In Search of Digital Dopamine: How Apps Can Motivate Depressed Patients, a Review and Conceptual Analysis
Abstract
:1. Introduction
1.1. Motivation Deficit in Depression: A Neglected Dimension
1.2. A Specific Approach Is Needed to Treat Conative Disorders
1.3. Digital Dopamine to the Rescue
1.4. The Role of Digital Technologies: Toward the “Light Side”
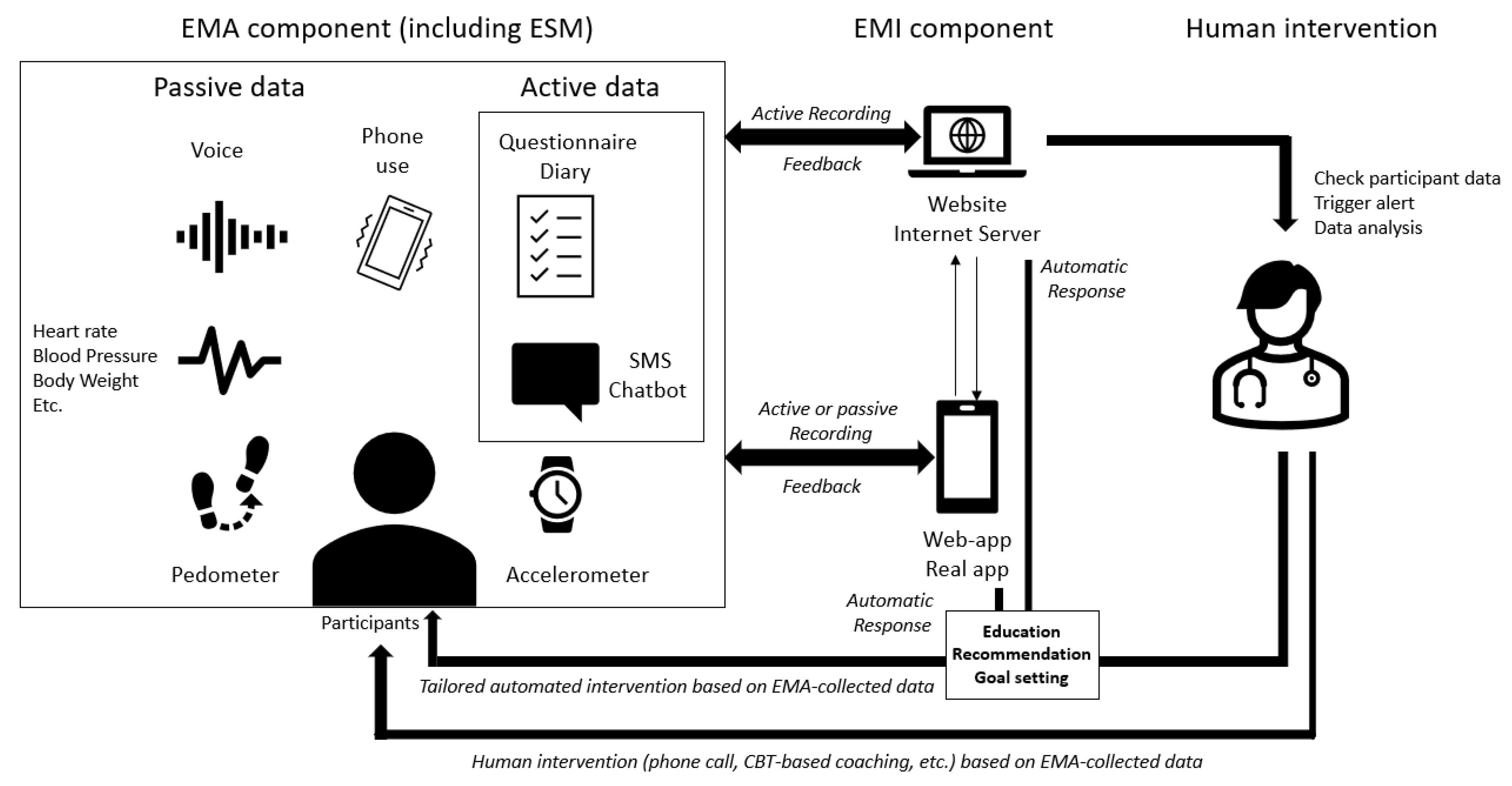
2. Materials and Methods
- -
- Both an EMA and an EMI component that are not connected to each other.
