Visual Short-Term Memory for Coherent and Sequential Motion: A rTMS Investigation
Abstract
1. Introduction
2. Experiment 1
2.1. Methods
2.1.1. Participants
2.1.2. Apparatus
2.1.3. Stimuli
2.1.4. Repetitive TMS
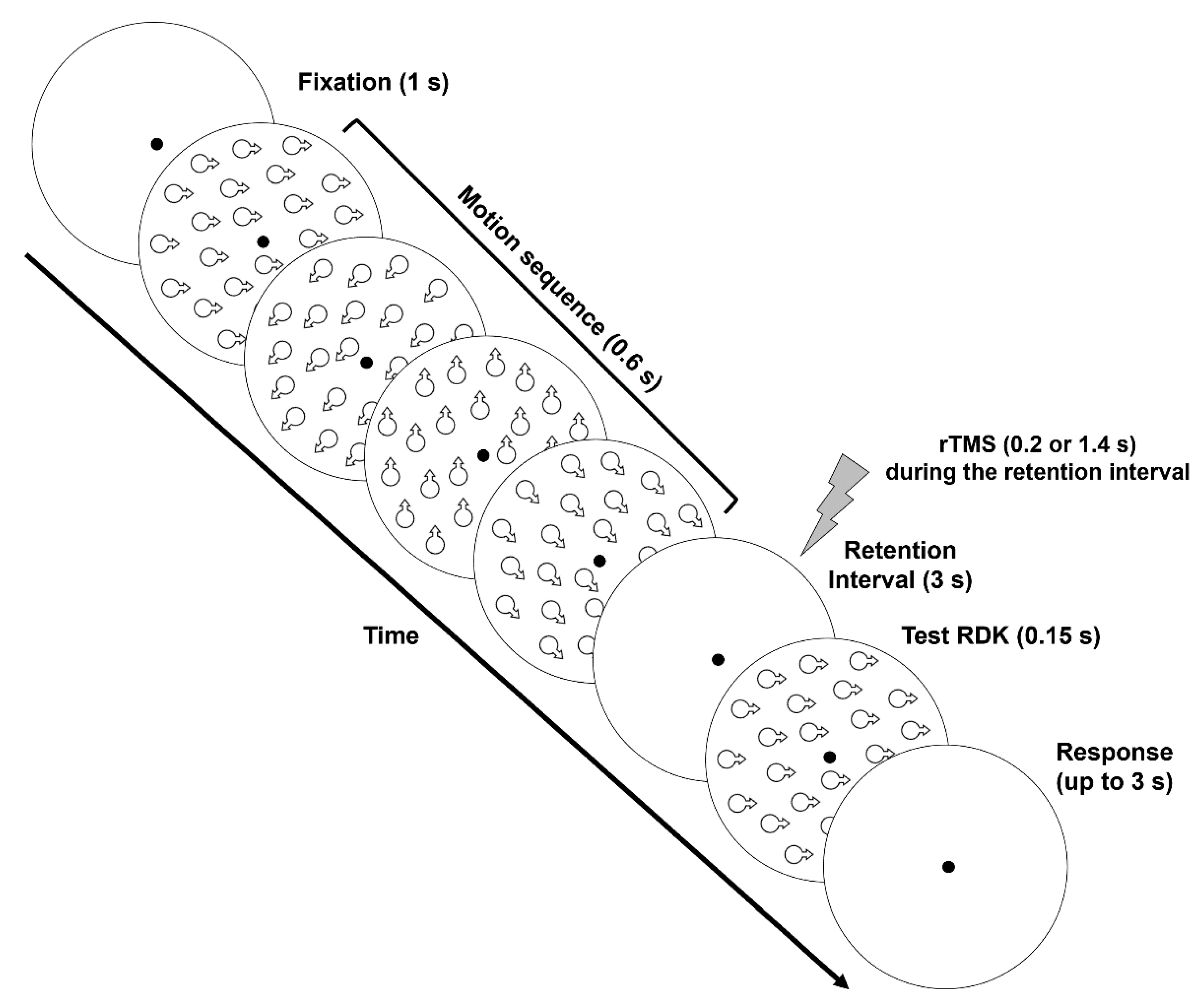
2.1.5. Procedure
2.1.6. Data Analysis
2.2. Results
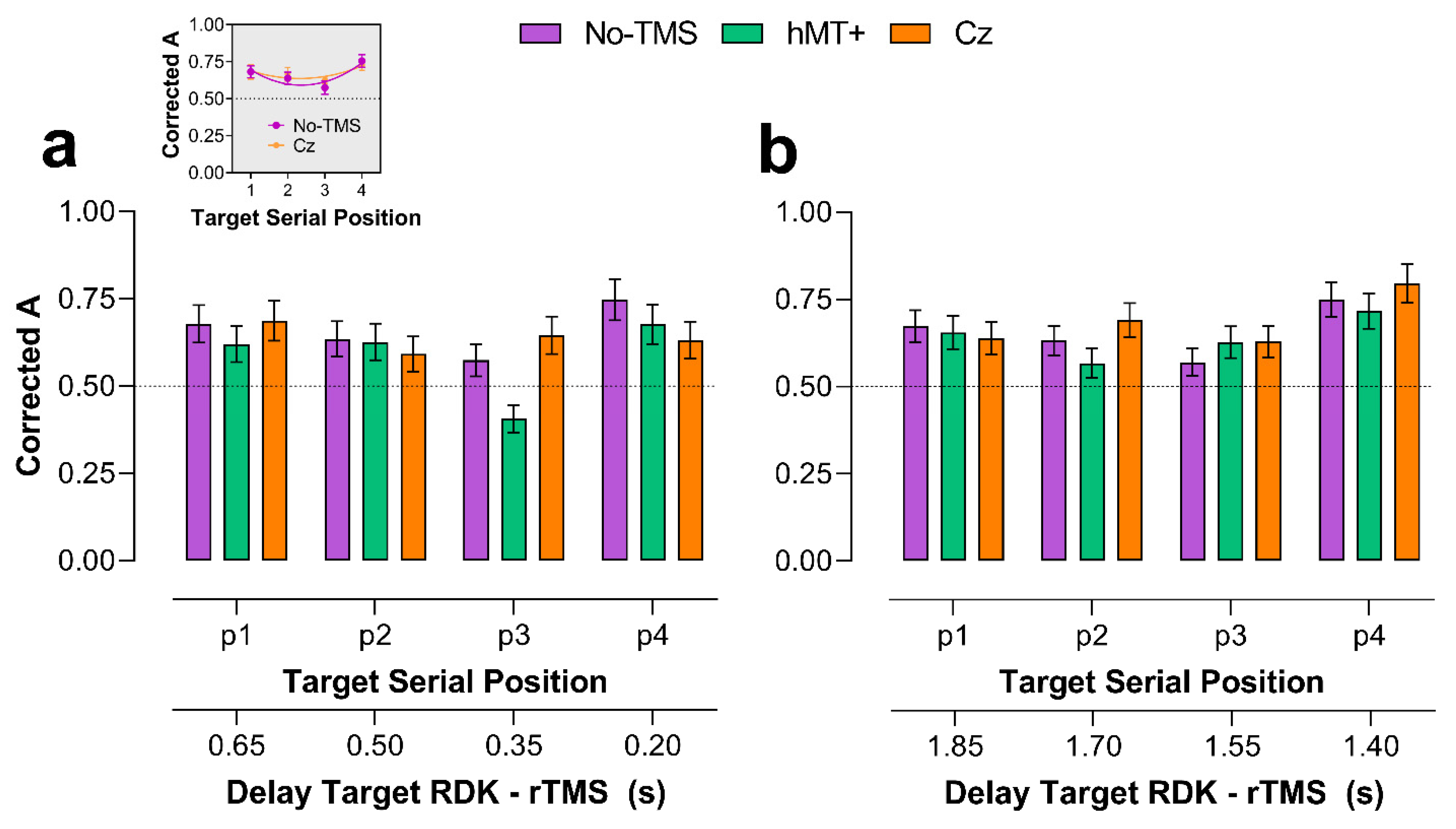
2.2.1. Main VSTM Experiment: Sensitivity (A)
2.2.2. Early rTMS
2.2.3. Late rTMS
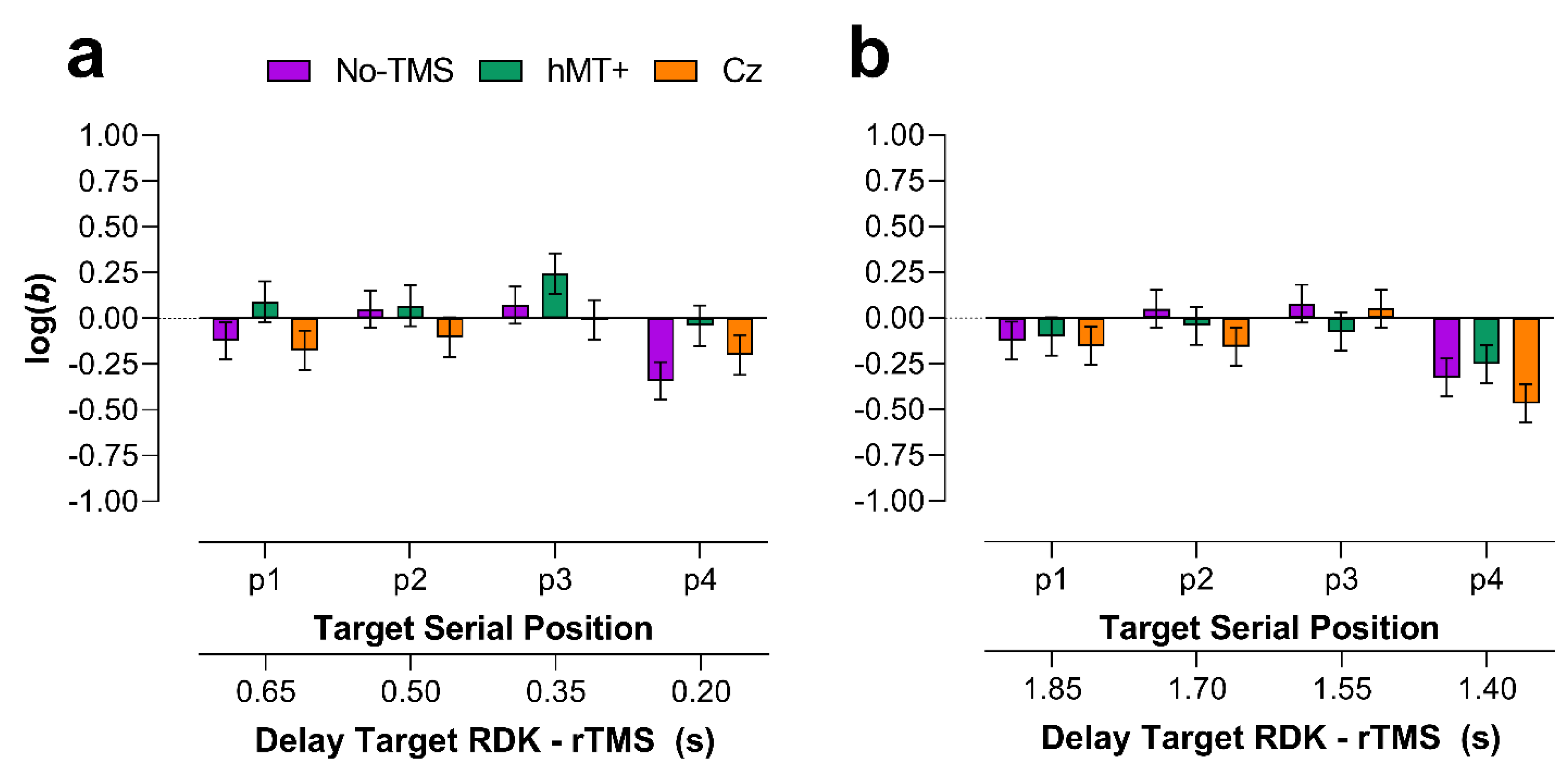
2.2.4. Main VSTM Experiment: Bias
Early rTMS
Late rTMS
2.3. Discussion
3. Experiment 2
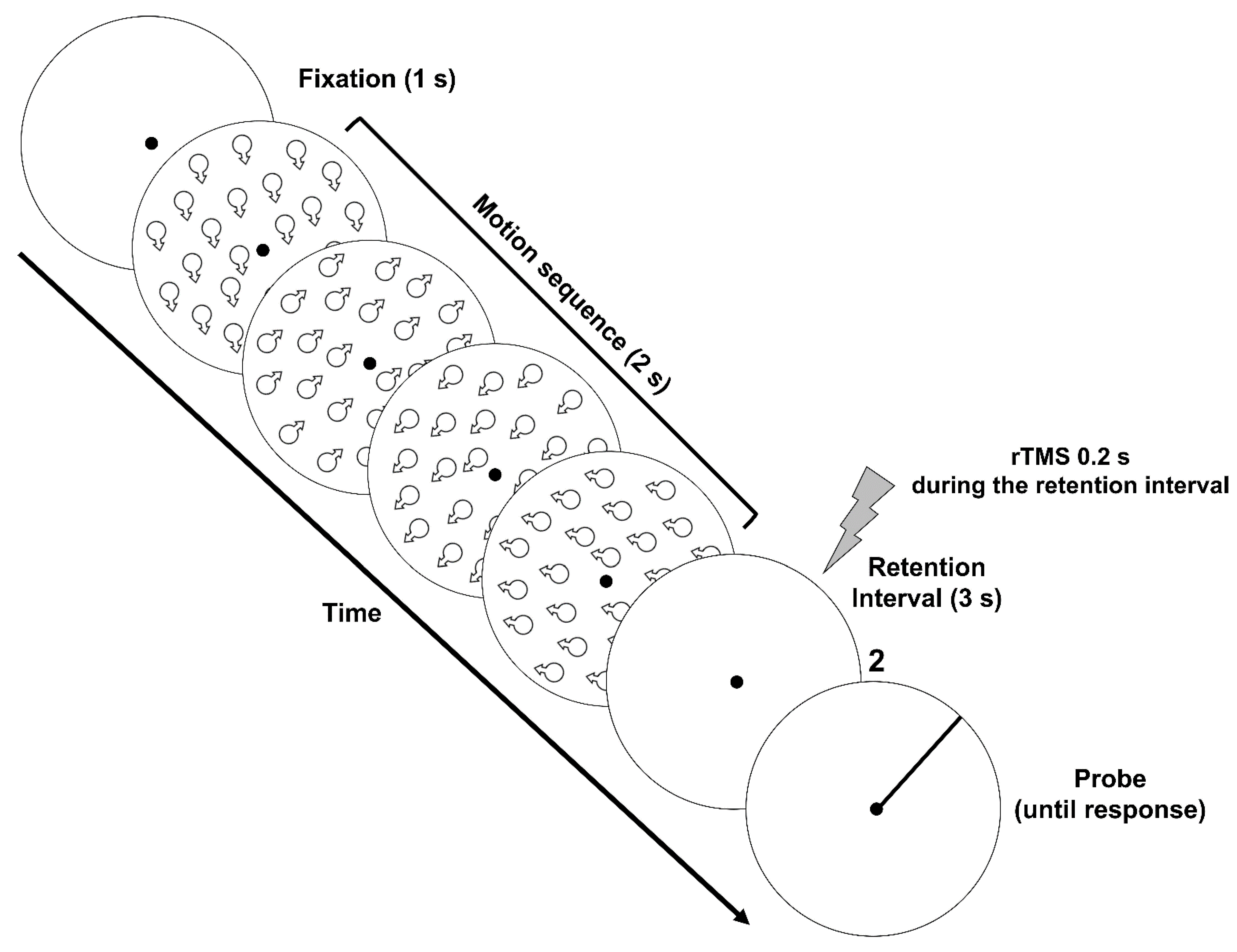
3.1. Methods
3.2. Precision Calculation
3.3. Results
3.3.1. Precision of VSTM for Motion Direction
3.3.2. Modeling of Visual Short-Term Memory
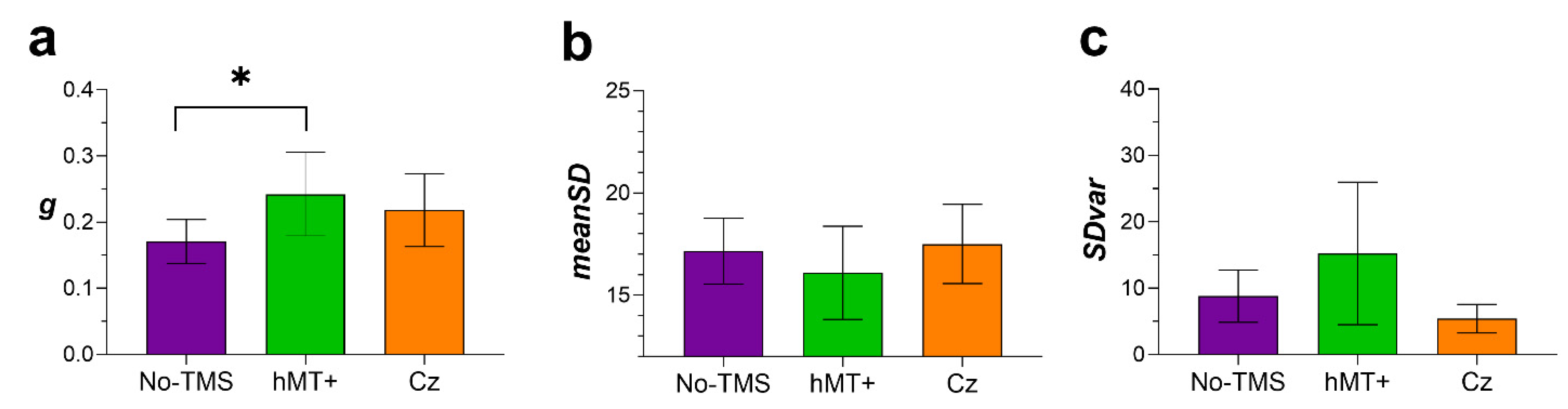
3.3.3. Results of the Variable Precision Model
4. Discussion
5. General Discussion
6. Limitations
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luck, S.J.; Vogel, E.K. The capacity of visual working memory for features and conjunctions. Nature 1997, 390, 279–281. [Google Scholar] [CrossRef]
- Luck, S.J.; Vogel, E.K. Visual working memory capacity: From psychophysics and neurobiology to individual differences. Trends Cogn. Sci. 2013, 17, 391–400. [Google Scholar] [CrossRef]
- van Lamsweerde, A.E.; Johnson, J.S. Assessing the Effect of Early Visual Cortex Transcranial Magnetic Stimulation on Working Memory Consolidation. J. Cogn. Neurosci. 2017, 29, 1226–1238. [Google Scholar] [CrossRef]
- McKeefry, D.J.; Burton, M.P.; Vakrou, C. Speed selectivity in visual short term memory for motion. Vis. Res. 2007, 47, 2418–2425. [Google Scholar] [CrossRef]
- Pashler, H. Familiarity and visual change detection. Percept. Psychophys. 1988, 44, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Vogel, E.K.; Woodman, G.F.; Luck, S.J. Storage of features, conjunctions and objects in visual working memory. J. Exp. Psychol. Hum. Percept. Perform. 2001, 27, 92–114. [Google Scholar] [CrossRef]
- Eng, H.Y.; Chen, D.; Jiang, Y. Visual working memory for simple and complex visual stimuli. Psychon. Bull. Rev. 2005, 12, 1127–1133. [Google Scholar] [CrossRef]
