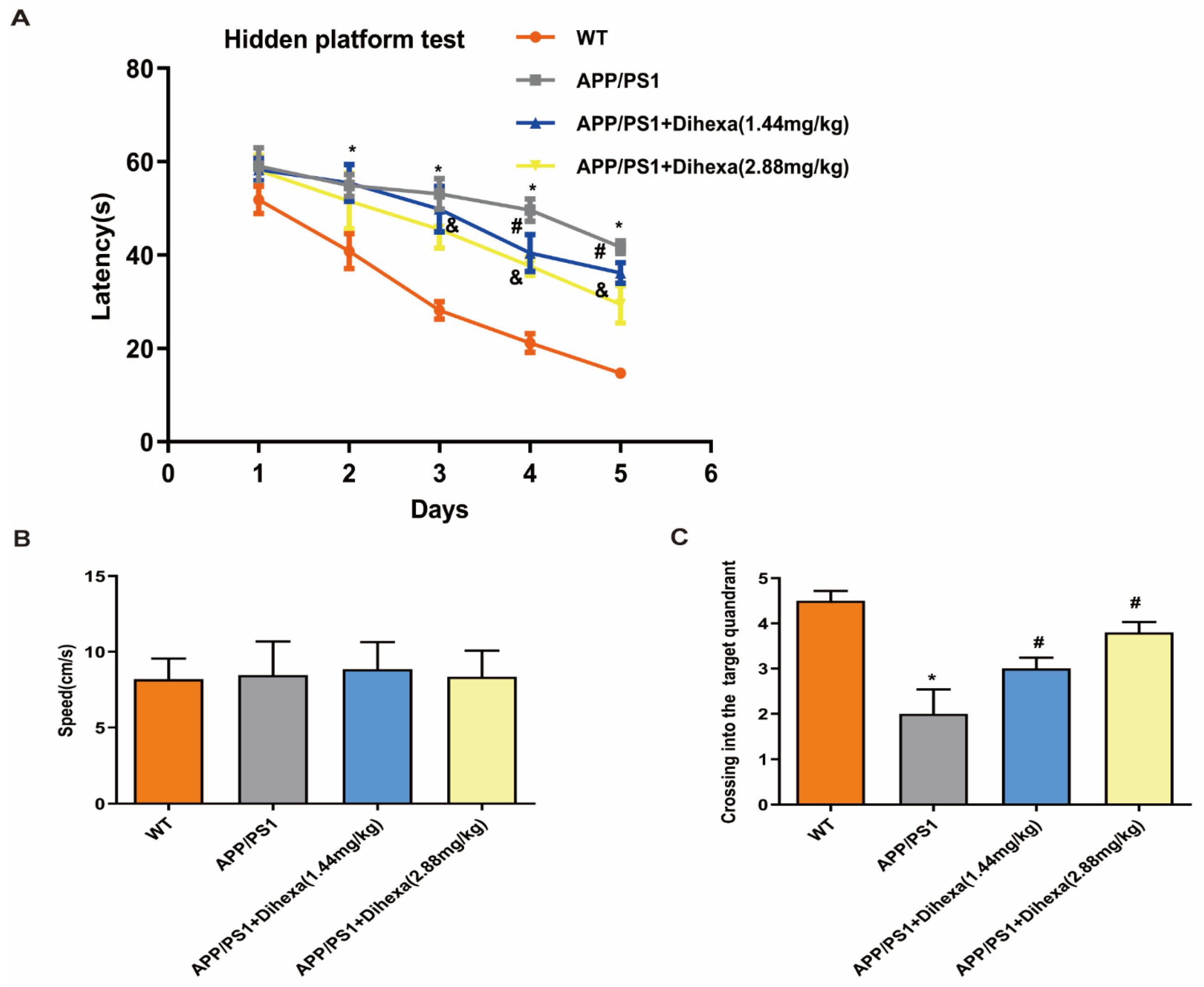
3.2. Dihexa Rescued the Cognitive Ability of APP/PS1 Mice
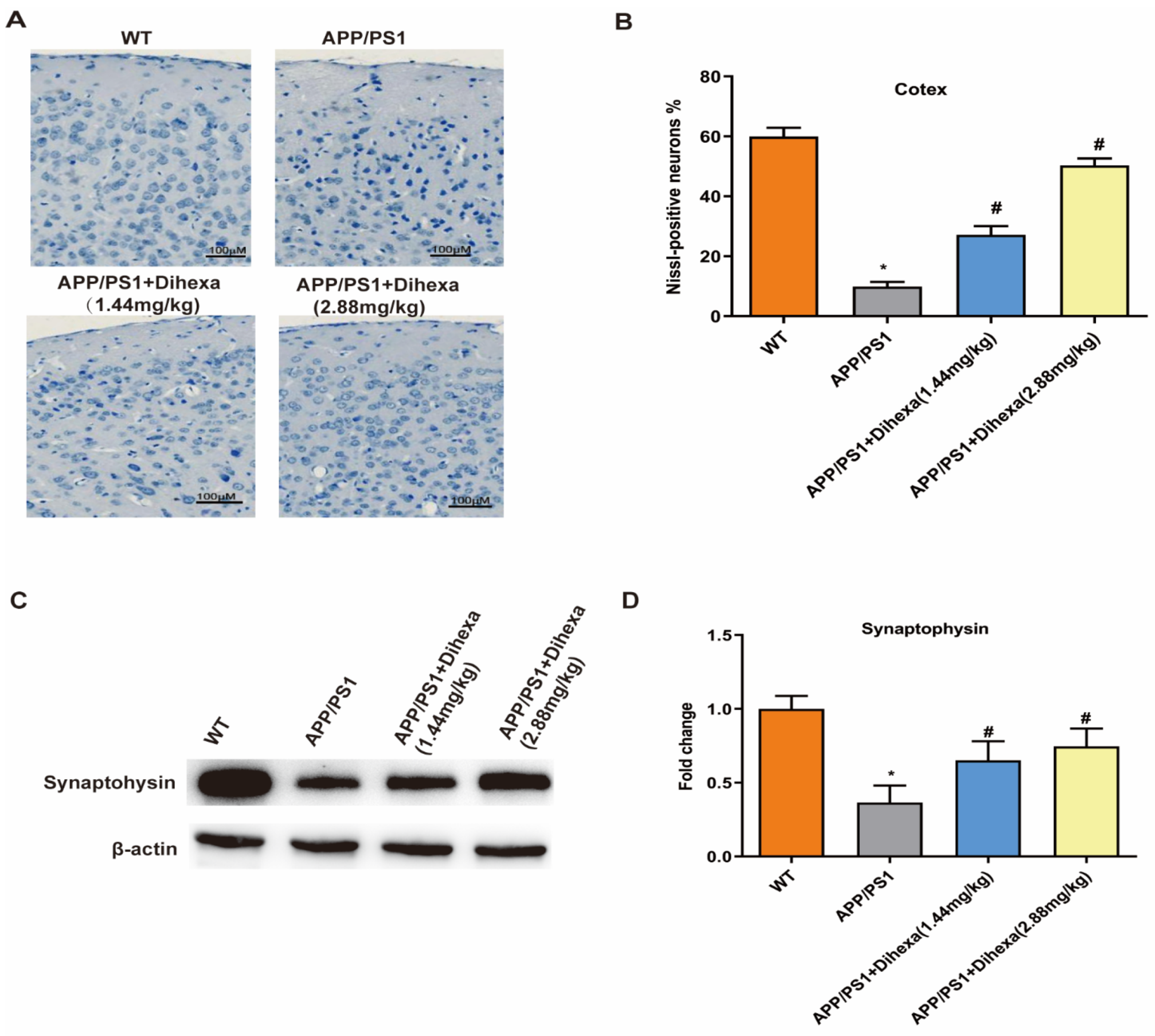
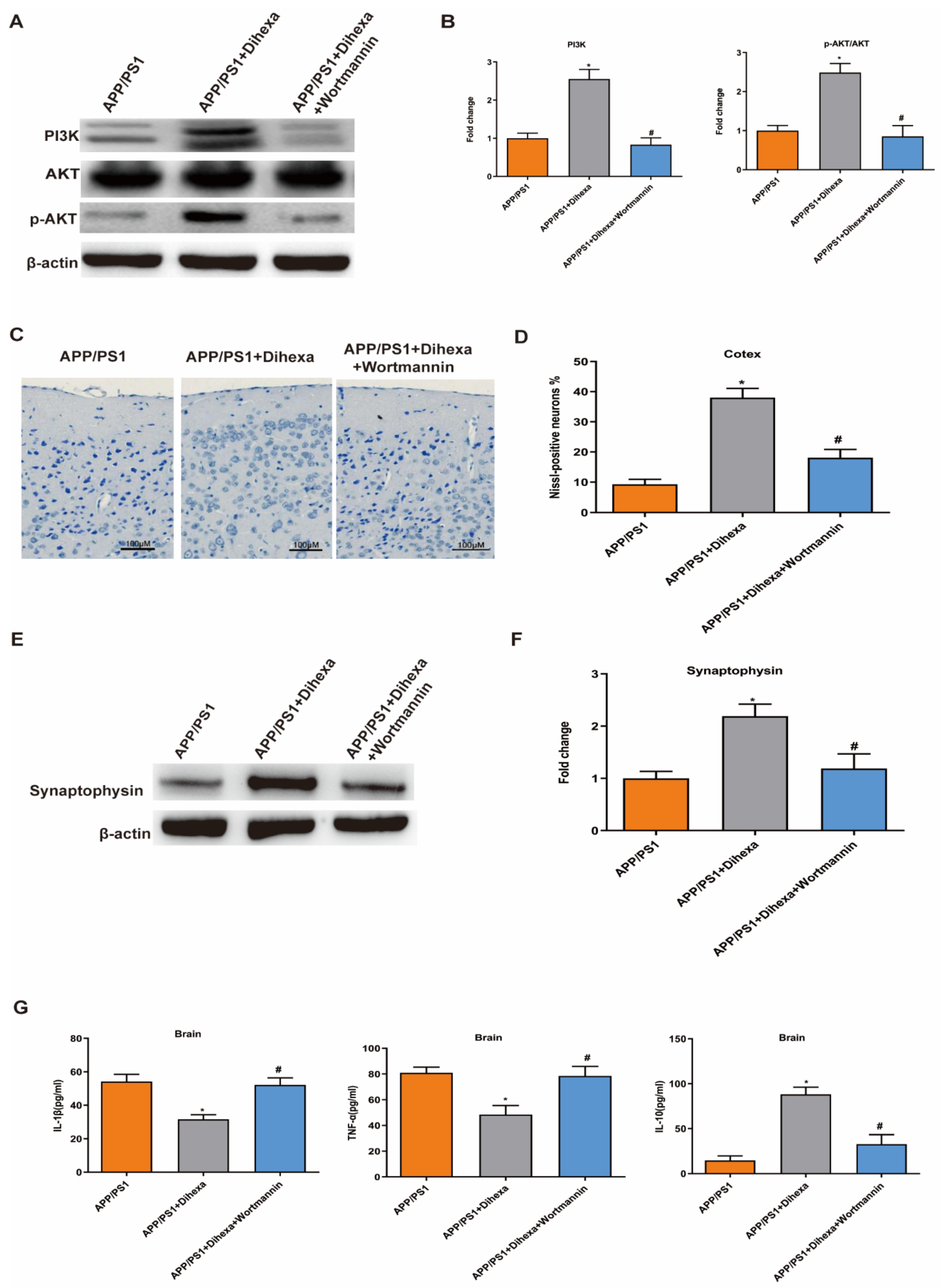
3.3. Dihexa Ameliorated Neuronal Loss in the Brains of APP/PS1 Mice
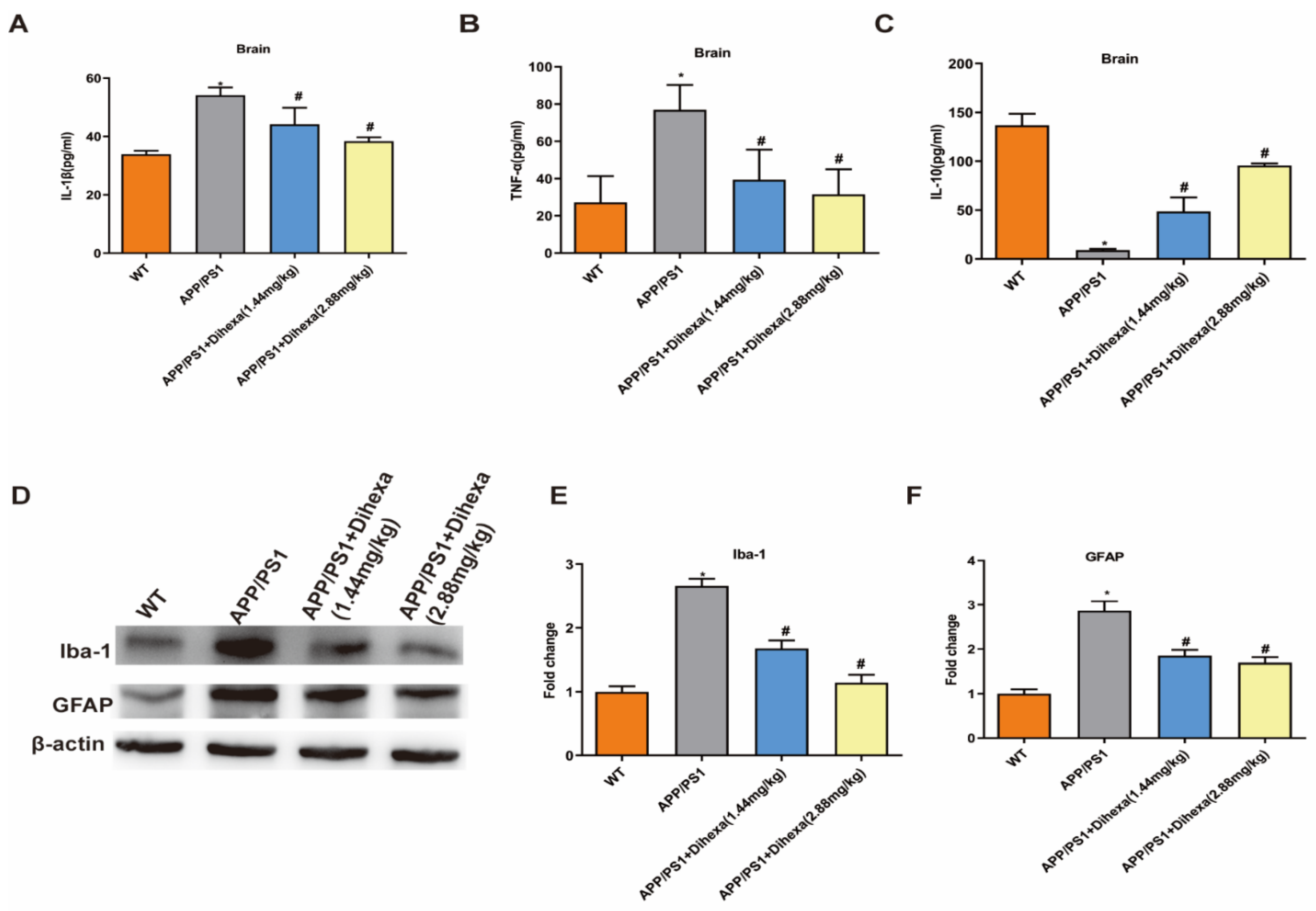
3.4. Dihexa Attenuated Neuroinflammation and Inhibited Glial Activation in the Brains of APP/PS1 Mice
3.5. Dihexa Activated the PI3K/AKT Signaling Pathway in the Brains of APP/PS1 Mice
3.6. PI3K Inhibitor Reversed the Effect of Dihexa in the Brains of APP/PS1 Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soria Lopez, J.A.; González, H.M.; Léger, G.C. Alzheimer’s disease. Handb. Clin. Neurol. 2019, 167, 231–255. [Google Scholar]
- Chakravorty, A.; Jetto, C.T.; Manjithaya, R. Dysfunctional Mitochondria and Mitophagy as Drivers of Alzheimer’s Disease Pathogenesis. Front. Aging Neurosci. 2019, 11, 311. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, F.; Ma, X.; Perry, G.; Zhu, X. Mitochondria dysfunction in the pathogenesis of Alzheimer’s disease: Recent advances. Mol. Neurodegener. 2020, 15, 30. [Google Scholar] [CrossRef] [PubMed]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Molina-Van den Bosch, M.; Jacobs-Cachá, C.; Vergara, A.; Serón, D.; Soler, M.J. The renin-angiotensin system and the brain. Hipertens. Y Riesgo Vasc. 2021, 38, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Bakhle, Y.S. How ACE inhibitors transformed the renin-angiotensin system. Br. J. Pharmacol. 2020, 177, 2657–2665. