Spontaneous and Perturbational Complexity in Cortical Cultures
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
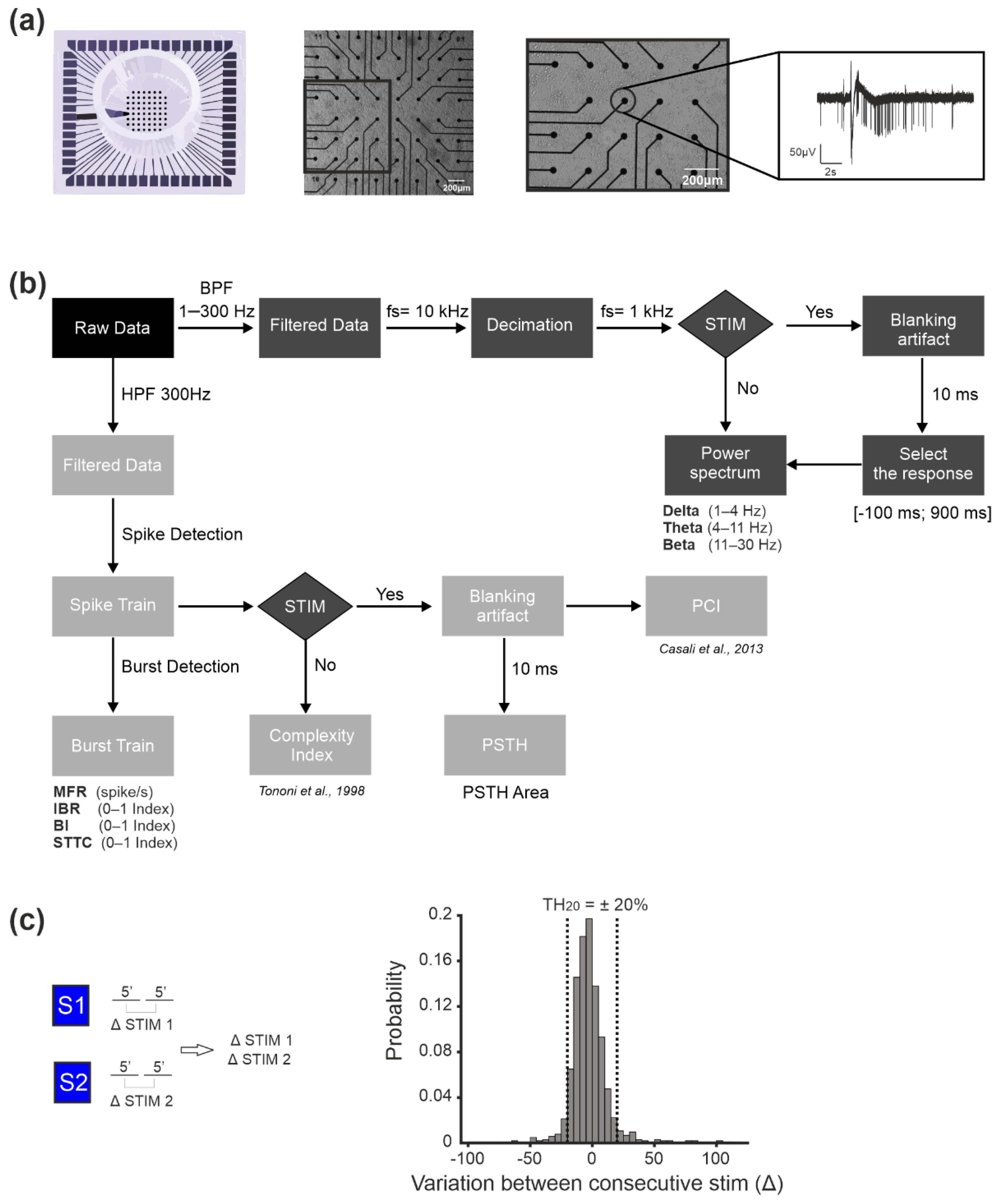
2.2. Experimental Set-Up and Micro-Electrode Array Recordings
2.3. Experimental Protocol
2.4. Data Analysis
2.4.1. Local Field Potential (LFP) Analysis
2.4.2. Multi-Unit Activity (MUA) Analysis
2.4.3. Complexity Indices
2.4.4. Statistical Analysis
3. Results
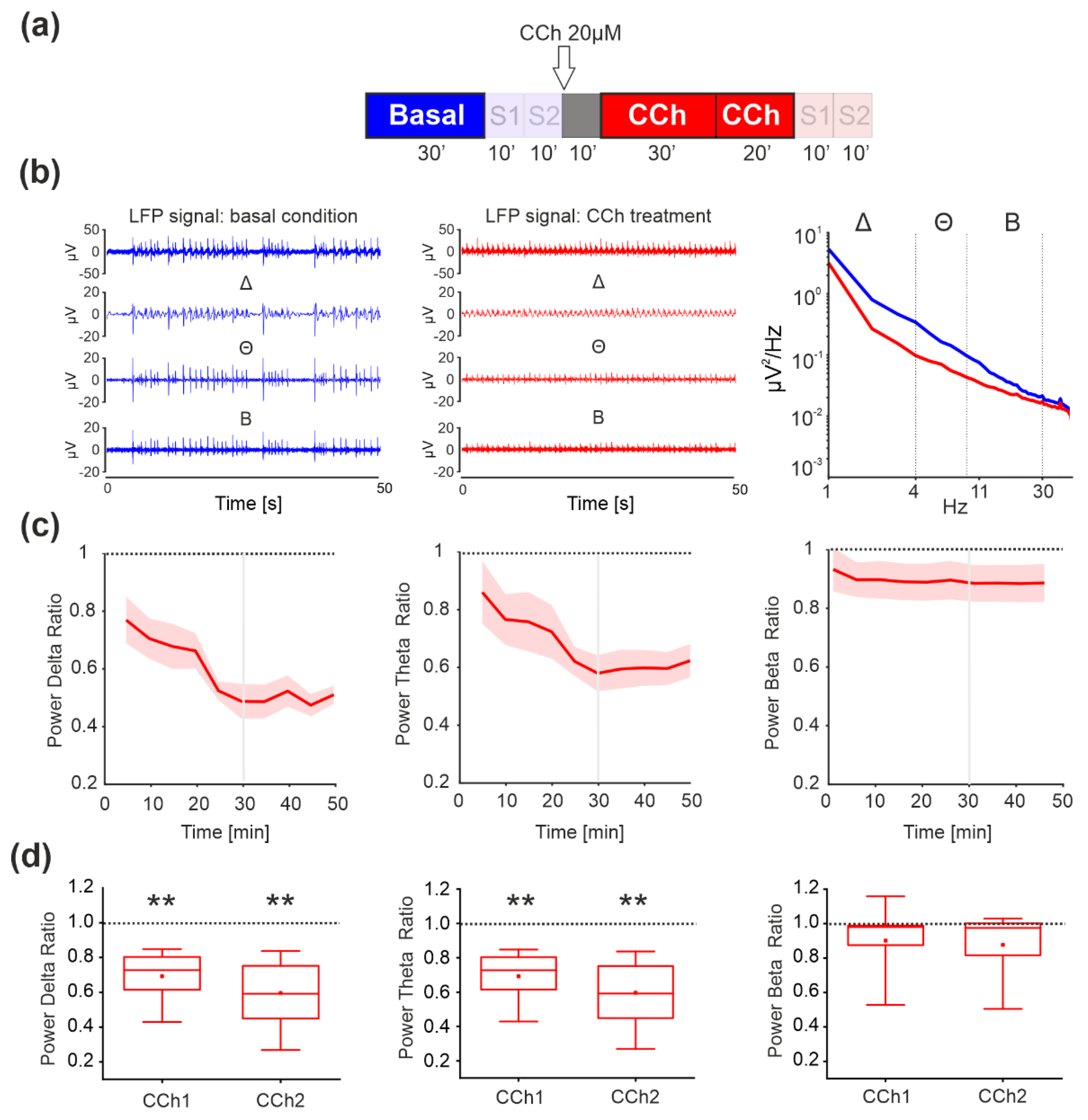
3.1. Spontaneous Activity—LFP Analysis
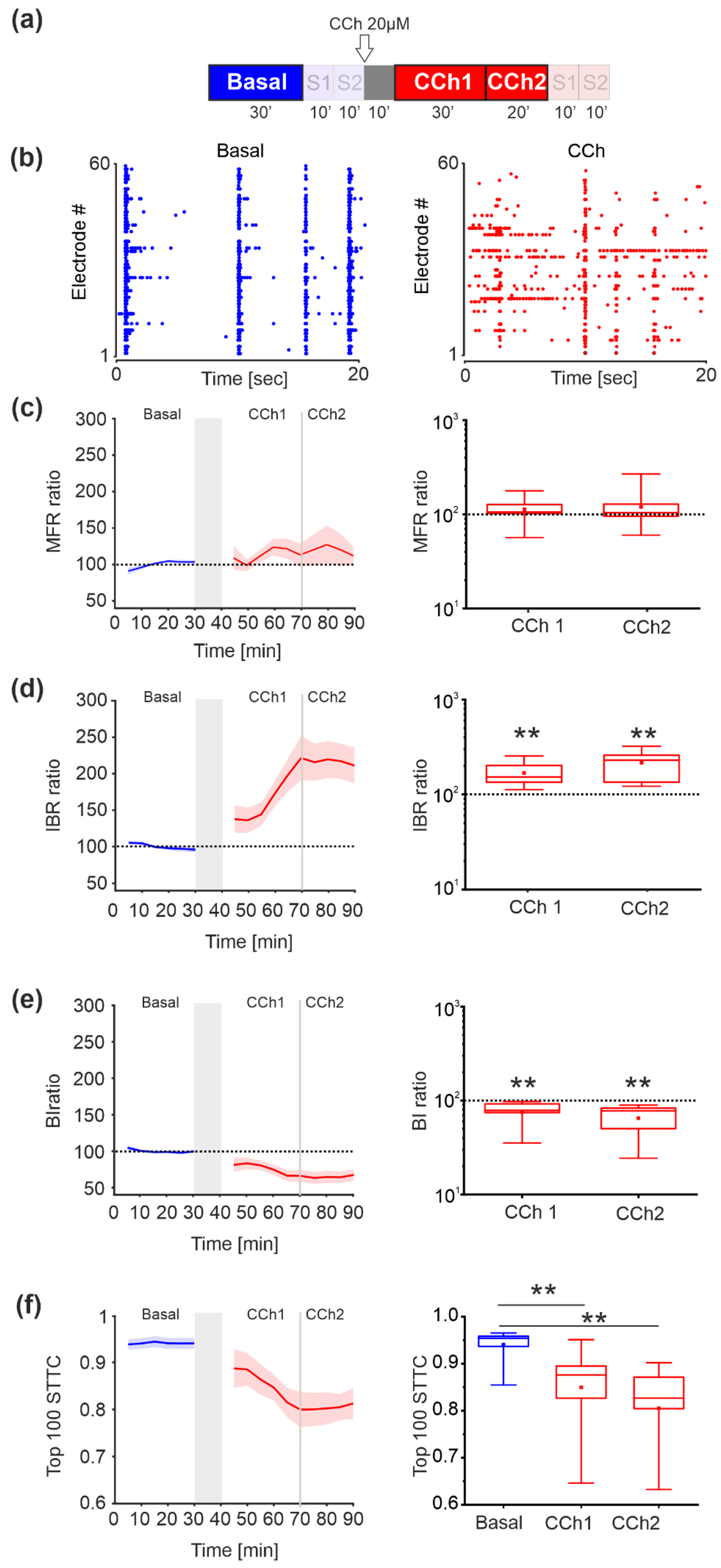
3.2. Spontaneous Activity—MUA Analysis
3.3. Complexity in Spontaneous Activity
3.4. Evoked Activity—LFP Analysis
3.5. Evoked Activity—MUA Analysis
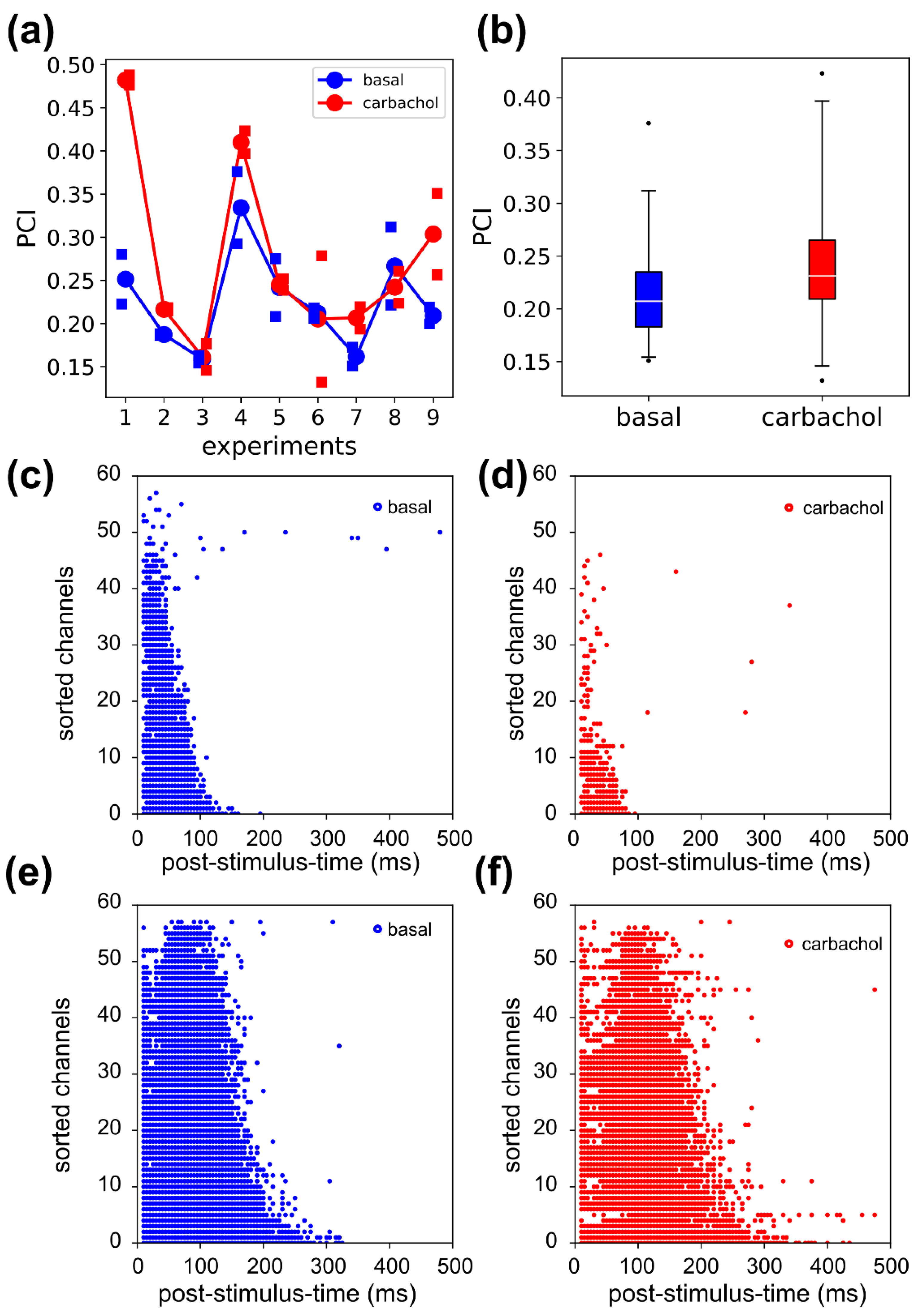
3.6. Complexity in Evoked Activity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kandel, E.R.; Schwartz, J.H.; Jessell, T.M.; Siegelbaum, S.; Hudspeth, A.J.; Mack, S. Principles of Neural Science; McGraw-Hill: New York, NY, USA, 2000; Volume 4. [Google Scholar]
- Reinartz, S.; Biro, I.; Gal, A.; Giugliano, M.; Marom, S. Synaptic dynamics contribute to long-term single neuron response fluctuations. Front. Neural Circuits 2014, 8, 71. [Google Scholar] [CrossRef]
- Gal, A.; Marom, S. Entrainment of the intrinsic dynamics of single isolated neurons by natural-like input. J. Neurosci. 2013, 33, 7912–7918. [Google Scholar] [CrossRef]
- Pasquale, V.; Martinoia, S.; Chiappalone, M. Stimulation triggers endogenous activity patterns in cultured cortical networks. Sci. Rep. 2017, 7, 9080. [Google Scholar] [CrossRef] [PubMed]
- Chiappalone, M.; Bove, M.; Vato, A.; Tedesco, M.; Martinoia, S. Dissociated cortical networks show spontaneously correlated activity patterns during in vitro development. Brain Res. 2006, 1093, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Chiappalone, M.; Massobrio, P.; Martinoia, S. Network plasticity in cortical assemblies. Eur. J. Neurosci. 2008, 28, 221–237. [Google Scholar] [CrossRef]
- Le Feber, J. In Vitro Models of Brain Disorders. In In Vitro Neuronal Networks; Springer: Cham, Switzerland, 2019; pp. 19–49. [Google Scholar]