- Pasternak, T.; Zaksas, D. Stimulus specificity and temporal dynamics of working memory for visual motion. J. Neurophysiol. 2003, 90, 2757–2762. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pasternak, T.; Greenlee, M.W. Working memory in primate sensory systems. Nat. Rev. Neurosci. 2005, 6, 97–107. [Google Scholar] [CrossRef]
- Pavan, A.; Langgartner, D.; Greenlee, M.W. Visual short-term memory for global motion revealed by directional and speed-tuned masking. Neuropsychologia 2013, 51, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Campana, G.; Cowey, A.; Walsh, V. Priming of motion direction and area V5/MT: A test of perceptual memory. Cereb. Cortex 2002, 12, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Campana, G.; Cowey, A.; Walsh, V. Visual area V5/MT remembers “what” but not “where”. Cereb Cortex 2006, 16, 1766–1770. [Google Scholar] [CrossRef]
- Sneve, M.H.; Alnaes, D.; Endestad, T.; Greenlee, M.W.; Magnussen, S. Modulation of activity in human visual area V1 during memory masking. PLoS ONE 2011, 6, e18651. [Google Scholar] [CrossRef]
- Blake, R.; Cepeda, N.J.; Hiris, E. Memory for visual motion. J. Exp. Psychol. Hum. Percept. Perform. 1997, 23, 353–369. [Google Scholar] [CrossRef]
- Magnussen, S.; Greenlee, M.W. Retention and disruption of motion information in visual short-term memory. J. Exp. Psychol. Learn. Mem. Cogn. 1992, 18, 151–156. [Google Scholar] [CrossRef]
- Magnussen, S.; Greenlee, M.W.; Thomas, J.P. Parallel processing in visual short-term memory. J. Exp. Psychol. Hum. Percept. Perform. 1996, 22, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Bisley, J.W.; Pasternak, T. The multiple roles of visual cortical areas MT/MST in remembering the direction of visual motion. Cereb. Cortex 2000, 10, 1053–1065. [Google Scholar] [CrossRef] [PubMed]
- Bisley, J.W.; Zaksas, D.; Pasternak, T. Microstimulation of cortical area MT affects performance on a visual working memory task. J. Neurophysiol. 2001, 85, 187–196. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zaksas, D.; Bisley, J.W.; Pasternak, T. Motion information is spatially localized in a visual working-memory task. J. Neurophysiol. 2001, 86, 912–921. [Google Scholar] [CrossRef]
- Magnussen, S.; Greenlee, M.W.; Asplund, R.; Dyrnes, S. Stimulus-specific mechanisms of visual short-term memory. Vis. Res. 1991, 31, 1213–1219. [Google Scholar] [CrossRef]