- -
- EMA triggered by another EMA component.
- -
- Fully or partially automated intervention based on the EMA components allowing tailored interventions in real time.
3. Results
3.1. Definitions
- -
- Engagement in pleasurable activities [44];
- -
- Increasing positive emotions;
- -
- Motivate the patient;
- -
- Encourage engagement in practicing or using previously learned skills.
3.2. Applications
- -
- MoodTracker, an EMA only app
- -
- ImproveYourMood, an EMA and EMI app
- -
- ImproveYourMood+, an EMA and EMI app + prompts
- EMA
- + EMI feedback
- EMA
- EMA (called “ESM” in this study) only.
- EMA
- Treatment As Usual (TAU).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klein, D.F. Endogenomorphic Depression: A Conceptual and Terminological Revision. Arch. Gen. Psychiatry 1974, 31, 447. [Google Scholar] [CrossRef] [PubMed]
- Vinckier, F.; Gourion, D.; Mouchabac, S. Anhedonia predicts poor psychosocial functioning: Results from a large cohort of patients treated for major depressive disorder by general practitioners. Eur. Psychiatr. 2017, 44, 1–8. [Google Scholar] [CrossRef]
- Kringelbach, M.L.; Berridge, K.C. Towards a functional neuroanatomy of pleasure and happiness. Trends Cogn. Sci. 2009, 13, 479–487. [Google Scholar] [CrossRef] [Green Version]
- Pessiglione, M.; Vinckier, F.; Bouret, S.; Daunizeau, J.; Le Bouc, R. Why not try harder? Computational approach to motivation deficits in neuro-psychiatric diseases. Brain 2018, 141, 629–650. [Google Scholar] [CrossRef] [Green Version]
- Pettorruso, M.; d’Andrea, G.; Martinotti, G.; Cocciolillo, F.; Miuli, A.; Di Muzio, I.; Collevecchio, R.; Verrastro, V.; De-Giorgio, F.; Janiri, L.; et al. Hopelessness, Dissociative Symptoms, and Suicide Risk in Major Depressive Disorder: Clinical and Biological Correlates. Brain Sci. 2020, 10, 519. [Google Scholar] [CrossRef]
- Belujon, P.; Grace, A.A. Dopamine System Dysregulation in Major Depressive Disorders. Int. J. Neuropsychopharmacol. 2017, 20, 1036–1046. [Google Scholar] [CrossRef] [Green Version]
- Bromberg-Martin, E.S.; Matsumoto, M.; Hikosaka, O. Dopamine in Motivational Control: Rewarding, Aversive, and Alerting. Neuron 2010, 68, 815–834. [Google Scholar] [CrossRef] [Green Version]
- Arias-Carrión, O.; Stamelou, M.; Murillo-Rodríguez, E.; Menéndez-González, M.; Pöppel, E. Dopaminergic reward system: A short integrative review. Int. Arch. Med. 2010, 3, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torrisi, S.A.; Laudani, S.; Contarini, G.; De Luca, A.; Geraci, F.; Managò, F.; Papaleo, F.; Salomone, S.; Drago, F.; Leggio, G.M. Dopamine, Cognitive Impairments and Second-Generation Antipsychotics: From Mechanistic Advances to More Personalized Treatments. Pharmaceuticals 2020, 13, 365. [Google Scholar] [CrossRef] [PubMed]
- Uphoff, E.; Ekers, D.; Robertson, L.; Dawson, S.; Sanger, E.; South, E.; Samaan, Z.; Richards, D.; Meader, N.; Churchill, R. Behavioural Activation Therapy for Depression in Adults. Cochrane Common Mental Disorders Group, editor. Cochrane Database of Systematic Reviews [Internet]. Available online: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD013461.pub2/full (accessed on 12 September 2021).