- Ju, H.; Dranias, M.R.; Banumurthy, G.; Van Dongen, A.M. Spatiotemporal memory is an intrinsic property of networks of dissociated cortical neurons. J. Neurosci. 2015, 35, 4040–4051. [Google Scholar] [CrossRef]
- Nieus, T.; D’Andrea, V.; Amin, H.; Di Marco, S.; Safaai, H.; Maccione, A.; Berdondini, L.; Panzeri, S. State-dependent representation of stimulus-evoked activity in high-density recordings of neural cultures. Sci. Rep. 2018, 8, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Buonomano, D.V.; Maass, W. State-dependent computations: Spatiotemporal processing in cortical networks. Nat. Rev. Neurosci. 2009, 10, 113–125. [Google Scholar] [CrossRef]
- Isomura, T.; Kotani, K.; Jimbo, Y. Cultured cortical neurons can perform blind source separation according to the free-energy principle. PLoS Comput. Biol. 2015, 11, e1004643. [Google Scholar] [CrossRef]
- Isomura, T.; Friston, K. In vitro neural networks minimise variational free energy. Sci. Rep. 2018, 8, 16926. [Google Scholar] [CrossRef]
- Tononi, G.; Boly, M.; Massimini, M.; Koch, C. Integrated information theory: From consciousness to its physical substrate. Nat. Rev. Neurosci. 2016, 17, 450–461. [Google Scholar] [CrossRef]
- Tononi, G.; Edelman, G.M.; Sporns, O. Complexity and coherency: Integrating information in the brain. Trends Cogn. Sci. 1998, 2, 474–484. [Google Scholar] [CrossRef]
- Sarasso, S.; Casali, A.G.; Casarotto, S.; Rosanova, M.; Sinigaglia, C.; Massimini, M. Consciousness and complexity: A consilience of evidence. Neurosci. Conscious. 2021, 7, 1–24. [Google Scholar]
- Grasso, M.; Haun, A.M.; Tononi, G. Of maps and grids. Neurosci. Conscious. 2021, 2021, niab022. [Google Scholar] [CrossRef]
- Haun, A.; Tononi, G. Why does space feel the way it does? Towards a principled account of spatial experience. Entropy 2019, 21, 1160. [Google Scholar] [CrossRef]
- Deco, G.; Jirsa, V.; McIntosh, A.R.; Sporns, O.; Kötter, R. Key role of coupling, delay, and noise in resting brain fluctuations. Proc. Natl. Acad. Sci. USA 2009, 106, 10302–10307. [Google Scholar] [CrossRef] [PubMed]