- Amemiya, T.; Beck, B.; Walsh, V.; Gomi, H.; Haggard, P. Visual area V5/hMT+ contributes to perception of tactile motion direction: A TMS study. Sci. Rep. 2017, 7, 40937. [Google Scholar] [CrossRef]
- Zeki, S. Area V5-a microcosm of the visual brain. Front. Integr. Neurosci. 2015, 9, 21. [Google Scholar] [CrossRef]
- Awh, E.; Vogel, E.K.; Oh, S.H. Interactions between attention and working memory. Neuroscience 2006, 139, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Wilken, P.; Ma, W.J. A detection theory account of change detection. J. Vis. 2004, 4, 1120–1135. [Google Scholar] [CrossRef]
- Kawasaki, M.; Watanabe, M.; Okuda, J.; Sakagami, M.; Aihara, K. Human posterior parietal cortex maintains color, shape and motion in visual short-term memory. Brain Res. 2008, 1213, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Luria, R.; Vogel, E.K. Shape and color conjunction stimuli are represented as bound objects in visual working memory. Neuropsychologia 2011, 49, 1632–1639. [Google Scholar] [CrossRef] [PubMed]
- Bays, P.M.; Catalao, R.F.; Husain, M. The precision of visual working memory is set by allocation of a shared resource. J. Vis. 2009, 9, 7. [Google Scholar] [CrossRef]
- Bays, P.M.; Gorgoraptis, N.; Wee, N.; Marshall, L.; Husain, M. Temporal dynamics of encoding, storage, and reallocation of visual working memory. J. Vis. 2011, 11, 10. [Google Scholar] [CrossRef]
- Bays, P.M.; Husain, M. Dynamic shifts of limited working memory resources in human vision. Science 2008, 321, 851–854. [Google Scholar] [CrossRef] [PubMed]
- Bays, P.M.; Wu, E.Y.; Husain, M. Storage and binding of object features in visual working memory. Neuropsychologia 2011, 49, 1622–1631. [Google Scholar] [CrossRef]
- Zokaei, N.; Gorgoraptis, N.; Bahrami, B.; Bays, P.M.; Husain, M. Precision of working memory for visual motion sequences and transparent motion surfaces. J. Vis. 2011, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Gorgoraptis, N.; Catalao, R.F.; Bays, P.M.; Husain, M. Dynamic updating of working memory resources for visual objects. J. Neurosci. 2011, 31, 8502–8511. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, R.; Shin, H.; Chou, W.C.; George, R.; Ma, W.J. Variability in encoding precision accounts for visual short-term memory limitations. Proc. Natl. Acad. Sci. USA 2012, 109, 8780–8785. [Google Scholar] [CrossRef]