- Grillner, S.; Robertson, B. The Basal Ganglia Over 500 Million Years. Curr. Biol. 2016, 26, R1088–R1100. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.; Lau, Y.; Chan, L.; Luk, J.W. Prevalence of social media addiction across 32 nations: Meta-analysis with subgroup analysis of classification schemes and cultural values. Addict. Behav. 2021, 117, 106845. [Google Scholar] [CrossRef] [PubMed]
- Turel, O.; He, Q.; Xue, G.; Xiao, L.; Bechara, A. Examination of Neural Systems Sub-Serving Facebook “Addiction”. Psychol Rep. 2014, 115, 675–695. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, F.; Bourla, A.; Mouchabac, S.; Karila, L. e-Addictology: An Overview of New Technologies for Assessing and Intervening in Addictive Behaviors. Front. Psychiatry 2018, 9, 51. [Google Scholar] [CrossRef]
- Ferreri, F.; Bourla, A.; Peretti, C.-S.; Segawa, T.; Jaafari, N.; Mouchabac, S. How New Technologies Can Improve Prediction, Assessment, and Intervention in Obsessive-Compulsive Disorder (e-OCD): Review. JMIR Ment. Health 2019, 6, e11643. [Google Scholar] [CrossRef]
- Bourla, A.; Mouchabac, S.; El Hage, W.; Ferreri, F. e-PTSD: An overview on how new technologies can improve prediction and assessment of Posttraumatic Stress Disorder (PTSD). Eur. J. Psychotraumatol. 2018, 9, 1424448. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Varma, D.S.; Prosperi, M. A systematic review of the effectiveness of mobile apps for monitoring and management of mental health symptoms or disorders. J. Psychiatr. Res. 2018, 107, 73–78. [Google Scholar] [CrossRef]
- Jain, S.H.; Powers, B.W.; Hawkins, J.B.; Brownstein, J.S. The digital phenotype. Nat. Biotechnol. 2015, 33, 462–463. [Google Scholar] [CrossRef] [PubMed]
- Moshe, I.; Terhorst, Y.; Opoku Asare, K.; Sander, L.B.; Ferreira, D.; Baumeister, H.; Mohr, D.C.; Pulkki-Raaback, L. Predicting Symptoms of Depression and Anxiety Using Smartphone and Wearable Data. Front. Psychiatry 2021, 12, 625247. [Google Scholar] [CrossRef]
- Bai, R.; Xiao, L.; Guo, Y.; Zhu, X.; Li, N.; Wang, Y.; Chen, Q.; Feng, L.; Wang, Y.; Yu, X.; et al. Tracking and Monitoring Mood Stability of Patients With Major Depressive Disorder by Machine Learning Models Using Passive Digital Data: Prospective Naturalistic Multicenter Study. JMIR mHealth uHealth 2021, 9, e24365. [Google Scholar] [CrossRef]
- Maatoug, R.; Peiffer-Smadja, N.; Delval, G.; Brochu, T.; Pitrat, B.; Millet, B. Ecological Momentary Assessment Using Smartphones in Patients with Depression: Feasibility Study. JMIR Form. Res. 2021, 5, e14179. [Google Scholar] [CrossRef]
- Shah, R.V.; Grennan, G.; Zafar-Khan, M.; Alim, F.; Dey, S.; Ramanathan, D.; Mishra, J. Personalized machine learning of depressed mood using wearables. Transl. Psychiatry 2021, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Henderson, C.; Evans-Lacko, S.; Thornicroft, G. Mental Illness Stigma, Help Seeking, and Public Health Programs. Am. J. Public Health 2013, 103, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Benarous, X.; Edel, Y.; Consoli, A.; Brunelle, J.; Etter, J.-F.; Cohen, D.; Khazaal, Y. Ecological Momentary Assessment and Smartphone Application Intervention in Adolescents with Substance Use and Comorbid Severe Psychiatric Disorders: Study Protocol. Front. Psychiatry 2016, 7, 157. Available online: http://journal.frontiersin.org/Article/10.3389/fpsyt.2016.00157/abstract (accessed on 12 October 2021). [CrossRef] [Green Version]
- Joseph, M.A.; Natarajan, J.; Buckingham, J.; Al Noumani, M. Using digital badges to enhance nursing students’ attendance and motivation. Nurse Educ. Pract. 2021, 52, 103033. [Google Scholar] [CrossRef]
- Ahtinen, A.; Mattila, E.; Välkkynen, P.; Kaipainen, K.; Vanhala, T.; Ermes, M.; Sairanen, E.; Myllymaki, T.; Lappalainen, R. Mobile Mental Wellness Training for Stress Management: Feasibility and Design Implications Based on a One-Month Field Study. JMIR mHealth uHealth 2013, 1, e11. [Google Scholar] [CrossRef]