- Massimini, M.; Ferrarelli, F.; Huber, R.; Esser, S.K.; Singh, H.; Tononi, G. Breakdown of cortical effective connectivity during sleep. Science 2005, 309, 2228–2232. [Google Scholar] [CrossRef]
- Casali, A.G.; Gosseries, O.; Rosanova, M.; Boly, M.; Sarasso, S.; Casali, K.R.; Casarotto, S.; Bruno, M.-A.; Laureys, S.; Tononi, G. A theoretically based index of consciousness independent of sensory processing and behavior. Sci. Transl. Med. 2013, 5, ra105–ra198. [Google Scholar] [CrossRef] [PubMed]
- Casarotto, S.; Comanducci, A.; Rosanova, M.; Sarasso, S.; Fecchio, M.; Napolitani, M.; Pigorini, A.G.; Casali, A.; Trimarchi, P.D.; Boly, M. Stratification of unresponsive patients by an independently validated index of brain complexity. Ann. Neurol. 2016, 80, 718–729. [Google Scholar] [CrossRef] [PubMed]
- Sarasso, S.; Boly, M.; Napolitani, M.; Gosseries, O.; Charland-Verville, V.; Casarotto, S.; Rosanova, M.; Casali, A.G.; Brichant, J.-F.; Boveroux, P. Consciousness and complexity during unresponsiveness induced by propofol, xenon, and ketamine. Curr. Biol. 2015, 25, 3099–3105. [Google Scholar] [CrossRef]
- Arena, A.; Comolatti, R.; Thon, S.; Casali, A.; Storm, J. General anesthesia disrupts complex cortical dynamics in response to intracranial electrical stimulation in rats. eNeuro 2021, 8. [Google Scholar] [CrossRef]
- Dasilva, M.; Camassa, A.; Navarro-Guzman, A.; Pazienti, A.; Perez-Mendez, L.; Zamora-López, G.; Mattia, M.; Sanchez-Vives, M.V. Modulation of cortical slow oscillations and complexity across anesthesia levels. Neuroimage 2021, 224, 117415. [Google Scholar] [CrossRef]
- D’Andola, M.; Rebollo, B.; Casali, A.G.; Weinert, J.F.; Pigorini, A.; Villa, R.; Massimini, M.; Sanchez-Vives, M.V. Bistability, causality, and complexity in cortical networks: An in vitro perturbational study. Cereb. Cortex 2018, 28, 2233–2242. [Google Scholar] [CrossRef] [PubMed]
- Barbero-Castillo, A.; Mateos-Aparicio, P.; Dalla Porta, L.; Camassa, A.; Perez-Mendez, L.; Sanchez-Vives, M.V. Impact of GABAA and GABAB inhibition on cortical dynamics and perturbational complexity during synchronous and desynchronized states. J. Neurosci. 2021, 41, 5029–5044. [Google Scholar] [CrossRef] [PubMed]