- World Medical, A. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Brainard, D.H. The Psychophysics Toolbox. Spat. Vis. 1997, 10, 433–436. [Google Scholar] [CrossRef]
- Kleiner, M.; Brainard, D.; Pelli, D.; Ingling, A.; Murray, R.; Broussard, C. What’s new in psychtoolbox-3. Perception 2007, 36, 1–16. [Google Scholar]
- Pelli, D.G. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spat. Vis. 1997, 10, 437–442. [Google Scholar] [CrossRef]
- Pavan, A.; Hobaek, M.; Blurton, S.P.; Contillo, A.; Ghin, F.; Greenlee, M.W. Visual short-term memory for coherent motion in video game players: Evidence from a memory-masking paradigm. Sci. Rep. 2019, 9, 6027. [Google Scholar] [CrossRef]
- Morgan, M.J.; Ward, R. Conditions for motion flow in dynamic visual noise. Vis. Res. 1980, 20, 431–435. [Google Scholar] [CrossRef]
- Newsome, W.T.; Pare, E.B. A selective impairment of motion perception following lesions of the middle temporal visual area (MT). J. Neurosci. 1988, 8, 2201–2211. [Google Scholar] [CrossRef] [PubMed]
- Pavan, A.; Boyce, M.; Ghin, F. Action Video Games Improve Direction Discrimination of Parafoveal Translational Global Motion but Not Reaction Times. Perception 2016, 45, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Antal, A.; Kincses, T.Z.; Nitsche, M.A.; Paulus, W. Modulation of moving phosphene thresholds by transcranial direct current stimulation of V1 in human. Neuropsychologia 2003, 41, 1802–1807. [Google Scholar] [CrossRef]
- Stewart, L.; Battelli, L.; Walsh, V.; Cowey, A. Motion perception and perceptual learning studied by magnetic stimulation. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999, 51, 334–350. [Google Scholar] [PubMed]
- Cowey, A.; Campana, G.; Walsh, V.; Vaina, L.M. The role of human extra-striate visual areas V5/MT and V2/V3 in the perception of the direction of global motion: A transcranial magnetic stimulation study. Exp. Brain Res. 2006, 171, 558–562. [Google Scholar] [CrossRef]
- Campana, G.; Maniglia, M.; Pavan, A. Common (and multiple) neural substrates for static and dynamic motion after-effects: A rTMS investigation. Cortex 2013, 49, 2590–2594. [Google Scholar] [CrossRef] [PubMed]
- Laycock, R.; Crewther, D.P.; Fitzgerald, P.B.; Crewther, S.G. Evidence for fast signals and later processing in human V1/V2 and V5/MT+: A TMS study of motion perception. J. Neurophysiol. 2007, 98, 1253–1262. [Google Scholar] [CrossRef]