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.J.; Arnold, A.C. The renin-angiotensin system in cardiovascular autonomic control: Recent developments and clinical implications. Clin. Auton. Res. 2019, 29, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Royea, J.; Martinot, P.; Hamel, E. Memory and cerebrovascular deficits recovered following angiotensin IV intervention in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 2020, 134, 104644. [Google Scholar] [CrossRef] [PubMed]
- Royea, J.; Hamel, E. Brain angiotensin II and angiotensin IV receptors as potential Alzheimer’s disease therapeutic targets. Geroscience 2020, 42, 1237–1256. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Albiston, A.L.; Allen, A.M.; Mendelsohn, F.A.; Ping, S.E.; Barrett, G.L.; Murphy, M.; Morris, M.J.; McDowall, S.G.; Chai, S.Y. Effect of I.C.V. injection of AT4 receptor ligands, NLE1-angiotensin IV and LVV-hemorphin 7, on spatial learning in rats. Neuroscience 2004, 124, 341–349. [Google Scholar] [CrossRef]
- Gard, P.R. Cognitive-enhancing effects of angiotensin IV. BMC Neurosci. 2008, 9 (Suppl. S2), S15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paris, J.J.; Eans, S.O.; Mizrachi, E.; Reilley, K.J.; Ganno, M.L.; McLaughlin, J.P. Central administration of angiotensin IV rapidly enhances novel object recognition among mice. Neuropharmacology 2013, 70, 247–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benoist, C.C.; Wright, J.W.; Zhu, M.; Appleyard, S.M.; Wayman, G.A.; Harding, J.W. Facilitation of hippocampal synaptogenesis and spatial memory by C-terminal truncated Nle1-angiotensin IV analogs. J. Pharmacol. Exp. Ther. 2011, 339, 