A Novel Thromboplastin-Based Rat Model of Ischemic Stroke
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
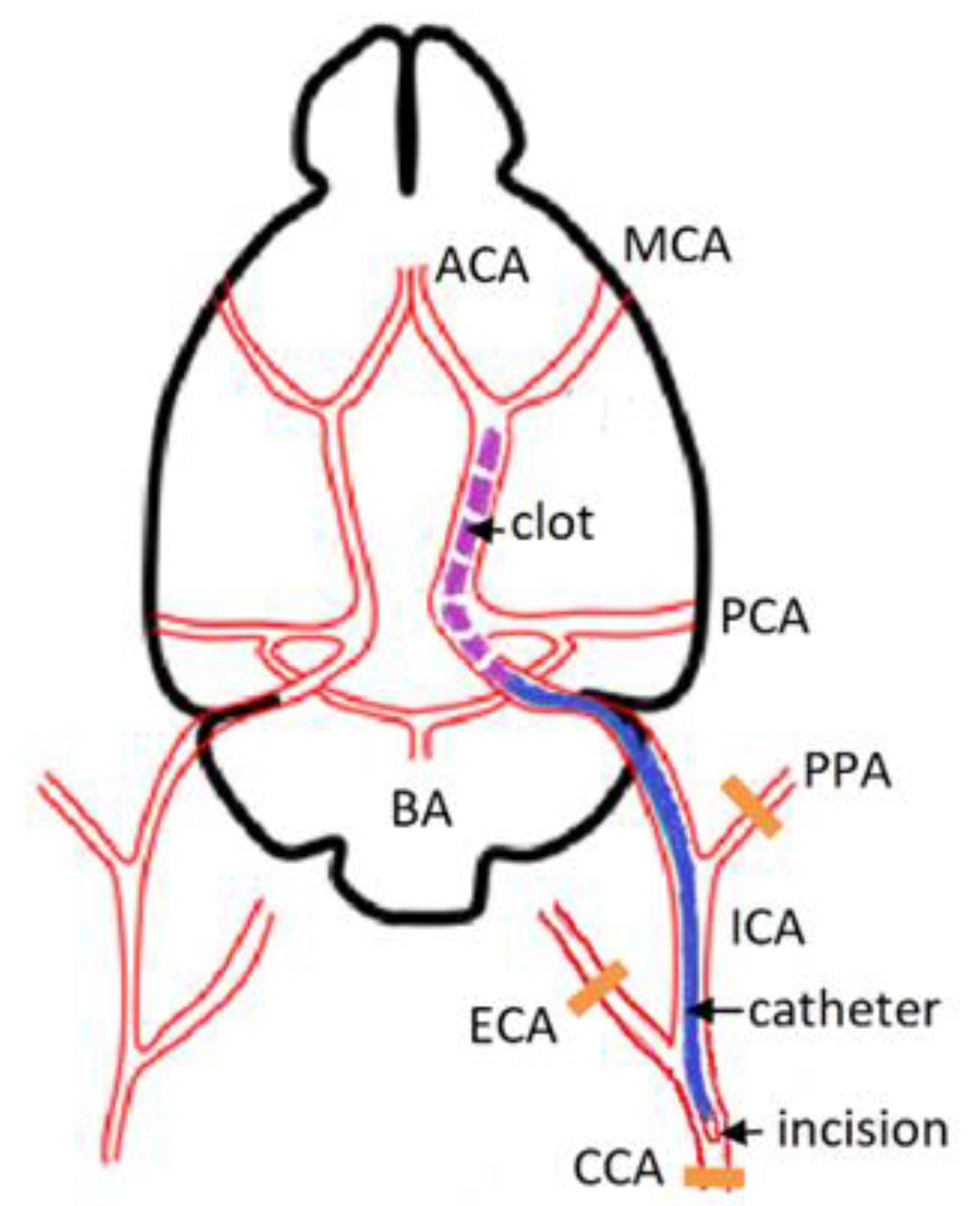
2.2. Animal Model
2.3. Physiological Measurements
2.4. Neurological Deficit Assessment
2.5. Histological Evaluation
2.6. Statistical Analysis
3. Results
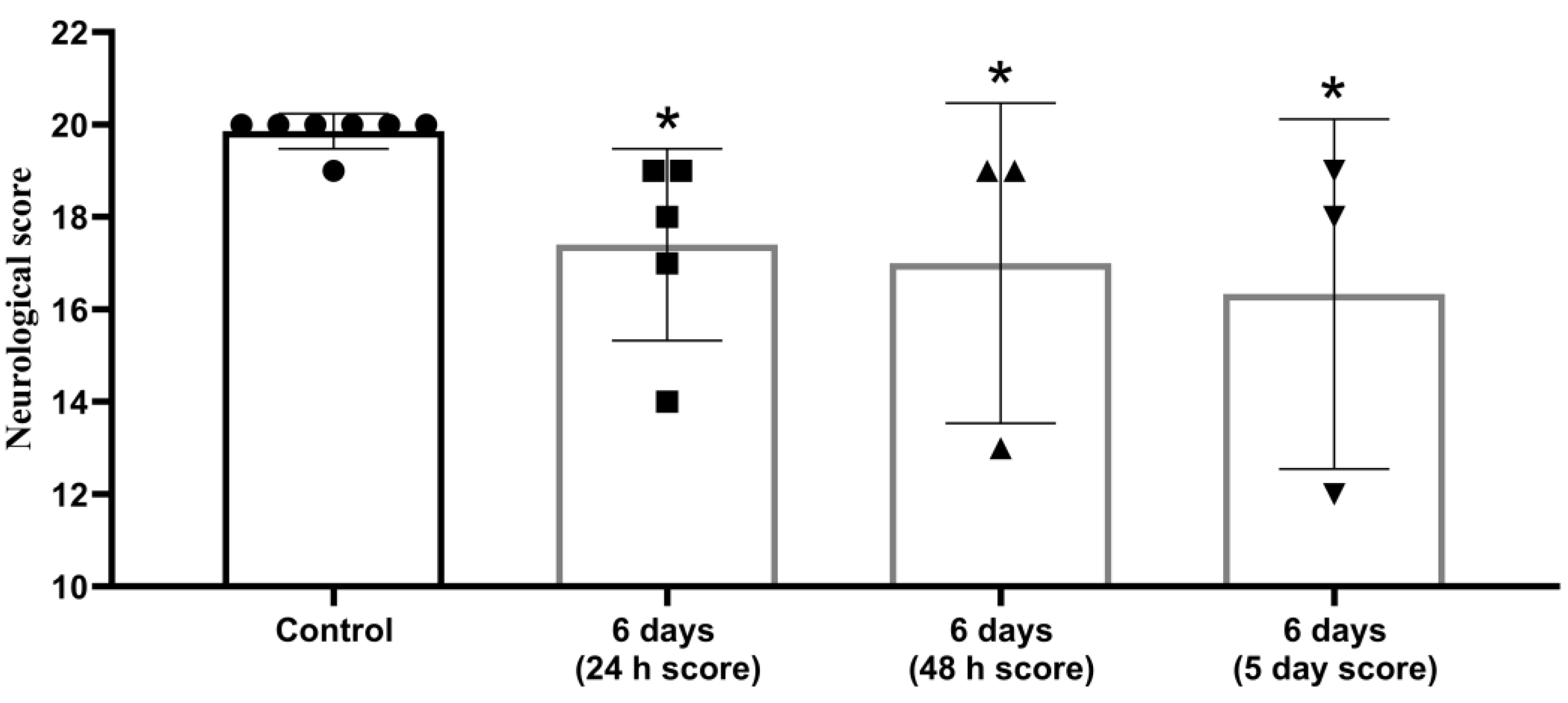
3.1. Neurological Status Assessment
3.2. Histology
4. Discussion
- 1.
- Despite the formation of a blood clot in vitro, the new thromboplastin-calcium model of ischemic stroke in rats is more feasible than similar models, better reflects the pathogenesis of the atherothrombotic ischemic stroke in humans, and simulates thrombogenesis, starting from the first stages of the external pathway of the coagulation cascade.
- 2.
- The high clinical relevance of the novel experimental model is based on taking into account the pathogenesis of perioperative ischemic stroke, which is a major clinical issue, especially in cardiovascular surgery [27].
- 1.
- Using thromboplastin instead of thrombin to trigger thrombus formation, which increases the relevance of our model due to greater similarity between the mechanisms of onset and development of ischemic stroke in the rat and in humans.
- 2.
- The use of autologous blood clots, which is more relevant than the use of allogeneic material.
- 3.
- Reduced cost of the experiment by eliminating the need for expensive equipment, in particular the operating microscope.
- 4.