- Pascual-Leone, A.; Bartres-Faz, D.; Keenan, J.P. Transcranial magnetic stimulation: Studying the brain-behaviour relationship by induction of ’virtual lesions’. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999, 354, 1229–1238. [Google Scholar] [CrossRef]
- Pascual-Leone, A.; Tarazona, F.; Keenan, J.; Tormos, J.M.; Hamilton, R.; Catala, M.D. Transcranial magnetic stimulation and neuroplasticity. Neuropsychologia 1999, 37, 207–217. [Google Scholar] [CrossRef]
- Pavan, A.; Alexander, I.; Campana, G.; Cowey, A. Detection of first- and second-order coherent motion in blindsight. Exp. Brain Res. 2011, 214, 261–271. [Google Scholar] [CrossRef]
- Pavan, A.; Ghin, F.; Donato, R.; Campana, G.; Mather, G. The neural basis of form and form-motion integration from static and dynamic translational Glass patterns: A rTMS investigation. Neuroimage 2017, 157, 555–560. [Google Scholar] [CrossRef]
- Schenk, T.; Ellison, A.; Rice, N.; Milner, A.D. The role of V5/MT+ in the control of catching movements: An rTMS study. Neuropsychologia 2005, 43, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Silvanto, J.; Cowey, A.; Lavie, N.; Walsh, V. Striate cortex (V1) activity gates awareness of motion. Nat. Neurosci. 2005, 8, 143–144. [Google Scholar] [CrossRef]
- Walsh, V.; Ellison, A.; Battelli, L.; Cowey, A. Task-specific impairments and enhancements induced by magnetic stimulation of human visual area V5. Proc. Biol. Sci. 1998, 265, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.; Aaen-Stockdale, C.; Koski, L.; Hess, R.F. A double dissociation between striate and extrastriate visual cortex for pattern motion perception revealed using rTMS. Hum. Brain Mapp. 2009, 30, 3115–3126. [Google Scholar] [CrossRef]
- Abrahamyan, A.; Clifford, C.W.; Ruzzoli, M.; Phillips, D.; Arabzadeh, E.; Harris, J.A. Accurate and rapid estimation of phosphene thresholds (REPT). PLoS ONE 2011, 6, e22342. [Google Scholar] [CrossRef]
- Mather, G.; Battaglini, L.; Campana, G. TMS reveals flexible use of form and motion cues in biological motion perception. Neuropsychologia 2016, 84, 193–197. [Google Scholar] [CrossRef]