- Colombi, I.; Tinarelli, F.; Pasquale, V.; Tucci, V.; Chiappalone, M. A Simplified In vitro Experimental Model Encompasses the Essential Features of Sleep. Front. Neurosci. 2016, 10, 315. [Google Scholar] [CrossRef]
- Le Feber, J.; Stoyanova, I.I.; Chiappalone, M. Connectivity, excitability and activity patterns in neuronal networks. Phys. Biol. 2014, 11, 036005. [Google Scholar] [CrossRef] [PubMed]
- Corner, M.A. From Neural Plate to Cortical Arousal—A Neuronal Network Theory of Sleep Derived from In Vitro “Model” Systems for Primordial Patterns of Spontaneous Bioelectric Activity in the Vertebrate Central Nervous System. Brain Sci. 2013, 3, 800–820. [Google Scholar] [CrossRef]
- Frega, M.; Pasquale, V.; Tedesco, M.; Marcoli, M.; Contestabile, A.; Nanni, M.; Bonzano, L.; Maura, G.; Chiappalone, M. Cortical cultures coupled to micro-electrode arrays: A novel approach to perform in vitro excitotoxicity testing. Neurotoxicol. Teratol. 2012, 34, 116–127. [Google Scholar] [CrossRef]
- Colombi, I.; Mahajani, S.; Frega, M.; Gasparini, L.; Chiappalone, M. Effects of antiepileptic drugs on hippocampal neurons coupled to micro-electrode arrays. Front. Neuroeng. 2013, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Chiappalone, M.; Vato, A.; Berdondini, L.; Koudelka-Hep, M.; Martinoia, S. Network dynamics and synchronous activity in cultured cortical neurons. Int. J. Neural Syst. 2007, 17, 87–103. [Google Scholar] [CrossRef]
- Wagenaar, D.A.; Pine, J.; Potter, S.M. Effective parameters for stimulation of dissociated cultures using multi-electrode arrays. J. Neurosci. Methods 2004, 138, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Shahaf, G.; Marom, S. Learning in networks of cortical neurons. J. Neurosci. 2001, 21, 8782–8788. [Google Scholar] [CrossRef]
- Chiappalone, M.; Pasquale, V.; Frega, M. In Vitro Neuronal Networks: From Culturing Methods to Neuro-Technological Applications; Springer: Cham, Switzerland, 2019; Volume 22. [Google Scholar]
