Comparative Efficacy of Levetiracetam for Epilepsy in School-Aged Children with Intellectual Disability and Normal Intelligence
Abstract
:1. Introduction
2. Methods
3. Results
3.1. Demographics and Clinical Characteristics
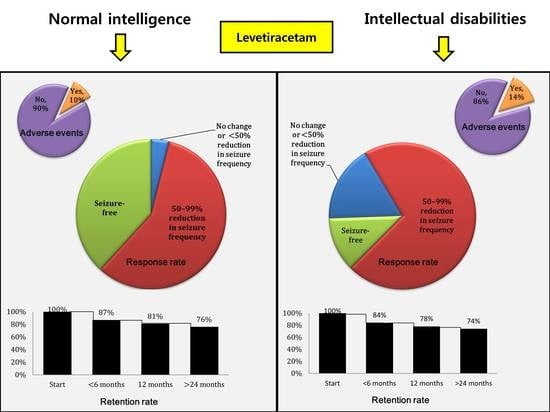
3.2. Adverse Effects
3.3. Response Rate
3.4. Retention Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berg, A.T. Risk of recurrence after a first unprovoked seizure. Epilepsia 2008, 49, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Coulter, D.L. Comprehensive management of epilepsy in persons with mental retardation. Epilepsia 1997, 38, S24–S31. [Google Scholar] [CrossRef]
- Berg, A.T.; Langfitt, J.T.; Testa, F.M.; Levy, S.R.; DiMario, F.; Westerveld, M.; Kulas, J. Global cognitive function in children with epilepsy: A community-based study. Epilepsia 2008, 49, 608–614. [Google Scholar] [CrossRef]
- Weijenberg, A.; Brouwer, O.F.; Callenbach, P.M. Levetiracetam Monotherapy in Children with Epilepsy: A Systematic Review. CNS Drugs 2015, 29, 371–382. [Google Scholar] [CrossRef] [Green Version]
- Lynch, B.A.; Lambeng, N.; Nocka, K.; Kensel-Hammes, P.; Bajjalieh, S.M.; Matagne, A.; Fuks, B. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc. Natl. Acad. Sci. USA 2004, 101, 9861–9866. [Google Scholar] [CrossRef] [Green Version]
- Levisohn, P.M.; Mintz, M.; Hunter, S.J.; Yang, H.; Jones, J. Neurocognitive effects of adjunctive levetiracetam in children with partial-onset seizures: A randomized, double-blind, placebo-controlled, noninferiority trial. Epilepsia 2009, 50, 2377–2389. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, J.H.; Elger, C.E.; Engel, J., Jr.; Forsgren, L.; French, J.A.; Glynn, M.; et al. ILAE official report: A practical clinical definition of epilepsy. Epilepsia 2014, 55, 475–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, R.S.; Cross, J.H.; French, J.A.; Higurashi, N.; Hirsch, E.; Jansen, F.E.; Lagae, L.; Moshé, S.L.; Peltola, J.; Roulet Perez, E.; et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 522–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lhatoo, S.D.; Sander, J.W. The epidemiology of epilepsy and learning disability. Epilepsia 2001, 42, 6–9. [Google Scholar] [CrossRef]
- Robertson, J.; Hatton, C.; Emerson, E.; Baines, S. Prevalence of epilepsy among people with intellectual disabilities: A systematic review. Seizure 2015, 29, 46–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doran, Z.; Shankar, R.; Keezer, M.R.; Dale, C.; McLean, B.; Kerr, M.P.; Devapriam, J.; Craig, J.; Sander, J.W. Managing anti-epileptic drug treatment in adult patients with intellectual disability: A serious conundrum. Eur. J. Neurol. 2016, 23, 1152–1157. [Google Scholar] [CrossRef]
- Forsgren, L.; Edvinsson, S.O.; Blomquist, H.K.; Heijbel, J.; Sidenvall, R. Epilepsy in a population of mentally retarded children and adults. Epilepsy Res. 1990, 6, 234–248. [Google Scholar] [CrossRef]
- Buelow, J.M.; Shore, C.P. Management challenges in children with both epilepsy and intellectual disability. Clin. Nurse Spec. CNS 2010, 24, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Ijff, D.M.; Aldenkamp, A.P. Cognitive side-effects of antiepileptic drugs in children. Handb Clin. Neurol. 2013, 111, 707–718. [Google Scholar] [CrossRef] [Green Version]
