Effects of tDCS on Language Recovery in Post-Stroke Aphasia: A Pilot Study Investigating Clinical Parameters and White Matter Change with Diffusion Imaging
Abstract
:1. Introduction
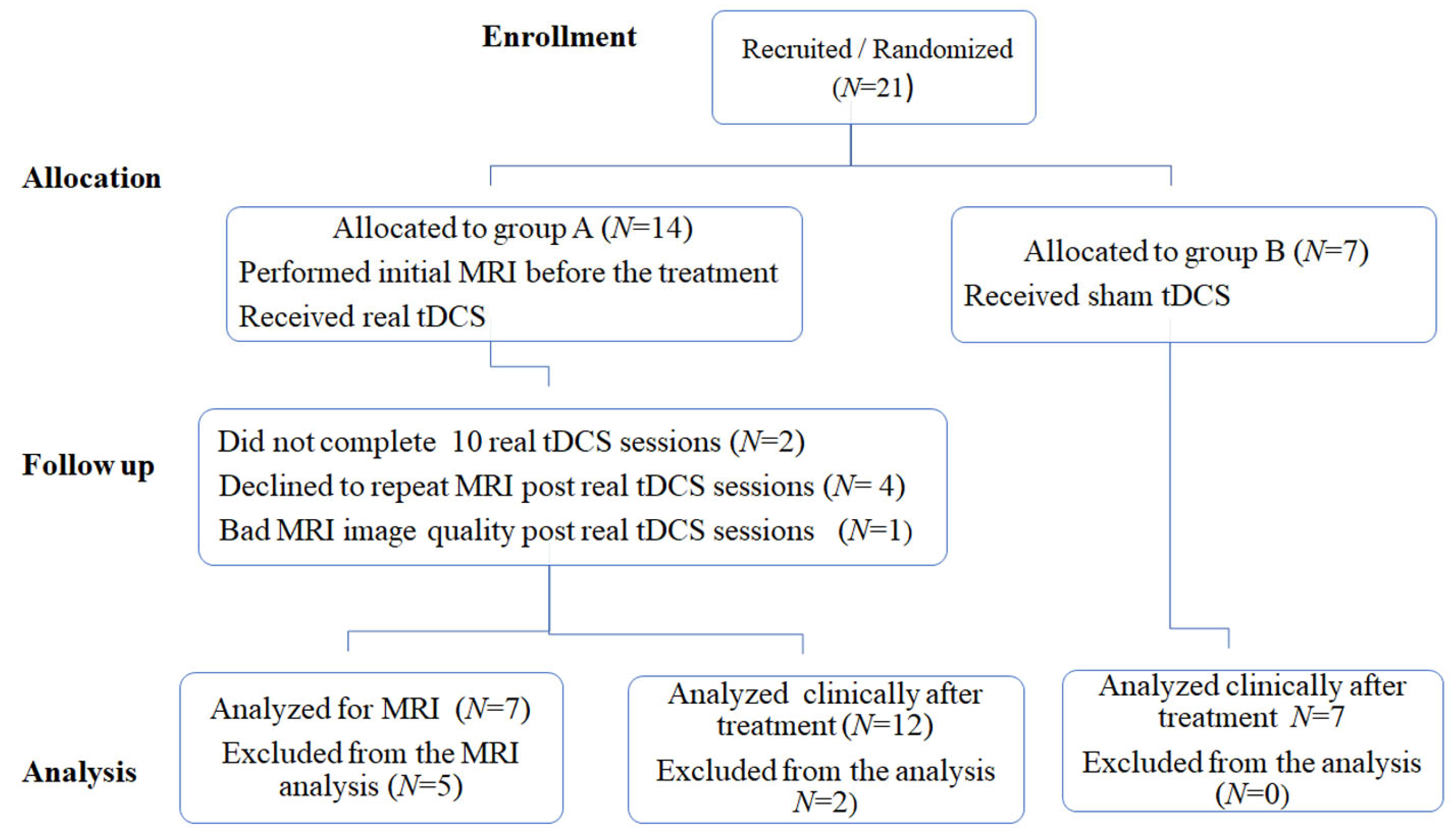
2. Materials and Methods
2.1. Patients
2.2. Clinical Assessment
2.3. tDSC Procedure
2.4. MRI Examination
2.4.1. MRI Protocol
2.4.2. Imaging Processing and Analysis
Processing of T1-WI Data
Processing of Diffusion Data
2.5. Statistical Analysis
3. Results
3.1. Demographic and Clinical Data
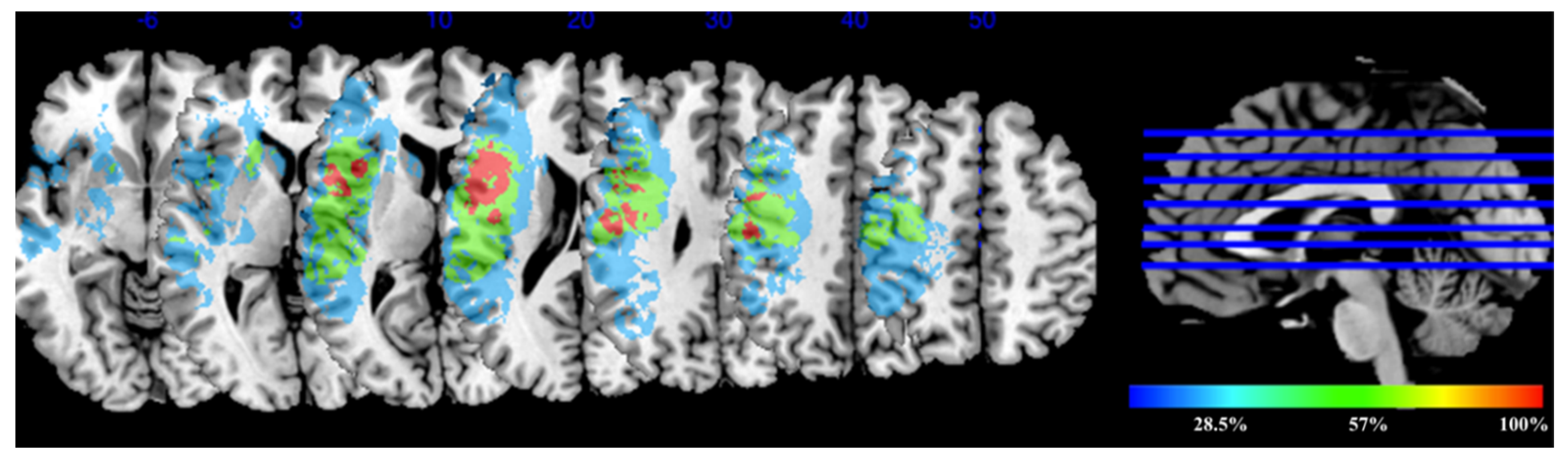
3.2. Lesion Overlay Map for the Experimental Group Receiving tDCS
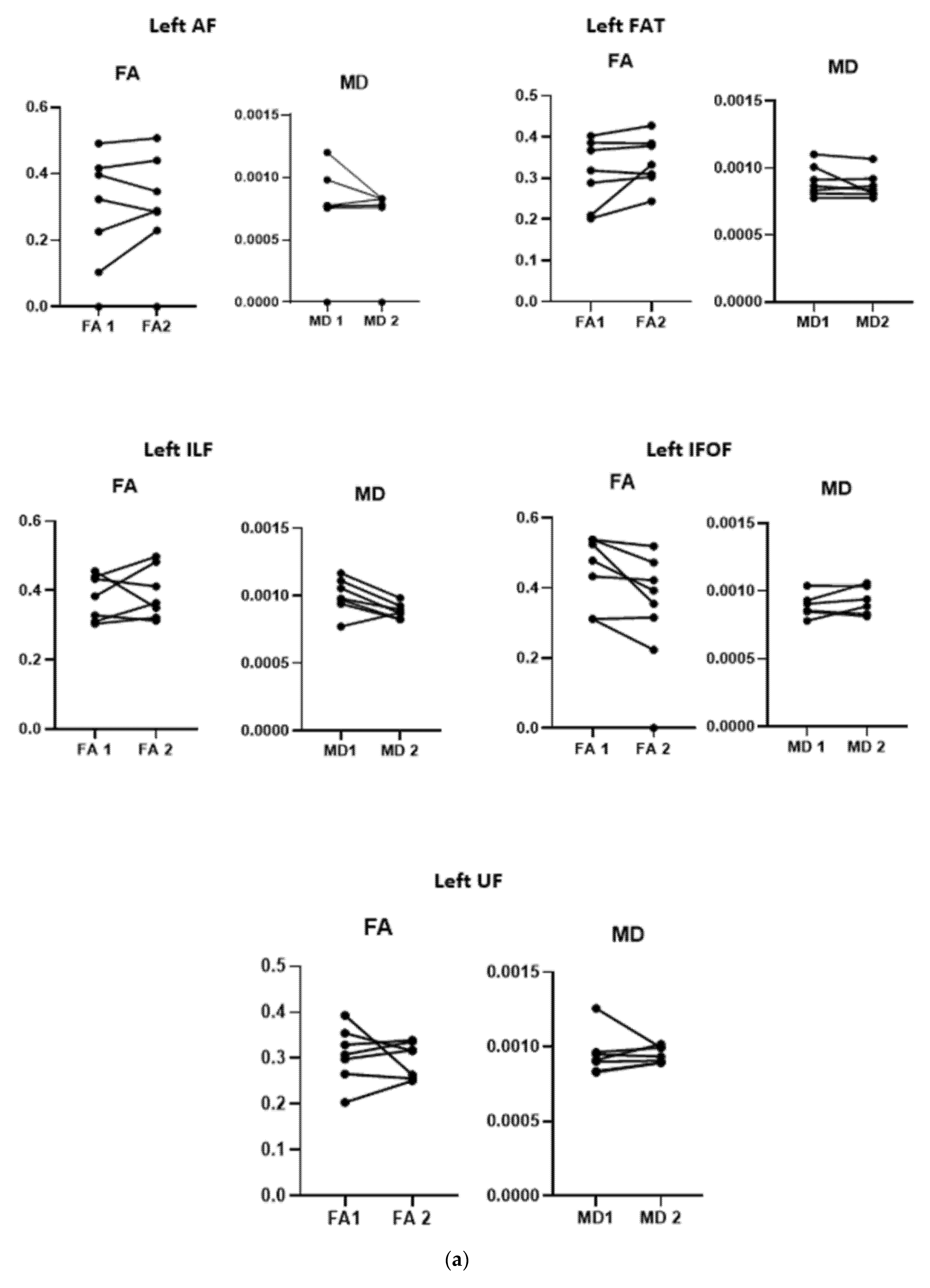
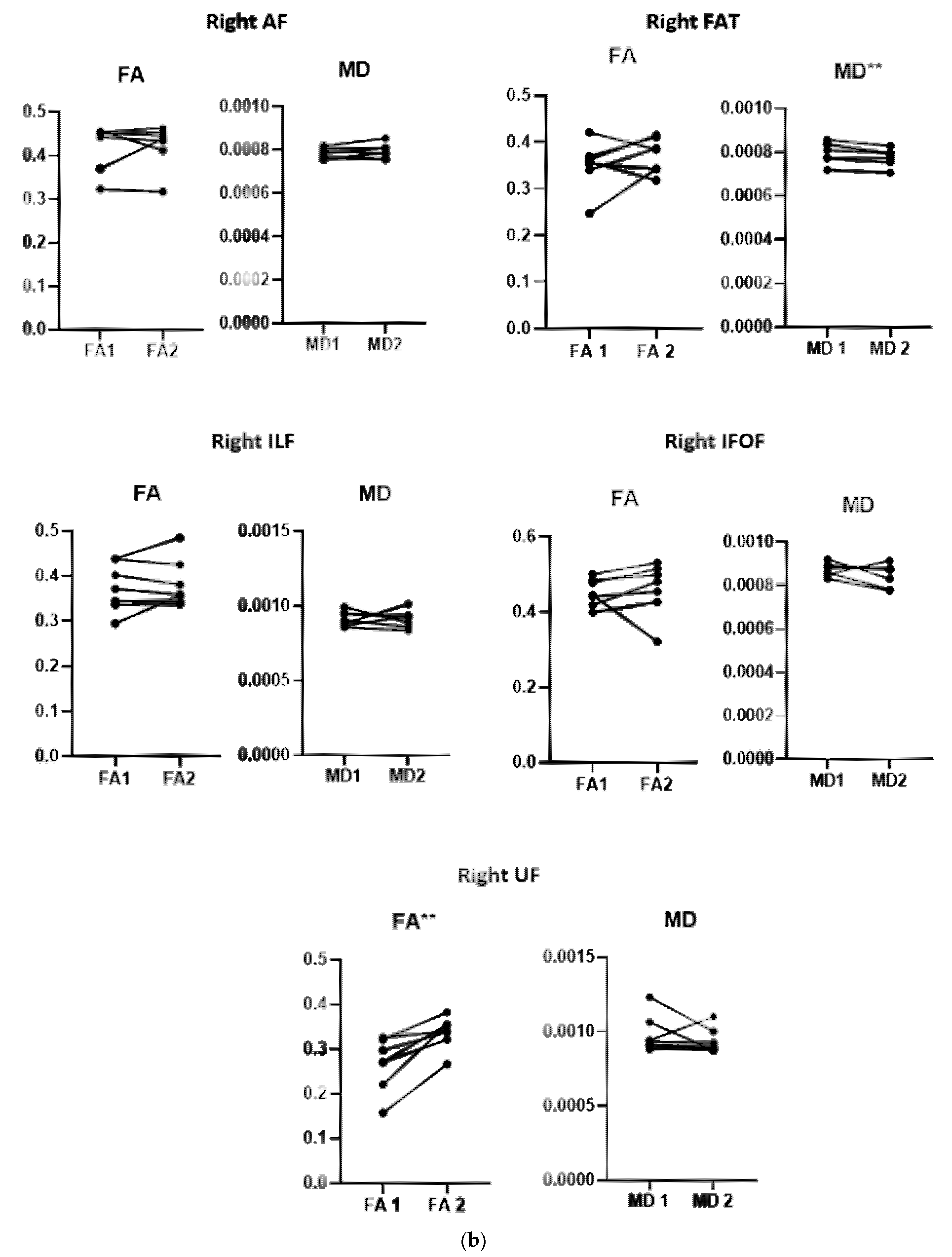
3.3. Diffusion Data
3.4. Correlation between Changes in DTI Measures and Behavioral Improvement
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lazar, R.M.; Minzer, B.; Antoniello, D.; Festa, J.R.; Krakauer, J.W.; Marshall, R.S. Improvement in aphasia scores after stroke is well predicted by initial severity. Stroke 2010, 41, 1485–1488. [Google Scholar] [CrossRef] [Green Version]
- Biou, E.; Cassoudesalle, H.; Cogné, M.; Sibon, I.; De Gabory, I.; Dehail, P.; Aupy, J.; Glize, B. Transcranial direct current stimulation in post-stroke aphasia rehabilitation: A systematic review. Ann. Phys. Rehabil. Med. 2019, 62, 104–121. [Google Scholar] [CrossRef] [PubMed]
- Bindman, L.J.; Lippold, O.C.; Redfearn, J.W. The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. J. Physiol. 1964, 172, 369–382. [Google Scholar] [CrossRef]
- Creutzfeldt, O.D.; Fromm, G.H.; Kapp, H. Influence of transcortical d-c currents on cortical neuronal activity. Exp. Neurol. 1962, 5, 436–452. [Google Scholar] [CrossRef]