- Buzsáki, G.; Anastassiou, C.A.; Koch, C. The origin of extracellular fields and currents—EEG, ECoG, LFP and spikes. Nat. Rev. Neurosci. 2012, 13, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, R.Q.; Panzeri, S. Extracting information from neuronal populations: Information theory and decoding approaches. Nat. Rev. Neurosci. 2009, 10, 173–185. [Google Scholar] [CrossRef]
- Bologna, L.L.; Pasquale, V.; Garofalo, M.; Gandolfo, M.; Baljon, P.L.; Maccione, A.; Martinoia, S.; Chiappalone, M. Investigating neuronal activity by SPYCODE multi-channel data analyzer. Neural Netw. 2010, 23, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Maccione, A.; Gandolfo, M.; Massobrio, P.; Novellino, A.; Martinoia, S.; Chiappalone, M. A novel algorithm for precise identification of spikes in extracellularly recorded neuronal signals. J. Neurosci. Methods 2009, 177, 241–249. [Google Scholar] [CrossRef]
- Pasquale, V.; Martinoia, S.; Chiappalone, M. A self-adapting approach for the detection of bursts and network bursts in neuronal cultures. J. Comput. Neurosci. 2010, 29, 213–229. [Google Scholar] [CrossRef]
- Wagenaar, D.A.; Madhavan, R.; Pine, J.; Potter, S.M. Controlling bursting in cortical cultures with closed-loop multi-electrode stimulation. J. Neurosci. 2005, 25, 680–688. [Google Scholar] [CrossRef]
- Cutts, C.S.; Eglen, S.J. Detecting pairwise correlations in spike trains: An objective comparison of methods and application to the study of retinal waves. J. Neurosci. 2014, 34, 14288–14303. [Google Scholar] [CrossRef]
- Denker, M.; Yegenoglu, A.; Grün, S. Collaborative HPC-Enabled Workflows on the HBP Collaboratory Using the Elephant Framework. In Proceedings of the Neuroinformatics 2018, Montreal, QC, Canada, 9–10 August 2018. [Google Scholar]
- Kreuz, T.; Mulansky, M.; Bozanic, N. SPIKY: A graphical user interface for monitoring spike train synchrony. J. Neurophysiol. 2015, 113, 3432–3445. [Google Scholar] [CrossRef] [PubMed]
- Mulansky, M.; Kreuz, T. PySpike—A Python library for analyzing spike train synchrony. SoftwareX 2016, 5, 183–189. [Google Scholar] [CrossRef]
- Schartner, M.; Seth, A.; Noirhomme, Q.; Boly, M.; Bruno, M.-A.; Laureys, S.; Barrett, A. Complexity of multi-dimensional spontaneous EEG decreases during propofol induced general anaesthesia. PLoS ONE 2015, 10, e0133532. [Google Scholar]