- Obtaining significant structural and functional changes in the brain.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fluri, F.; Schuhmann, M.K.; Kleinschnitz, C. Animal models of ischemic stroke and their application in clinical research. Drug Des. Dev. Ther. 2015, 20, 3445–3454. [Google Scholar]
- Chuang, B.T.C.; Liu, X.; Lundberg, A.J.; Toung, T.J.K.; Ulatowski, J.A.; Koehler, R.C. Refinement of embolic stroke model in rats: Effect of post-embolization anesthesia duration on arterial blood pressure, cerebral edema and mortality. J. Neurosci. Methods 2018, 307, 8–13. [Google Scholar] [CrossRef]
- Sommer, C.J. Ischemic stroke: Experimental models and reality. Acta Neuropathol. 2017, 133, 245–261. [Google Scholar] [CrossRef] [Green Version]
- Braeuninger, S.; Kleinschnitz, C. Rodent models of focal cerebral ischemia: Procedural pitfalls and translational problems. Exp. Transl. Stroke Med. 2009, 1, 8. [Google Scholar] [CrossRef] [Green Version]
- Gafarova, M.E.; Naumova, G.M.; Gulyaev, M.V.; Koshelev, V.B.; Sokolova, I.A.; Domashenko, M.A. Erythrocyte (dis)aggregation in stroke model in rats. Reg. Blood Circ. Microcirc. 2015, 14, 63–69. (In Russian) [Google Scholar] [CrossRef]
- Kudo, M.; Aoyama, A.; Ichimori, S.; Fukunaga, N. An animal model of cerebral infarction. Homologous blood clot emboli in rats. Stroke 1982, 13, 505–508. [Google Scholar] [CrossRef] [Green Version]
- Niessen, F.; Hilger, T.; Hoehn, M.; Hossmann, K.A. Differences in clot preparation determine outcome of recombinant tissue plasminogen activator treatment in experimental thromboembolic stroke. Stroke 2003, 34, 2019–2024. [Google Scholar] [CrossRef] [Green Version]
- Busch, E.; Krüger, R.; Hossmann, K.A. Improved model of thromboembolic stroke and rt-PA induced reperfusion in the rat. Brain Res. 1997, 778, 16–24. [Google Scholar] [CrossRef]
- Orset, C.; Macrez, R.; Young, A.R.; Panthou, D.; Angles-Cano, E.; Maubert, E.; Agin, V.; Vivien, D. Mouse model of in situ thromboembolic stroke and reperfusion. Stroke 2007, 38, 2771–2778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arkelius, K.; Vivien, D.; Orset, C.; Ansar, S. Validation of a stroke model in rat compatible with rt-PA-induced thrombolysis: New hope for successful translation to the clinic. Sci. Rep. 2020, 10, 12191. [Google Scholar] [CrossRef] [PubMed]
- Macrae, I.M. Preclinical stroke research—Advantages and disadvantages of the most common rodent models of focal ischaemia. Br. J. Pharmacol. 2011, 164, 1062–1078. [Google Scholar] [CrossRef] [Green Version]
- Ansar, S.; Chatzikonstantinou, E.; Wistuba-Schier, A.; Mirau-Weber, S.; Fatar, M.; Hennerici, M.G.; Meairs, S. Characterization of a New Model of Thromboembolic Stroke in C57 black/6J mice. Transl. Stroke Res. 2014, 5, 526–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Zhang, R.L.; Jiang, Q.; Raman, S.B.; Cantwell, L.; Chopp, M. A new rat model of thrombotic focal cerebral ischemia. J. Cereb. Blood Flow Metab. 1997, 17, 123–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunter, A.J.; Hatcher, J.; Virley, D.; Nelson, P.; Irving, E.; Hadingham, S.J.; Parsons, A.A. Functional assessments in mice and rats after focal stroke. Neuropharmacology 2000, 39, 806–816. [Google Scholar] [CrossRef]
- Ostrova, I.V.; Grebenchikov, O.A.; Golubeva, N.V. Neuroprotective Effect of Lithium Chloride in Rat Model of Cardiac Arrest. Gen. Reanimatol. 2019, 15, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Weiss, C.; Jelkmann, W. Functions of the blood. In Human Physiology; Schmidt, R.F., Thews, G., Eds.; Springer: Berlin/Heidelberg, Germany, 1983. [Google Scholar] [CrossRef]