- Dagöö, J.; Asplund, R.P.; Bsenko, H.A.; Hjerling, S.; Holmberg, A.; Westh, S.; Oberg, L.; Ljotsson, B.; Carlbring, P.; Furmark, T.; et al. Cognitive behavior therapy versus interpersonal psychotherapy for social anxiety disorder delivered via smartphone and computer: A randomized controlled trial. J. Anxiety Disord. 2014, 28, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Burns, M.N.; Begale, M.; Duffecy, J.; Gergle, D.; Karr, C.J.; Giangrande, E.; Mohr, D.C. Harnessing Context Sensing to Develop a Mobile Intervention for Depression. J. Med. Internet Res. 2011, 13, e55. [Google Scholar] [CrossRef] [Green Version]
- Colombo, D.; Fernández-Álvarez, J.; Patané, A.; Semonella, M.; Kwiatkowska, M.; García-Palacios, A.; Cipresso, P.; Riva, G.; Botella, C. Current State and Future Directions of Technology-Based Ecological Momentary Assessment and Intervention for Major Depressive Disorder: A Systematic Review. JCM 2019, 8, 465. [Google Scholar] [CrossRef] [Green Version]
- Versluis, A.; Verkuil, B.; Spinhoven, P.; van der Ploeg, M.M.; Brosschot, J.F. Changing Mental Health and Positive Psychological Well-Being Using Ecological Momentary Interventions: A Systematic Review and Meta-analysis. J. Med. Internet Res. 2016, 18, e152. [Google Scholar] [CrossRef] [Green Version]
- Everitt, N.; Broadbent, J.; Richardson, B.; Smyth, J.M.; Heron, K.; Teague, S.; Fuller-Tyszkiewicz, M. Exploring the features of an app-based just-in-time intervention for depression. J. Affect. Disord. 2021, 291, 279–287. [Google Scholar] [CrossRef]
- Depp, C.A.; Perivoliotis, D.; Holden, J.; Dorr, J.; Granholm, E.L. Single-Session Mobile-Augmented Intervention in Serious Mental Illness: A Three-Arm Randomized Controlled Trial. Schizophr. Bull. 2019, 45, 752–762. [Google Scholar] [CrossRef]
- Snippe, E.; Simons, C.J.P.; Hartmann, J.A.; Menne-Lothmann, C.; Kramer, I.; Booij, S.H.; Viechtbauer, W.; Delespaul, P.; Myin-Germeys, I.; Wichers, M. Change in daily life behaviors and depression: Within-person and between-person associations. Health Psychol. 2016, 35, 433–441. [Google Scholar] [CrossRef] [Green Version]
- Burton, C.; Szentagotai Tatar, A.; McKinstry, B.; Matheson, C.; Matu, S.; Moldovan, R.; Macnab, M.; Farrow, E.; David, D.; Pagliari, C.; et al. Pilot randomised controlled trial of Help4Mood, an embodied virtual agent-based system to support treatment of depression. J. Telemed. Telecare 2016, 22, 348–355. [Google Scholar] [CrossRef] [Green Version]
- Jonathan, G.K.; Dopke, C.A.; Michaels, T.; Bank, A.; Martin, C.R.; Adhikari, K.; Krakauer, R.L.; Ryan, C.; McBride, A.; Babington, P.; et al. A Smartphone-Based Self-management Intervention for Bipolar Disorder (LiveWell): User-Centered Development Approach. JMIR Ment. Health 2021, 8, e20424. [Google Scholar] [CrossRef]
- Soares Teles, A.; Rocha, A.; José da Silva e Silva, F.; Correia Lopes, J.; O’Sullivan, D.; Van de Ven, P.; Endler, M. Enriching Mental Health Mobile Assessment and Intervention with Situation Awareness. Sensors 2017, 17, 127. [Google Scholar] [CrossRef] [Green Version]
- Riese, H.; von Klipstein, L.; Schoevers, R.A.; van der Veen, D.C.; Servaas, M.N. Personalized ESM monitoring and feedback to support psychological treatment for depression: A pragmatic randomized controlled trial (Therap-i). BMC Psychiatry 2021, 21, 143. [Google Scholar] [CrossRef]