- Loring, D.W.; Meador, K.J. Cognitive side effects of antiepileptic drugs in children. Neurology 2004, 62, 872–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickrell, W.O.; Lacey, A.S.; Thomas, R.H.; Lyons, R.A.; Smith, P.E.; Rees, M.I. Trends in the first antiepileptic drug prescribed for epilepsy between 2000 and 2010. Seizure 2014, 23, 77–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Menachem, E.; Gilland, E. Efficacy and tolerability of levetiracetam during 1-year follow-up in patients with refractory epilepsy. Seizure 2003, 12, 131–135. [Google Scholar] [CrossRef] [Green Version]
- Cortes, C.; Manterola, C. Behavioral alterations associated with levetiracetam in pediatric epilepsy. Epilepsy Behav. 2020, 112, 107472. [Google Scholar] [CrossRef] [PubMed]
- Harden, C. Safety profile of levetiracetam. Epilepsia 2001, 42, 36–39. [Google Scholar] [CrossRef]
- Glauser, T.A. Behavioral and psychiatric adverse events associated with antiepileptic drugs commonly used in pediatric patients. J. Child Neurol. 2004, 19, S25–S38. [Google Scholar] [CrossRef]
- Szucs, A.; Clemens, Z.; Jakus, R.; Rásonyi, G.; Fabó, D.; Holló, A.; Barcs, G.; Kelemen, A.; Janszky, J. The risk of paradoxical levetiracetam effect is increased in mentally retarded patients. Epilepsia 2008, 49, 1174–1179. [Google Scholar] [CrossRef]
- Zhou, S.; Zhan, Q.; Wu, X. Effect of Levetiracetam on Cognitive Function and Clonic Seizure Frequency in Children with Epilepsy. Curr. Mol. Med. 2019, 19, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Aldenkamp, A.; Besag, F.; Gobbi, G.; Caplan, R.; Dunn, D.W.; Sillanpää, M. Psychiatric and Behavioural Disorders in Children with Epilepsy (ILAE Task Force Report): Adverse cognitive and behavioural effects of antiepileptic drugs in children. Epileptic Disord. Int. Epilepsy J. Videotape 2016, 18, S55–S67. [Google Scholar] [CrossRef]
- Brodtkorb, E.; Klees, T.M.; Nakken, K.O.; Lossius, R.; Johannessen, S.I. Levetiracetam in adult patients with and without learning disability: Focus on behavioral adverse effects. Epilepsy Behav. 2004, 5, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Tekgül, H.; Gencpinar, P.; Çavuşoğlu, D.; Dündar, N.O. The efficacy, tolerability and safety of levetiracetam therapy in a pediatric population. Seizure 2016, 36, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Lagae, L.; Buyse, G.; Ceulemans, B. Clinical experience with levetiracetam in childhood epilepsy: An add-on and mono-therapy trial. Seizure 2005, 14, 66–71. [Google Scholar] [CrossRef] [Green Version]
- Gümüş, H.; Kumandaş, S.; Per, H. Levetiracetam monotherapy in newly diagnosed cryptogenic West syndrome. Pediatric Neurol. 2007, 37, 350–353. [Google Scholar] [CrossRef]
- Li, J.; Xiao, N.; Chen, S. Efficacy and tolerability of levetiracetam in children with epilepsy. Brain Dev. 2011, 33, 145–151. [Google Scholar] [CrossRef]
- Huber, B.; Bömmel, W.; Hauser, I.; Horstmann, V.; Liem, S.; May, T.; Meinert, T.; Robertson, E.; Schulz, L.; Seidel, M.; et al. Efficacy and tolerability of levetiracetam in patients with therapy-resistant epilepsy and learning disabilities. Seizure 2004, 13, 168–175. [Google Scholar] [CrossRef] [Green Version]
- Peake, D.; Mordekar, S.; Gosalakkal, J.; Mukhtyar, B.; Buch, S.; Crane, J.; Wheway, R.; Rittey, C.; Donnelly, J.; Whitehouse, W.P.; et al. Retention rate of levetiracetam in children with intractable epilepsy at 1 year. Seizure 2007, 16, 185–189. [Google Scholar] [CrossRef] [Green Version]
- Symonds, J.D.; Zuberi, S.M.; Johnson, M.R. Advances in epilepsy gene discovery and implications for epilepsy diagnosis and treatment. Curr. Opin. Neurol. 2017, 30, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Perucca, P.; Bahlo, M.; Berkovic, S.F. The Genetics of Epilepsy. Annu. Rev. Genom. Hum. Genet. 2020, 21, 205–230. [Google Scholar] [CrossRef] [PubMed]