35–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCoy, A.T.; Benoist, C.C.; Wright, J.W.; Kawas, L.H.; Bule-Ghogare, J.M.; Zhu, M.; Appleyard, S.M.; Wayman, G.A.; Harding, J.W. Evaluation of metabolically stabilized angiotensin IV analogs as procognitive/antidementia agents. J. Pharmacol. Exp. Ther. 2013, 344, 141–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, M.S.; Chen, J. PLD regulates myoblast differentiation through the mTOR-IGF2 pathway. J. Cell Sci. 2008, 121, 282–289. [Google Scholar] [CrossRef] [Green Version]
- Cui, W.; Wang, S.; Wang, Z.; Wang, Z.; Sun, C.; Zhang, Y. Inhibition of PTEN Attenuates Endoplasmic Reticulum Stress and Apoptosis via Activation of PI3K/AKT Pathway in Alzheimer’s Disease. Neurochem. Res. 2017, 42, 3052–3060. [Google Scholar] [CrossRef]
- Kong, J.; Zhang, K.; Meng, X.; Zhang, Y.; Zhang, C. Dose-Dependent Bidirectional Effect of Angiotensin IV on Abdominal Aortic Aneurysm via Variable Angiotensin Receptor Stimulation. Hypertension 2015, 66, 617–626. [Google Scholar] [CrossRef] [Green Version]
- Haugabook, S.J.; Le, T.; Yager, D.; Zenk, B.; Healy, B.M.; Eckman, E.A.; Prada, C.; Younkin, L.; Murphy, P.; Pinnix, I.; et al. Reduction of Abeta accumulation in the Tg2576 animal model of Alzheimer’s disease after oral administration of the phosphatidyl-inositol kinase inhibitor wortmannin. FASEB J. 2001, 15, 16–18. [Google Scholar] [CrossRef]
- Park, B.M.; Cha, S.A.; Lee, S.H.; Kim, S.H. Angiotensin IV protects cardiac reperfusion injury by inhibiting apoptosis and inflammation via AT4R in rats. Peptides 2016, 79, 66–74. [Google Scholar] [CrossRef]