- Donato, R.; Pavan, A.; Nucci, M.; Campana, G. The neural mechanisms underlying directional and apparent circular motion assessed with repetitive transcranial magnetic stimulation (rTMS). Neuropsychologia 2020, 149, 107656. [Google Scholar] [CrossRef] [PubMed]
- Stablein, M.; Sieprath, L.; Knochel, C.; Landertinger, A.; Schmied, C.; Ghinea, D.; Mayer, J.S.; Bittner, R.A.; Reif, A.; Oertel-Knochel, V. Impaired working memory for visual motion direction in schizophrenia: Absence of recency effects and association with psychopathology. Neuropsychology 2016, 30, 653–663. [Google Scholar] [CrossRef]
- van de Ven, V.; Jacobs, C.; Sack, A.T. Topographic contribution of early visual cortex to short-term memory consolidation: A transcranial magnetic stimulation study. J. Neurosci. 2012, 32, 4–11. [Google Scholar] [CrossRef]
- Nettekoven, C.; Pieczewski, J.; Neuschmelting, V.; Jonas, K.; Goldbrunner, R.; Grefkes, C.; Weiss Lucas, C. Improving the efficacy and reliability of rTMS language mapping by increasing the stimulation frequency. Hum. Brain Mapp. 2021, 42, 5309–5321. [Google Scholar] [CrossRef]
- Pollack, I.; Norman, D.A. A non-parametric analysis of recognition experiments. Psychon. Sci. 1964, 1, 125–126. [Google Scholar] [CrossRef]
- Smith, W.D. Clarification of Sensitivity Measure A’. J. Math. Psychol. 1995, 39, 82–89. [Google Scholar] [CrossRef]
- Zhang, J.; Mueller, S.T. A note on ROC analysis and non-parametric estimate of sensitivity. Psychometrika 2005, 70, 203–212. [Google Scholar] [CrossRef]
- Stanislaw, H.; Todorov, N. Calculation of signal detection theory measures. Behav. Res. Methods Instrum. Comput. 1999, 31, 137–149. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 12. [Google Scholar] [CrossRef]
- Deese, J.; Kaufman, R.A. Serial effects in recall of unorganized and sequentially organized verbal material. J. Exp. Psychol. 1957, 54, 180–187. [Google Scholar] [CrossRef]
- Murdock, B.B. The serial position effect of free recall. J. Exp. Psychol. 1962, 64, 482–488. [Google Scholar] [CrossRef]
- Larsen, A.; Markussen, B.; Bundesen, C. Comparing exponential race and signal detection models of encoding stimuli into visual short-term memory. J. Exp. Psychol. Hum. Percept. Perform. 2020, 46, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Vogel, E.K. Visual short-term memory capacity predicts the “bandwidth” of visual long-term memory encoding. Mem. Cognit. 2019, 47, 1481–1497. [Google Scholar] [CrossRef] [PubMed]