- Dilena, R.; Striano, P.; Traverso, M.; Viri, M.; Cristofori, G.; Tadini, L.; Barbieri, S.; Romeo, A.; Zara, F. Dramatic effect of levetiracetam in early-onset epileptic encephalopathy due to STXBP1 mutation. Brain Dev. 2016, 38, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, M.; Barth, M.; Gueden, S.; Desbordes de Cepoy, P.; Aeby, A.; Vilain, C.; Hirsch, E.; de Saint Martin, A.; Portes, V.D.; Lesca, G.; et al. CACNA1A-associated epilepsy: Electroclinical findings and treatment response on seizures in 18 patients. Eur. J. Paediatr. Neurol. 2021, 33, 75–85. [Google Scholar] [CrossRef] [PubMed]


| Variables | Normal Intelligence n = 147 (49%) | Intellectual Disability n = 151 (51%) | p Value |
|---|---|---|---|
| Gender, n (%) | 0.918 | ||
| Female: Male | 70 (48%): 77 (52%) | 71 (47%): 80 (53%) | |
| Mean age, years (±SD) | 12.3 (±4.6); | 12.0 (±4.7); | 0.422 |
| Mean age at seizure onset, years (±SD) | 5.5 (±4.6) | 4.5 (±4.3) | 0.232 |
| Seizure type, n (%) | 0.390 | ||
| Localized | 28 (19%) | 17 (11%) | |
| Generalized | 83 (56%) | 81 (54%) | |
| Mixed/Unclassified | 36 (25%) | 53 (35%) | |
| Etiology of epilepsy, n (%) | 0.074 | ||
| Cerebral infection | 4 (2.5%) | 4 (2.5%) | |
| Perinatal problems | 7 (5%) | 30 (20%) | |
| Structural malformation | 6 (4%) | 43 (29%) | |
| Metabolic abnormality | 3 (2%) | 5 (3.5%) | |
| Vascular abnormality | 6 (4%) | 8 (5%) | |
| Tumor | 4 (2.5%) | 4 (2.5%) | |
| Trauma | 3 (2%) | 5 (3.5%) | |
| Genetic | 10 (7%) | 12 (8%) | |
| Idiopathic/Unknown | 104 (71%) | 40 (26%) | |
| Brain MRI, n (%) | 0.044 | ||
| Normal | 101 (69%) | 47 (31%) | |
| Abnormal | 44 (30%) | 99 (66%) | |
| Missing data | 2 (1%) | 5 (3%) | |
| Mean maximal dosage of LEV (mg/kg/day) | 33.0 | 33.5 | 0.517 |
| Monotherapy, n (%) | 51 (35%) | 36 (24%) | 0.047 |
| Polytherapy, n (%) | 96 (65%) | 115 (76%) | |
| Number of concomitant ASMs | |||
| 1 | 68 | 50 | |
| 2 | 21 | 36 | |
| ≥3 | 7 | 29 |
| Variables | Normal Intelligence (n = 147) | Intellectual Disability (n = 151) | p Value |
|---|---|---|---|
| Response to LEV (a) | 0.031 | ||
| No change or <50% | 5 (4%) | 25 (17%) | |
| 50–99% | 87 (59%) | 108 (71%) | |
| Seizure-free | 55 (37%) | 18 (12%) | |
| Adverse event | 0.397 | ||
| No | 127 (86%) | 136 (90%) | |
| Yes | 20 (14%) | 15 (10%) | |
| Retention rate (%) | 0.612 | ||
| <6 months | 128 (87%) | 129 (84%) | |
| 12 months | 120 (81%) | 118 (78%) | |
| >24 months | 112 (76%) | 111 (74%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, J.U.; Han, J.Y. Comparative Efficacy of Levetiracetam for Epilepsy in School-Aged Children with Intellectual Disability and Normal Intelligence. Brain Sci. 2021, 11, 1452. https://doi.org/10.3390/brainsci11111452
Moon JU, Han JY. Comparative Efficacy of Levetiracetam for Epilepsy in School-Aged Children with Intellectual Disability and Normal Intelligence. Brain Sciences. 2021; 11(11):1452. https://doi.org/10.3390/brainsci11111452
Chicago/Turabian StyleMoon, Ja Un, and Ji Yoon Han. 2021. "Comparative Efficacy of Levetiracetam for Epilepsy in School-Aged Children with Intellectual Disability and Normal Intelligence" Brain Sciences 11, no. 11: 1452. https://doi.org/10.3390/brainsci11111452
APA StyleMoon, J. U., & Han, J. Y. (2021). Comparative Efficacy of Levetiracetam for Epilepsy in School-Aged Children with Intellectual Disability and Normal Intelligence. Brain Sciences, 11(11), 1452. https://doi.org/10.3390/brainsci11111452