- Xu, F.; Ma, R.; Zhang, G.; Wang, S.; Yin, J.; Wang, E.; Xiong, E.; Zhang, Q.; Li, Y. Estrogen and propofol combination therapy inhibits endoplasmic reticulum stress and remarkably attenuates cerebral ischemia-reperfusion injury and OGD injury in hippocampus. Biomed. Pharmacother. 2018, 108, 1596–1606. [Google Scholar] [CrossRef]
- Duan, R.; Xue, X.; Zhang, Q.Q.; Wang, S.Y.; Gong, P.Y.; Yan, E.; Jiang, T.; Zhang, Y.D. ACE2 activator diminazene aceturate ameliorates Alzheimer’s disease-like neuropathology and rescues cognitive impairment in SAMP8 mice. Aging (Albany NY) 2020, 12, 14819–14829. [Google Scholar] [CrossRef]
- Sun, X.J.; Zhang, P.; Li, H.H.; Jiang, Z.W.; Jiang, C.C.; Liu, H. Cisplatin combined with metformin inhibits migration and invasion of human nasopharyngeal carcinoma cells by regulating E-cadherin and MMP-9. Asian Pac. J. Cancer Prev. 2014, 15, 4019–4023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-González, I.; Schlüter, A.; Aso, E.; Garcia-Esparcia, P.; Ansoleaga, B.; LLorens, F.; Carmona, M.; Moreno, J.; Fuso, A.; Portero-Otin, M.; et al. Neuroinflammatory signals in Alzheimer disease and APP/PS1 transgenic mice: Correlations with plaques, tangles, and oligomeric species. J. Neuropathol. Exp. Neurol. 2015, 74, 319–344. [Google Scholar] [CrossRef] [Green Version]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef]
- Golding, B.J.; Overall, A.D.; Brown, G.; Gard, P.R. Strain differences in the effects of angiotensin IV on mouse cognition. Eur. J. Pharmacol. 2010, 641, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Bromley-Brits, K.; Deng, Y.; Song, W. Morris water maze test for learning and memory deficits in Alzheimer’s disease model mice. J. Vis. Exp. 2011, 53, 2920. [Google Scholar] [CrossRef] [PubMed]