- Gartside, I.B. Mechanisms of sustained increases of firing rate of neurones in the rat cerebral cortex after polarization: Role of protein synthesis. Nature 1968, 220, 383–384. [Google Scholar] [CrossRef] [PubMed]
- Terzuolo, C.A.; Bullock, T.H. Measurement of imposed voltage gradient adequate to modulate neuronal firing. Proc. Natl. Acad. Sci. USA 1956, 42, 687–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolzoni, F.; Pettersson, L.G.; Jankowska, E. Evidence for long-lasting subcortical facilitation by transcranial direct current stimulation in the cat. J. Physiol. 2013, 591, 3381–3399. [Google Scholar] [CrossRef]
- Zheng, X.; Schlaug, G. Structural white matter changes in descending motor tracts correlate with improvements in motor impairment after undergoing a treatment course of tDCS and physical therapy. Front. Hum. Neurosci. 2015, 9, 229. [Google Scholar] [CrossRef] [Green Version]
- Saur, D.; Lange, R.; Baumgaertner, A.; Schraknepper, V.; Willmes, K.; Rijntjes, M.; Weiller, C. Dynamics of language reorganization after stroke. Brain J. Neurol. 2006, 129, 1371–1384. [Google Scholar] [CrossRef] [Green Version]
- Warren, J.E.; Crinion, J.T.; Lambon Ralph, M.A.; Wise, R.J. Anterior temporal lobe connectivity correlates with functional outcome after aphasic stroke. Brain J. Neurol. 2009, 132, 3428–3442. [Google Scholar] [CrossRef] [Green Version]
- Allendorfer, J.B.; Storrs, J.M.; Szaflarski, J.P. Changes in white matter integrity follow excitatory rTMS treatment of post-stroke aphasia. Restor. Neurol. Neurosci. 2012, 30, 103–113. [Google Scholar] [CrossRef] [Green Version]