- Schlosser, D.A.; Campellone, T.R.; Truong, B.; Etter, K.; Vergani, S.; Komaiko, K.; Vinogradov, S. Efficacy of PRIME, a Mobile App Intervention Designed to Improve Motivation in Young People with Schizophrenia. Schizophr. Bull. 2018, 44, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Fulford, D.; Mote, J.; Gard, D.E.; Mueser, K.T.; Gill, K.; Leung, L.; Dillaway, K. Development of the Motivation and Skills Support (MASS) social goal attainment smartphone app for (and with) people with schizophrenia. J. Behav. Cogn. Ther. 2020, 30, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Koontz, B.F.; Levine, E.; McSherry, F.; Niedzwiecki, D.; Sutton, L.; Dale, T.; Streicher, M.; Rushing, C.; Owen, L.; Kraus, W.E.; et al. Increasing physical activity in Cancer Survivors through a Text-messaging Exercise motivation Program (ICanSTEP). Supportive Care Cancer 2021, 29, 7339–7349. Available online: https://link.springer.com/10.1007/s00520-021-06281-y (accessed on 12 October 2021). [CrossRef]
- Takeyama, N.; Moriyama, M.; Kazawa, K.; Steenkamp, M.; Rahman, M.M. A Health Guidance App to Improve Motivation, Adherence to Lifestyle Changes and Indicators of Metabolic Disturbances among Japanese Civil Servants. IJERPH 2020, 17, 8147. [Google Scholar] [CrossRef] [PubMed]
- Schueller, S.M.; Aguilera, A.; Mohr, D.C. Ecological momentary interventions for depression and anxiety. Depress. Anxiety 2017, 34, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Mohr, D.C.; Schueller, S.M.; Montague, E.; Burns, M.N.; Rashidi, P. The Behavioral Intervention Technology Model: An Integrated Conceptual and Technological Framework for eHealth and mHealth Interventions. J. Med. Internet Res. 2014, 16, e146. [Google Scholar] [CrossRef]
- Ly, K.H.; Trüschel, A.; Jarl, L.; Magnusson, S.; Windahl, T.; Johansson, R.; Carlbring, P.; Andersson, G. Behavioural activation versus mindfulness-based guided self-help treatment administered through a smartphone application: A randomised controlled trial. BMJ Open 2014, 4, e003440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramer, I.; Simons, C.J.P.; Hartmann, J.A.; Menne-Lothmann, C.; Viechtbauer, W.; Peeters, F.; Schruers, K.; van Bemmel, A.L.; Myin-Germeys, I.; Delespaul, P.; et al. A therapeutic application of the experience sampling method in the treatment of depression: A randomized controlled trial. World Psychiatry 2014, 13, 68–77. [Google Scholar] [CrossRef]
- Sankaran, S.; Dendale, P.; Coninx, K. Evaluating the Impact of the HeartHab App on Motivation, Physical Activity, Quality of Life, and Risk Factors of Coronary Artery Disease Patients: Multidisciplinary Crossover Study. JMIR mHealth uHealth 2019, 7, e10874. [Google Scholar] [CrossRef]
- Höchsmann, C.; Infanger, D.; Klenk, C.; Königstein, K.; Walz, S.P.; Schmidt-Trucksäss, A. Effectiveness of a Behavior Change Technique–Based Smartphone Game to Improve Intrinsic Motivation and Physical Activity Adherence in Patients With Type 2 Diabetes: Randomized Controlled Trial. JMIR Serious Games 2019, 7, e11444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, E.; Park, H.-A. Experiences of Patients with a Diabetes Self-Care App Developed Based on the Information-Motivation-Behavioral Skills Model: Before-and-After Study. JMIR Diabetes 2019, 4, e11590. [Google Scholar] [CrossRef] [PubMed]
- Brunet, J.; Tulloch, H.E.; Wolfe Phillips, E.; Reid, R.D.; Pipe, A.L.; Reed, J.L. Motivation Predicts Change in Nurses’ Physical Activity Levels During a Web-Based Worksite Intervention: Results from a Randomized Trial. J. Med. Internet Res. 2020, 22, e11543. [Google Scholar] [CrossRef]
- Vankipuram, M.; McMahon, S.; Fleury, J. ReadySteady: App for Accelerometer-based Activity Monitoring and Wellness-Motivation Feedback System for Older Adults. AMIA Annu. Symp. Proc. 2012, 2012, 931–939. [Google Scholar]
- Bos, F.M.; Snippe, E.; Bruggeman, R.; Wichers, M.; van der Krieke, L. Insights of Patients and Clinicians on the Promise of the Experience Sampling Method for Psychiatric Care. Psychiatr. Serv. 2019, 70, 983–991. [Google Scholar] [CrossRef]
- Baumel, A.; Muench, F.J. Effort-Optimized Intervention Model: Framework for Building and Analyzing Digital Interventions That Require Minimal Effort for Health-Related Gains. J. Med. Internet Res. 2021, 23, e24905. [Google Scholar] [CrossRef] [PubMed]