- Swaab, D.F.; Bao, A.M. (Re-)activation of neurons in aging and dementia: Lessons from the hypothalamus. Exp. Gerontol. 2011, 46, 178–184. [Google Scholar] [CrossRef]
- Xu, X.; An, L.; Mi, X.; Zhang, T. Impairment of cognitive function and synaptic plasticity associated with alteration of information flow in theta and gamma oscillations in melamine-treated rats. PLoS ONE 2013, 8, e77796. [Google Scholar]
- Head, E.; Corrada, M.M.; Kahle-Wrobleski, K.; Kim, R.C.; Sarsoza, F.; Goodus, M.; Kawas, C.H. Synaptic proteins, neuropathology and cognitive status in the oldest-old. Neurobiol. Aging 2009, 30, 1125–1134. [Google Scholar] [CrossRef] [Green Version]
- Raz, L.; Knoefel, J.; Bhaskar, K. The neuropathology and cerebrovascular mechanisms of dementia. J. Cereb. Blood Flow Metab. 2016, 36, 172–186. [Google Scholar] [CrossRef] [Green Version]
- Kolos, Y.A.; Grigoriyev, I.P.; Korzhevskyi, D.E. A synaptic marker synaptophysin. Morfologiia 2015, 147, 78–82. [Google Scholar]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, J.A.; Arvanitakis, Z.; Leurgans, S.E.; Bennett, D.A. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann. Neurol. 2009, 66, 200–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, F.; Bai, F.; Zhang, Z. Inflammatory Cytokines and Alzheimer’s Disease: A Review from the Perspective of Genetic Polymorphisms. Neurosci. Bull. 2016, 32, 