- McKinnon, E.T.; Fridriksson, J.; Glenn, G.R.; Jensen, J.H.; Helpern, J.A.; Basilakos, A.; Rorden, C.; Shih, A.Y.; Spampinato, M.V.; Bonilha, L. Structural plasticity of the ventral stream and aphasia recovery. Ann. Neurol. 2017, 82, 147–151. [Google Scholar] [CrossRef]
- Schlaug, G.; Marchina, S.; Norton, A. Evidence for plasticity in white-matter tracts of patients with chronic Broca’s aphasia undergoing intense intonation-based speech therapy. Ann. N. Y. Acad. Sci. 2009, 1169, 385–394. [Google Scholar] [CrossRef] [Green Version]
- van Hees, S.; McMahon, K.; Angwin, A.; de Zubicaray, G.; Read, S.; Copland, D.A. Changes in white matter connectivity following therapy for anomia post stroke. Neurorehabilit. Neural Repair 2014, 28, 325–334. [Google Scholar] [CrossRef]
- Wan, C.Y.; Zheng, X.; Marchina, S.; Norton, A.; Schlaug, G. Intensive therapy induces contralateral white matter changes in chronic stroke patients with Broca’s aphasia. Brain Lang. 2014, 136, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Zipse, L.; Norton, A.; Marchina, S.; Schlaug, G. When right is all that is left: Plasticity of right-hemisphere tracts in a young aphasic patient. Ann. N. Y. Acad. Sci. 2012, 1252, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.L.; Lee, J.E.; Lazar, M.; Field, A.S. Diffusion tensor imaging of the brain. Neurother. J. Am. Soc. Exp. NeuroTherapeutics 2007, 4, 316–329. [Google Scholar] [CrossRef] [Green Version]
- Beaulieu, C. The basis of anisotropic water diffusion in the nervous system—A technical review. NMR Biomed. 2002, 15, 435–455. [Google Scholar] [CrossRef] [PubMed]