- Fougnie, D.; Suchow, J.W.; Alvarez, G.A. Variability in the quality of visual working memory. Nat. Commun. 2012, 3, 1229. [Google Scholar] [CrossRef]
- van den Berg, R.; Awh, E.; Ma, W.J. Factorial comparison of working memory models. Psychol. Rev. 2014, 121, 124–149. [Google Scholar] [CrossRef]
- Wobbrock, J.O.; Findlater, L.; Gergle, D.; Higgins, J.J. The Aligned Rank Transform for nonparametric factorial analyses using only ANOVA procedures. In Proceedings of the Conference on Human Factors in Computing Systems, Vancouver, BC, Canada, 7–12 May 2011; pp. 143–146. [Google Scholar]
- Cohen, D.; Goddard, E.; Mullen, K.T. Reevaluating hMT+ and hV4 functional specialization for motion and static contrast using fMRI-guided repetitive transcranial magnetic stimulation. J. Vis. 2019, 19, 11. [Google Scholar] [CrossRef]
- Maunsell, J.H.; van Essen, D.C. The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J. Neurosci. 1983, 3, 2563–2586. [Google Scholar] [CrossRef]
- Maunsell, J.H.; Van Essen, D.C. Functional properties of neurons in middle temporal visual area of the macaque monkey. II. Binocular interactions and sensitivity to binocular disparity. J. Neurophysiol. 1983, 49, 1148–1167. [Google Scholar] [CrossRef]
- Maunsell, J.H.; Van Essen, D.C. Functional properties of neurons in middle temporal visual area of the macaque monkey. I. Selectivity for stimulus direction, speed, and orientation. J. Neurophysiol. 1983, 49, 1127–1147. [Google Scholar] [CrossRef] [PubMed]
- Born, R.T.; Bradley, D.C. Structure and function of visual area MT. Annu. Rev. Neurosci. 2005, 28, 157–189. [Google Scholar] [CrossRef] [PubMed]
- Rust, N.C.; Mante, V.; Simoncelli, E.P.; Movshon, J.A. How MT cells analyze the motion of visual patterns. Nat. Neurosci. 2006, 9, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Galeano Weber, E.M.; Peters, B.; Hahn, T.; Bledowski, C.; Fiebach, C.J. Superior Intraparietal Sulcus Controls the Variability of Visual Working Memory Precision. J. Neurosci. 2016, 36, 5623–5635. [Google Scholar] [CrossRef]