- Eysenbach, G. The Law of Attrition. J. Med. Internet Res. 2005, 7, e11. [Google Scholar] [CrossRef] [PubMed]
- Holdener, M.; Gut, A.; Angerer, A. Applicability of the User Engagement Scale to Mobile Health: A Survey-Based Quantitative Study. JMIR mHealth uHealth 2020, 8, e13244. [Google Scholar] [CrossRef] [PubMed]
- Bonnot, O.; Adrien, V.; Venelle, V.; Bonneau, D.; Gollier-Briant, F.; Mouchabac, S. Mobile App for Parental Empowerment for Caregivers of Children with Autism Spectrum Disorders: Prospective Open Trial. JMIR Ment. Health 2021, 8, e27803. [Google Scholar] [CrossRef]
- Kelders, S.M.; Sommers-Spijkerman, M.; Goldberg, J. Investigating the Direct Impact of a Gamified Versus Nongamified Well-Being Intervention: An Exploratory Experiment. J. Med. Internet Res. 2018, 20, e247. [Google Scholar] [CrossRef]
- Floryan, M.; Chow, P.I.; Schueller, S.M.; Ritterband, L.M. The Model of Gamification Principles for Digital Health Interventions: Evaluation of Validity and Potential Utility. J. Med. Internet Res. 2020, 22, e16506. [Google Scholar] [CrossRef]
- Cheng, V.W.S.; Davenport, T.; Johnson, D.; Vella, K.; Hickie, I.B. Gamification in Apps and Technologies for Improving Mental Health and Well-Being: Systematic Review. JMIR Ment. Health 2019, 6, e13717. [Google Scholar] [CrossRef] [Green Version]
- Lumsden, J.; Edwards, E.A.; Lawrence, N.S.; Coyle, D.; Munafò, M.R. Gamification of Cognitive Assessment and Cognitive Training: A Systematic Review of Applications and Efficacy. JMIR Serious Games 2016, 4, e11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, A.; Patel, M. Prescribing Behavior Change: Opportunities and Challenges for Clinicians to Embrace Digital and Mobile Health. JMIR mHealth uHealth 2020, 8, e17281. [Google Scholar] [CrossRef]
- Patoz, M.-C.; Hidalgo-Mazzei, D.; Blanc, O.; Verdolini, N.; Pacchiarotti, I.; Murru, A.; Zukerwar, L.; Vieta, E.; Llorca, P.-M.; Samalin, L. Patient and physician perspectives of a smartphone application for depression: A qualitative study. BMC Psychiatry 2021, 21, 65. [Google Scholar] [CrossRef]
- Lipschitz, J.M.; Connolly, S.L.; Miller, C.J.; Hogan, T.P.; Simon, S.R.; Burdick, K.E. Patient interest in mental health mobile app interventions: Demographic and symptom-level differences. J. Affect. Disord. 2020, 263, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Torrisi, S.A.; Lavanco, G.; Maurel, O.M.; Gulisano, W.; Laudani, S.; Geraci, F.; Grasso, M.; Barbagallo, C.; Caraci, F.; Bucolo, C.; et al. A novel arousal-based individual screening reveals susceptibility and resilience to PTSD-like phenotypes in mice. Neurobiol. Stress 2021, 14, 100286. [Google Scholar] [CrossRef] [PubMed]
- Myin-Germeys, I.; Klippel, A.; Steinhart, H.; Reininghaus, U. Ecological momentary interventions in psychiatry. Curr. Opin. Psychiatry 2016, 29, 258–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Definition | References | |
|---|---|---|
| EMA | Smartphone-based evaluation of day-to-day symptoms, in the habitual environment of the patient, with the possibility of withdrawing from recall biases since they evaluate themselves “Right then, not later; Right there, not elsewhere”. Experience Sampling Method (ESM) is a passive data-based EMA. | n.s. |
| EMI | Smartphone-based intervention involving the delivery of psychoeducation, advice, or recommendations about how to behave according to the patient’s immediate environment. | n.s. |
| EMA + EMI | Application that integrates both an EMA and an EMI component that are not connected to each other. | IYM [31] CBT2go [32] PsyMate [33] Help4Mood [34] LiveWell [35] |
| Smart-EMA | EMA triggered by another EMA component (for example a questionnaire that is triggered by a certain location, or by a cutoff on another questionnaire). | n.s. |