469–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, Q.; Alam, M.Z.; Mushtaq, G.; Damanhouri, G.A.; Rasool, M.; Kamal, M.A.; Haque, A. Inflammatory Process in Alzheimer’s and Parkinson’s Diseases: Central Role of Cytokines. Curr. Pharm. Des. 2016, 22, 541–548. [Google Scholar] [CrossRef]
- Wang, Y.; Che, M.; Xin, J.; Zheng, Z.; Li, J.; Zhang, S. The role of IL-1β and TNF-α in intervertebral disc degeneration. Biomed. Pharmacother. 2020, 131, 110660. [Google Scholar] [CrossRef]
- Worthen, R.J.; Garzon Zighelboim, S.S.; Torres Jaramillo, C.S.; Beurel, E. Anti-inflammatory IL-10 administration rescues depression-associated learning and memory deficits in mice. J. Neuroinflamm. 2020, 17, 246. [Google Scholar] [CrossRef]
- Karve, I.P.; Taylor, J.M.; Crack, P.J. The contribution of astrocytes and microglia to traumatic brain injury. Br. J. Pharmacol. 2016, 173, 692–702. [Google Scholar] [CrossRef] [Green Version]
- Kong, J.; Ren, G.; Jia, N.; Wang, Y.; Zhang, H.; Zhang, W.; Chen, B.; Cao, Y. Effects of nicorandil in neuroprotective activation of PI3K/AKT pathways in a cellular model of Alzheimer’s disease. Eur. Neurol. 2013, 70, 233–241. [Google Scholar] [CrossRef]
- Sancheti, H.; Akopian, G.; Yin, F.; Brinton, R.D.; Walsh, J.P.; Cadenas, E. Age-dependent modulation of synaptic plasticity and insulin mimetic effect of lipoic acid on a mouse model of Alzheimer’s disease. PLoS ONE 2013, 8, e69830. [Google Scholar] [CrossRef]