- Ince, R.A.; Petersen, R.S.; Swan, D.C.; Panzeri, S. Python for information theoretic analysis of neural data. Front. Neuroinform. 2009, 3, 4. [Google Scholar] [CrossRef]
- Bayne, T.; Seth, A.K.; Massimini, M. From Complexity to Consciousness. Trends Neurosci. 2020, 43, 546–547. [Google Scholar] [CrossRef]
- Lo, C.-C.; Chou, T.; Penzel, T.; Scammell, T.E.; Strecker, R.E.; Stanley, H.E.; Ivanov, P.C. Common scale-invariant patterns of sleep–wake transitions across mammalian species. Proc. Natl. Acad. Sci. USA 2004, 101, 17545–17548. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.J.; Robinson, P.A.; Kedziora, D.J.; Abeysuriya, R.G. Mammalian sleep dynamics: How diverse features arise from a common physiological framework. PLoS Comput. Biol. 2010, 6, e1000826. [Google Scholar] [CrossRef]
- Brown, R.E.; Basheer, R.; McKenna, J.T.; Strecker, R.E.; McCarley, R.W. Control of sleep and wakefulness. Physiol. Rev. 2012, 92, 1087–1187. [Google Scholar] [CrossRef]
- Steriade, M.; McCormick, D.A.; Sejnowski, T.J. Thalamocortical oscillations in the sleeping and aroused brain. Science 1993, 262, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Pace, M.; Colombi, I.; Falappa, M.; Freschi, A.; Bandarabadi, M.; Armirotti, A.; Encarnación, B.M.; Adamantidis, A.R.; Amici, R.; Cerri, M. Loss of Snord116 alters cortical neuronal activity in mice: A preclinical investigation of Prader–Willi syndrome. Hum. Mol. Genet. 2020, 29, 2051–2064. [Google Scholar] [CrossRef]
- Hinard, V.; Mikhail, C.; Pradervand, S.; Curie, T.; Houtkooper, R.H.; Auwerx, J.; Franken, P.; Tafti, M. Key electrophysiological, molecular, and metabolic signatures of sleep and wakefulness revealed in primary cortical cultures. J. Neurosci. 2012, 32, 12506–12517. [Google Scholar] [CrossRef]
- Kaufman, M.; Corner, M.A.; Ziv, N.E. Long-term relationships between cholinergic tone, synchronous bursting and synaptic remodeling. PLoS ONE 2012, 7, e40980. [Google Scholar] [CrossRef]