- Catani, M.; Mesulam, M.M.; Jakobsen, E.; Malik, F.; Martersteck, A.; Wieneke, C.; Thompson, C.K.; Thiebaut de Schotten, M.; Dell’Acqua, F.; Weintraub, S.; et al. A novel frontal pathway underlies verbal fluency in primary progressive aphasia. Brain J. Neurol. 2013, 136, 2619–2628. [Google Scholar] [CrossRef] [Green Version]
- Kemerdere, R.; de Champfleur, N.M.; Deverdun, J.; Cochereau, J.; Moritz-Gasser, S.; Herbet, G.; Duffau, H. Role of the left frontal aslant tract in stuttering: A brain stimulation and tractographic study. J. Neurol. 2016, 263, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Lawes, I.N.; Barrick, T.R.; Murugam, V.; Spierings, N.; Evans, D.R.; Song, M.; Clark, C.A. Atlas-based segmentation of white matter tracts of the human brain using diffusion tensor tractography and comparison with classical dissection. NeuroImage 2008, 39, 62–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Ficek, B.; Webster, K.; Frangakis, C.; Caffo, B.; Hillis, A.E.; Faria, A.; Tsapkini, K. White Matter Integrity Predicts Electrical Stimulation (tDCS) and Language Therapy Effects in Primary Progressive Aphasia. Neurorehabilit. Neural Repair 2021, 35, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.J.; Meador, K.J.; Sethi, K.D.; Grotta, J.C.; Thomson, D.S. Graded neurologic scale for use in acute hemispheric stroke treatment protocols. Stroke 1987, 18, 665–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gough, P.M.; Nobre, A.C.; Devlin, J.T. Dissociating linguistic processes in the left inferior frontal cortex with transcranial magnetic stimulation. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 8010–8016. [Google Scholar] [CrossRef] [Green Version]