- Mattson, M.P. Apoptosis in neurodegenerative disorders. Nat. Rev. Mol. Cell Biol. 2000, 1, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.K.; Zhang, H.B.; Shi, S.S.; Liang, R.S.; Wang, C.H.; Chen, C.M.; Yang, W.Z. 5-LOX Inhibitor Zileuton Reduces Inflammatory Reaction and Ischemic Brain Damage Through the Activation of PI3K/Akt Signaling Pathway. Neurochem. Res. 2016, 41, 2779–2787. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.R.; Li, B.; Wang, M.G.; Meng, F.G.; Yu, J.J.; Guo, F.; Li, Y. Activation of the PI3K-Akt pathway promotes neuroprotection of the δ-opioid receptor agonist against cerebral ischemia-reperfusion injury in rat models. Biomed. Pharmacother. 2017, 93, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Chen, J.; Gao, J. Nanocarriers as a powerful vehicle to overcome blood–brain barrier in treating neurodegenerative diseases: Focus on recent advances. Asian J. Pharm Sci. 2019, 14, 480–496. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Deng, Y.; Fu, X.; Wang, S.; Duan, R.; Zhang, Y. AngIV-Analog Dihexa Rescues Cognitive Impairment and Recovers Memory in the APP/PS1 Mouse via the PI3K/AKT Signaling Pathway. Brain Sci. 2021, 11, 1487. https://doi.org/10.3390/brainsci11111487
Sun X, Deng Y, Fu X, Wang S, Duan R, Zhang Y. AngIV-Analog Dihexa Rescues Cognitive Impairment and Recovers Memory in the APP/PS1 Mouse via the PI3K/AKT Signaling Pathway. Brain Sciences. 2021; 11(11):1487. https://doi.org/10.3390/brainsci11111487
Chicago/Turabian StyleSun, Xiaojin, Yang Deng, Xinxin Fu, Siyu Wang, Rui Duan, and Yingdong Zhang. 2021. "AngIV-Analog Dihexa Rescues Cognitive Impairment and Recovers Memory in the APP/PS1 Mouse via the PI3K/AKT Signaling Pathway" Brain Sciences 11, no. 11: 1487. https://doi.org/10.3390/brainsci11111487