| Smart-EMI | Fully or partially automated intervetion based on the EMA components allowing tailored interventions in real time. | MoodBuster [36] Mobilyze! [28] Therap-I [37] PRIME [38] MASS [39] ICanStep [40] Hiroshima HN [41] |
| App | Objectives | Population and Method | Result |
|---|---|---|---|
| SituMan | Accuracy of Situation Identification (SituMan component) EMA: Situation awarness Active data Passive data SituMan EMI: Feedback graph | 12 healthy volunteers using SituMan for 7 days | Accuracy was 100% for three participants, >90% for five, >80% for three, >70% for one |
| MoodBuster [32] | |||
| MoodTracker | Efficacy of microintervention content, just-in-time approaches, and the potential efficacy of symptom monitoring EMA: Mood active data EMI (IYM or IYM+ only): Breathing exercises Mindful body scan Gratitude exercise | Naturalistic trial on 235 healthy volunteers over 3 weeks, randomized into four groups: waitlist (control group), MoodTracker, IYM, IYM+ | Participants in the IYM condition exhibited significantly greater improvements in depressive and anxietysymptoms (only at follow-up) and automatic negative thoughts (both postintervention and follow-up). EMI use resulted in immediate improvement in mood state, suggesting the resources had their intended effect in-the-moment |
| ImproveYourMood (IYM or IYM+) [27] | |||
| CBT2go [28] | Assess the efficacy of interventions on Brief Psychiatric Rating Scale—expanded measuring psychopathologic symptoms (anxiety, depression, mania, delusions/hallucinations, unusual behavior, and negative symptoms) EMA: Maladaptive beliefs, socialization, and medication adherence Active data EMI: Psychoeducation about the topic and queried participants about their experience and current strategies for self-management | RCT on 255 participants diagnosed with schizophrenia, schizoaffective disorder, or bipolar I disorder randomized into three groups: Treatment as usual (n = 83), CBT2go (n = 77), Self-Monitoring (n = 69), | Participants who received interventions experienced greater improvement in global psychopathology than TAU. Community functioning improved more in the CBT2go vs. TAU condition |
| Mobilyze! [24] | Investigate the technical feasibility, functional reliability, and patient satisfaction EMA: Situation Symptom tracker Active data Passive data EMI: Behavioral activation approach | Eight adults with major depressive disorder in a single-arm pilot study for 8 weeks. | Intent-to-treat analyses revealed that depressive symptoms self-reported on the PHQ-9 decreased significantly over time |
| PsyMate [42] | Assess if ESM-derived personalized feedback can be used, in combination with standard antidepressant medication, as an effective add-on treatment for depressive symptoms EMA: Symptom tracker Active data (ESM) EMI: ESM-derived feedback (face-to-face contact) | Controlled trial on depressed patients randomly assigned to three arms: experimental (n = 33), pseudoexperimental (n = 36) or control group (n = 33) for 6 weeks. | The experimental group demonstrated a significantly greater weekly decline in depressive symptoms over the complete study period compared to the control group |
| Help4Mood [30] | Evaluate system use and acceptability, explore likely recruitment and retention rates and to obtain an estimate of potential treatment response EMA: Symptom tracker Active data Passive data EMI: Cognitive Behavioral Therapy | Multicentric RCT on 27 patients with MDD randomized to intervention + TAU or TAU alone for 4 weeks | ITT analysis showed a small difference in change of BDI-2 scores, but post hoc on-treatment analysis suggested that participants who used Help4Mood regularly experienced a median change in BDI-2 of -8 points |
| Therap-i [33] | Test the efficacy of the Therap-i module as a supportive tool in psychotherapeutic TAU in MDD patients EMA: Personalized items Active data EMI: EMA-derived feedback | Pragmatic RCT on 100 MDD patient randomized in the intervention group or TAU for 8 weeks | Data collection is ongoing |