- Zhao, Y.; Kuai, S.; Zanto, T.P.; Ku, Y. Neural Correlates Underlying the Precision of Visual Working Memory. Neuroscience 2020, 425, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Zokaei, N.; Manohar, S.; Husain, M.; Feredoes, E. Causal evidence for a privileged working memory state in early visual cortex. J. Neurosci. 2014, 34, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Rademaker, R.L.; van de Ven, V.G.; Tong, F.; Sack, A.T. The impact of early visual cortex transcranial magnetic stimulation on visual working memory precision and guess rate. PLoS ONE 2017, 12, e0175230. [Google Scholar] [CrossRef]
- Zhang, W.; Luck, S.J. Discrete fixed-resolution representations in visual working memory. Nature 2008, 453, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Dumoulin, S.O.; Bittar, R.G.; Kabani, N.J.; Baker, C.L., Jr.; Le Goualher, G.; Bruce Pike, G.; Evans, A.C. A new anatomical landmark for reliable identification of human area V5/MT: A quantitative analysis of sulcal patterning. Cereb. Cortex 2000, 10, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Pertzov, Y.; Bays, P.M.; Joseph, S.; Husain, M. Rapid forgetting prevented by retrospective attention cues. J. Exp. Psychol. Hum. Percept. Perform. 2013, 39, 1224–1231. [Google Scholar] [CrossRef]
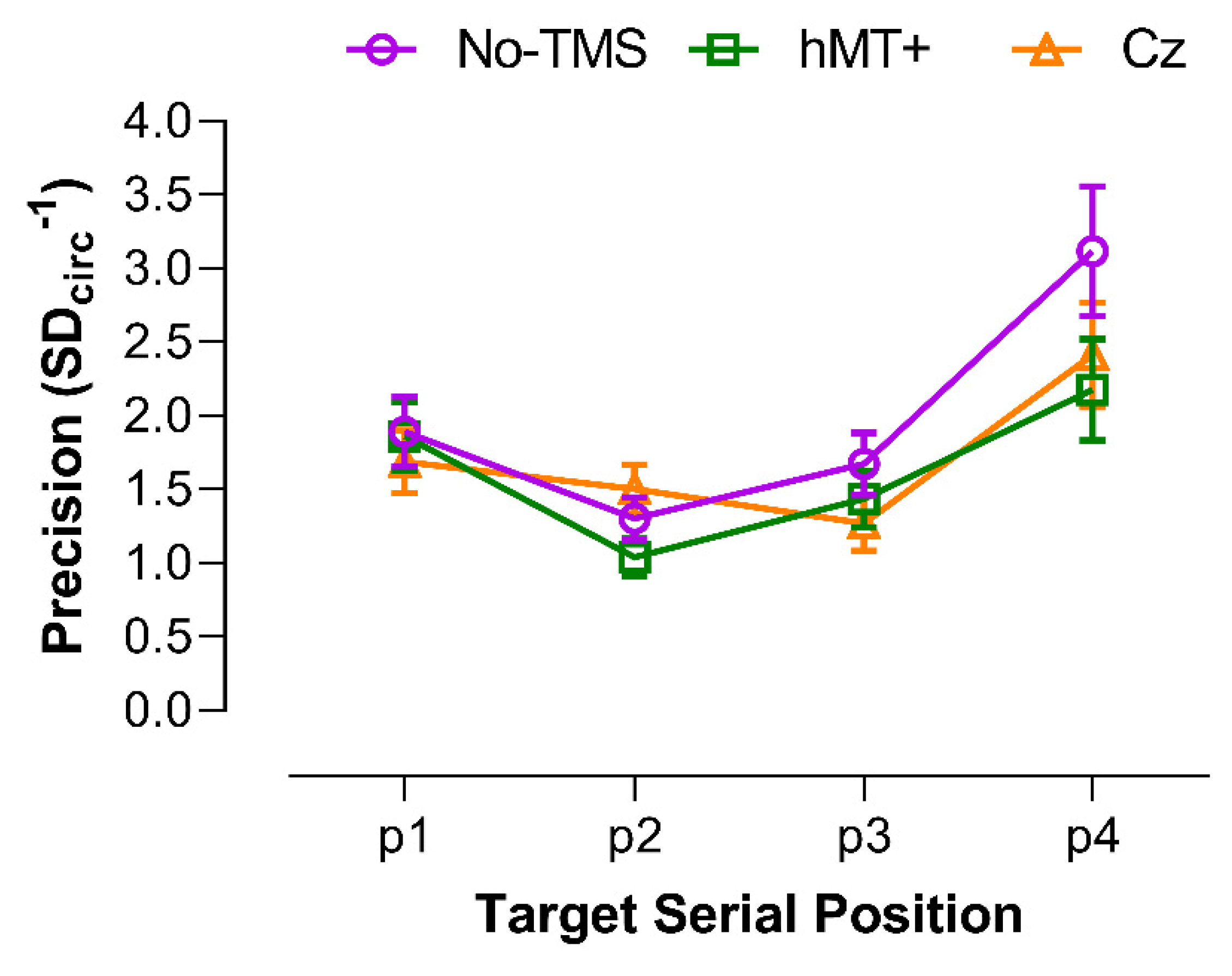
| Target Serial Position | ||||
|---|---|---|---|---|
| Stimulation Type Comparison | P1 | P2 | P3 | P4 |
| hMT+ vs. No-TMS | 0.894 | 0.042 * | 0.189 | 0.015 * |
| Cz vs. No-TMS | 0.338 | 0.288 | 0.015 * | 0.084 |
| Cz vs. hMT+ | 0.375 | 0.006 * | 0.204 | 0.287 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavan, A.; Ghin, F.; Campana, G. Visual Short-Term Memory for Coherent and Sequential Motion: A rTMS Investigation. Brain Sci. 2021, 11, 1471. https://doi.org/10.3390/brainsci11111471
Pavan A, Ghin F, Campana G. Visual Short-Term Memory for Coherent and Sequential Motion: A rTMS Investigation. Brain Sciences. 2021; 11(11):1471. https://doi.org/10.3390/brainsci11111471
Chicago/Turabian StylePavan, Andrea, Filippo Ghin, and Gianluca Campana. 2021. "Visual Short-Term Memory for Coherent and Sequential Motion: A rTMS Investigation" Brain Sciences 11, no. 11: 1471. https://doi.org/10.3390/brainsci11111471
APA StylePavan, A., Ghin, F., & Campana, G. (2021). Visual Short-Term Memory for Coherent and Sequential Motion: A rTMS Investigation. Brain Sciences, 11(11), 1471. https://doi.org/10.3390/brainsci11111471