- Asada, Y.; Yamashita, A.; Sato, Y.; Hatakeyama, K. Pathophysiology of atherothrombosis: Mechanisms of thrombus formation on disrupted atherosclerotic plaques. Pathol. Int. 2020, 70, 309–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grover, S.P.; Mackman, N. Tissue Factor: An Essential Mediator of Hemostasis and Trigger of Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 709–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tatsumi, K.; Mackman, N. Tissue Factor and Atherothrombosis. J. Atheroscler. Thromb. 2015, 22, 543–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, D.S.; Sergeeva, E.G.; Ionova, Z.I.; Gorbach, A.V. Tissue factor and atherothrombosis. Curr. Pharm. Des. 2015, 21, 1152–1157. [Google Scholar] [CrossRef]
- Alexandrov, A.V.; Alagona, P. Stroke and atherothrombosis: An update on the role of antiplatelet therapy. Int. J. Stroke 2008, 3, 175–181. [Google Scholar] [CrossRef]
- Ding, W.Y.; Gupta, D.; Lip, G.Y.H. Atrial fibrillation and the prothrombotic state: Revisiting Virchow’s triad in 2020. Heart 2020, 106, 1463–1468. [Google Scholar] [CrossRef] [PubMed]
- Undas, A. Altered fibrin clot properties and fibrinolysis in patients with atrial fibrillation: Practical implications. Europace 2020, 22, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Beguin, S.; Hemker, H.C. The effect of fibrin clots and clot-bound thrombin on the development of platelet procoagulant activity. Thromb. Haemost. 1995, 74, 962–968. [Google Scholar] [CrossRef] [Green Version]
- Wang-Fischer, Y. Manual of Stroke Models in Rat; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar] [CrossRef]
- Vlisides, P.; Mashour, G.A. Perioperative stroke. Can. J. Anaesth. 2016, 63, 193–204. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Li, W.; Satani, N.; Nghiem, D.M.; Xi, X.; Aronowski, J.; Savitz, S.I. Protective Effects of Autologous Bone Marrow Mononuclear Cells after Administering t-PA in an Embolic Stroke Model. Transl. Stroke Res. 2018, 9, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.L.; Chopp, M.; Zhang, Z.G.; Jian, Q.; Ewing, J.R. A rat model of focal embolic cerebral ischemia. Brain Res. 1997, 766, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J. Animal models of stroke. Anim. Model. Exp. Med. 2021, 4, 204–219. [Google Scholar] [CrossRef] [PubMed]


| Group | Weight, g | pH | РaСО2, mm Hg | РaО2, mm Hg | НСО3 | SO2 | Lactate | HR | RR | Rectal Temperature, °С |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 321 ± 18 | 7.34 ± 0.01 | 43.5 ± 1.1 | 64.5 ± 4.5 | 23.6 ± 0.2 | 90.2 ± 1.8 | 1.1 ± 0.4 | 374 ± 27 | 86 ± 9 | 35.2 ± 0.5 |
| 6 h | 339 ± 0.7 | 7.32 ± 0.01 | 40.3 ± 3.2 | 57.6 ± 3.3 | 20.7 ± 1.2 | 87.3 ± 1.8 | 1.4 ± 0.1 | 380 ± 20 | 108 ± 11 | 35.1 ± 0.0 |
| 24 h | 357 ± 21 | 7.32 ± 0.01 | 40.1 ± 0.8 | 66.0 ± 6.8 | 20.8 ± 0.8 | 89.5 ± 3.3 | 0.9 ± 0.1 | 367 ± 28 | 82.7 ± 6 | 34.8 ± 0.1 |
| 72 h | 324 ± 6.8 | 7.31 ± 0.01 | 42.4 ± 2.9 | 67.6 ± 4.8 | 21.1 ± 1.6 | 90.2 ± 1.9 | 1.0 ± 0.2 | 366 ± 23 | 78.8 ± 6 | 34.7 ± 0.4 |
| 6 days | 329 ± 22 | 7.31 ± 0.01 | 42.6 ± 4.9 | 58.6 ± 7.4 | 21.9 ± 2.9 | 86 ± 5.0 | 0.9 ± 0.4 | 409 ± 18 | 86.2 ± 8 | 34.8 ± 0.3 |
| Hippocampal Field | Control | 6 h | 24 h | 72 h | 6 days |
|---|---|---|---|---|---|
| CA1 | 206.5 (199.1; 209.5) | 202.5 (97.3; 216.8) | 171.9 * (133.1; 188.5) | 152.5 * (64.9; 178.7) | 81.5 * (13.0; 124.6) |
| CA4 | 118.4 (118.4; 119.5) | 103.5 * (87.5; 110.5) | 83,3 * (71.7; 103.5) | 107.4 # (63.4; 123.2) | 94.9 * (84.8; 98.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostrova, I.V.; Kalabushev, S.N.; Ryzhkov, I.A.; Tsokolaeva, Z.I. A Novel Thromboplastin-Based Rat Model of Ischemic Stroke. Brain Sci. 2021, 11, 1475. https://doi.org/10.3390/brainsci11111475
Ostrova IV, Kalabushev SN, Ryzhkov IA, Tsokolaeva ZI. A Novel Thromboplastin-Based Rat Model of Ischemic Stroke. Brain Sciences. 2021; 11(11):1475. https://doi.org/10.3390/brainsci11111475
Chicago/Turabian StyleOstrova, Irina V., Sergei N. Kalabushev, Ivan A. Ryzhkov, and Zoya I. Tsokolaeva. 2021. "A Novel Thromboplastin-Based Rat Model of Ischemic Stroke" Brain Sciences 11, no. 11: 1475. https://doi.org/10.3390/brainsci11111475
APA StyleOstrova, I. V., Kalabushev, S. N., Ryzhkov, I. A., & Tsokolaeva, Z. I. (2021). A Novel Thromboplastin-Based Rat Model of Ischemic Stroke. Brain Sciences, 11(11), 1475. https://doi.org/10.3390/brainsci11111475