- Kaufman, M.; Reinartz, S.; Ziv, N.E. Adaptation to prolonged neuromodulation in cortical cultures: An invariable return to network synchrony. BMC Biol. 2014, 12, 83. [Google Scholar] [CrossRef][Green Version]
- Colombo, M.A.; Napolitani, M.; Boly, M.; Gosseries, O.; Casarotto, S.; Rosanova, M.; Brichant, J.-F.; Boveroux, P.; Rex, S.; Laureys, S. The spectral exponent of the resting EEG indexes the presence of consciousness during unresponsiveness induced by propofol, xenon, and ketamine. Neuroimage 2019, 189, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Reinartz, S. Long-term activity dynamics of single neurons and networks. In In Vitro Neuronal Networks; Springer: Cham, Switzerland, 2019; pp. 331–350. [Google Scholar]
- Jimbo, Y.; Tateno, T.; Robinson, H. Simultaneous induction of pathway-specific potentiation and depression in networks of cortical neurons. Biophys. J. 1999, 76, 670–678. [Google Scholar] [CrossRef]
- Lonardoni, D.; Amin, H.; Di Marco, S.; Maccione, A.; Berdondini, L.; Nieus, T. Recurrently connected and localized neuronal communities initiate coordinated spontaneous activity in neuronal networks. PLoS Comput. Biol. 2017, 13, e1005672. [Google Scholar] [CrossRef] [PubMed]
- Bisio, M.; Bosca, A.; Pasquale, V.; Berdondini, L.; Chiappalone, M. Emergence of bursting activity in connected neuronal sub-populations. PLoS ONE 2014, 9, e107400. [Google Scholar] [CrossRef] [PubMed]
- Marconi, E.; Nieus, T.; Maccione, A.; Valente, P.; Simi, A.; Messa, M.; Dante, S.; Baldelli, P.; Berdondini, L.; Benfenati, F. Emergent functional properties of neuronal networks with controlled topology. PLoS ONE 2012, 7, e34648. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Moriya, S.; Ide, K.; Hayakawa, T.; Akima, H.; Sato, S.; Kubota, S.; Tanii, T.; Niwano, M.; Teller, S. Impact of modular organization on dynamical richness in cortical networks. Sci. Adv. 2018, 4, eaau4914. [Google Scholar] [CrossRef]
- Bosi, S.; Rauti, R.; Laishram, J.; Turco, A.; Lonardoni, D.; Nieus, T.; Prato, M.; Scaini, D.; Ballerini, L. From 2D to 3D: Novel nanostructured scaffolds to investigate signalling in reconstructed neuronal networks. Sci. Rep. 2015, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Palazzolo, G.; Moroni, M.; Soloperto, A.; Aletti, G.; Naldi, G.; Vassalli, M.; Nieus, T.; Difato, F. Fast wide-volume functional imaging of engineered in vitro brain tissues. Sci. Rep. 2017, 7, 8499. [Google Scholar] [CrossRef]