| LiveWell [31] | Support the ongoing improvement and dissemination of technology-based mental health interventions. EMA: Wellness Plan Daily Check-in Active data EMI: Information on bipolar disorder self-management, Toolbox (skills practice), and Daily Review, lifestyle personalized plan for reducing risk | 12 individuals with bipolar disorder participated in a field trial and an 8-week pilot study | Users reported that they were more aware of early warning signs and symptoms |
| PRIME [34] | Evaluate the efficacy of PRIME by assessing changes in components of motivated behavior using a modified version of the Trust Task EMA: Self-determined goals Active data EMI: EMA-triggered display of brief challenges, CBT, behavioral activation, mindfulness, and psychoeducation | RCT, 43 people with recent-onset schizophrenia spectrum disorders were randomized into the PRIME (n = 22) or TAU/waitlist (WL) (n = 21) during 12 weeks | Participants in the PRIME condition showed a greater increase from baseline to 12 weeks compared to WL |
| MASS [35] | Ongoing EMA: Social goals Steps Motivation Active data EMI: Custom feedback, encouragement and video clip made for improving social skills | Ongoing | Ongoing |
| ICanSTEP [36] | Evaluate whether wearable activity tracker with personalized text message feedback would increase physical activity. EMA: Activity Tracker Passive data EMI: Daily text messages personalized to their activity level | Pilot study on 30 patients with solid tumor cancers in a nonrandomized, prospective intervention trial lasting 3 months | 39% of participants increased their steps taken by at least 20%, and 23% increased their 6 MW distance by 20%. At 3 months, there was a significant improvement in median BDI-II. |
| Hiroshima Health Note [37] | Evaluate whether an Information and Communication Technology (ICT) application motivated to increase adherence to lifestyle changes, and to improve indicators of metabolic disturbances EMA: Physiological signs Active data Passive data EMI: Tailored feedback Reminders encouraging participants to review their own data and to continue with behavioral changes | Nonrandomized, open-label, parallel-group study on 102 overweight or elevated glucose-concentration participants over 6 months. In total, 63 were allocated to intervention (ICT) and 39 to the control group | ICT group showed a significant decrease in male waist circumference, diastolic BP, and HbA1c and increase in HDL cholesterol |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mouchabac, S.; Maatoug, R.; Conejero, I.; Adrien, V.; Bonnot, O.; Millet, B.; Ferreri, F.; Bourla, A. In Search of Digital Dopamine: How Apps Can Motivate Depressed Patients, a Review and Conceptual Analysis. Brain Sci. 2021, 11, 1454. https://doi.org/10.3390/brainsci11111454
Mouchabac S, Maatoug R, Conejero I, Adrien V, Bonnot O, Millet B, Ferreri F, Bourla A. In Search of Digital Dopamine: How Apps Can Motivate Depressed Patients, a Review and Conceptual Analysis. Brain Sciences. 2021; 11(11):1454. https://doi.org/10.3390/brainsci11111454
Chicago/Turabian StyleMouchabac, Stephane, Redwan Maatoug, Ismael Conejero, Vladimir Adrien, Olivier Bonnot, Bruno Millet, Florian Ferreri, and Alexis Bourla. 2021. "In Search of Digital Dopamine: How Apps Can Motivate Depressed Patients, a Review and Conceptual Analysis" Brain Sciences 11, no. 11: 1454. https://doi.org/10.3390/brainsci11111454
APA StyleMouchabac, S., Maatoug, R., Conejero, I., Adrien, V., Bonnot, O., Millet, B., Ferreri, F., & Bourla, A. (2021). In Search of Digital Dopamine: How Apps Can Motivate Depressed Patients, a Review and Conceptual Analysis. Brain Sciences, 11(11), 1454. https://doi.org/10.3390/brainsci11111454