- Frega, M.; Tedesco, M.; Massobrio, P.; Pesce, M.; Martinoia, S. Network dynamics of 3D engineered neuronal cultures: A new experimental model for in vitro electrophysiology. Sci. Rep. 2014, 4, 1–14. [Google Scholar] [CrossRef]
- Kanagasabapathi, T.T.; Massobrio, P.; Barone, R.A.; Tedesco, M.; Martinoia, S.; Wadman, W.J.; Decré, M.M. Functional connectivity and dynamics of cortical–thalamic networks co-cultured in a dual compartment device. J. Neural Eng. 2012, 9, 036010. [Google Scholar] [CrossRef] [PubMed]
- Lavazza, A.; Massimini, M. Cerebral organoids: Ethical issues and consciousness assessment. J. Med. Ethics 2018, 44, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Aparicio, P.; Bello, S.A.; Rodríguez-Moreno, A. Challenges in Physiological Phenotyping of hiPSC-Derived Neurons: From 2D Cultures to 3D Brain Organoids. Front. Cell Dev. Biol. 2020, 8, 797. [Google Scholar] [CrossRef]
- Tasnim, K.; Liu, J. Emerging bioelectronics for brain organoid electrophysiology. J. Mol. Biol. 2021, 167165. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombi, I.; Nieus, T.; Massimini, M.; Chiappalone, M. Spontaneous and Perturbational Complexity in Cortical Cultures. Brain Sci. 2021, 11, 1453. https://doi.org/10.3390/brainsci11111453
Colombi I, Nieus T, Massimini M, Chiappalone M. Spontaneous and Perturbational Complexity in Cortical Cultures. Brain Sciences. 2021; 11(11):1453. https://doi.org/10.3390/brainsci11111453
Chicago/Turabian StyleColombi, Ilaria, Thierry Nieus, Marcello Massimini, and Michela Chiappalone. 2021. "Spontaneous and Perturbational Complexity in Cortical Cultures" Brain Sciences 11, no. 11: 1453. https://doi.org/10.3390/brainsci11111453
APA StyleColombi, I., Nieus, T., Massimini, M., & Chiappalone, M. (2021). Spontaneous and Perturbational Complexity in Cortical Cultures. Brain Sciences, 11(11), 1453. https://doi.org/10.3390/brainsci11111453