- Gandiga, P.C.; Hummel, F.C.; Cohen, L.G. Transcranial DC stimulation (tDCS): A tool for double-blind sham-controlled clinical studies in brain stimulation. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2006, 117, 845–850. [Google Scholar] [CrossRef]
- Godde, B.; Dadashev, L.; Karim, A.A. Effects of tDCS on Tactile Perception Depend on Tactile Expertise in Both Musicians and Non-Musicians. Brain Sci. 2020, 10. [Google Scholar] [CrossRef]
- Leemans, A.; Jeurissen, B.; Sijbers, J.; Jones, D. ExploreDTI: A graphical toolbox for processing, analyzing, and visualizing diffusion MR data. Proc. Intl. Soc. Mag. Reson. Med. 2009, 17, 3537. [Google Scholar]
- Perrone, D.; Aelterman, J.; Pižurica, A.; Jeurissen, B.; Philips, W.; Leemans, A. The effect of Gibbs ringing artifacts on measures derived from diffusion MRI. NeuroImage 2015, 120, 441–455. [Google Scholar] [CrossRef]
- Rohde, G.K.; Barnett, A.S.; Basser, P.J.; Marenco, S.; Pierpaoli, C. Comprehensive approach for correction of motion and distortion in diffusion-weighted MRI. Magn. Reson. Med. 2004, 51, 103–114. [Google Scholar] [CrossRef]
- Veraart, J.; Sijbers, J.; Sunaert, S.; Leemans, A.; Jeurissen, B. Weighted linear least squares estimation of diffusion MRI parameters: Strengths, limitations, and pitfalls. NeuroImage 2013, 81, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Tax, C.M.; Jeurissen, B.; Vos, S.B.; Viergever, M.A.; Leemans, A. Recursive calibration of the fiber response function for spherical deconvolution of diffusion MRI data. NeuroImage 2014, 86, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Tournier, J.D.; Calamante, F.; Connelly, A. Robust determination of the fibre orientation distribution in diffusion MRI: Non-negativity constrained super-resolved spherical deconvolution. NeuroImage 2007, 35, 1459–1472. [Google Scholar] [CrossRef] [PubMed]
- Fekonja, L.; Wang, Z.; Bährend, I.; Rosenstock, T.; Rösler, J.; Wallmeroth, L.; Vajkoczy, P.; Picht, T. Manual for clinical language tractography. Acta Neurochir. 2019, 161, 1125–1137. [Google Scholar] [CrossRef] [Green Version]
- Krogsrud, S.K.; Fjell, A.M.; Tamnes, C.K.; Grydeland, H.; Mork, L.; Due-Tønnessen, P.; Bjørnerud, A.; Sampaio-Baptista, C.; Andersson, J.; Johansen-Berg, H.; et al. Changes in white matter microstructure in the developing brain--A longitudinal diffusion tensor imaging study of children from 4 to 11years of age. NeuroImage 2016, 124, 473–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Z.; Ma, Y.; Gong, G.; He, Y.; Caramazza, A.; Bi, Y. White matter structural connectivity underlying semantic processing: Evidence from brain damaged patients. Brain J. Neurol. 2013, 136, 2952–2965. [Google Scholar] [CrossRef] [Green Version]
- Duffau, H.; Gatignol, P.; Moritz-Gasser, S.; Mandonnet, E. Is the left uncinate fasciculus essential for language? A cerebral stimulation study. J. Neurol. 2009, 256, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Hau, J.; Sarubbo, S.; Perchey, G.; Crivello, F.; Zago, L.; Mellet, E.; Jobard, G.; Joliot, M.; Mazoyer, B.M.; Tzourio-Mazoyer, N.; et al. Cortical Terminations of the Inferior Fronto-Occipital and Uncinate Fasciculi: Anatomical Stem-Based Virtual Dissection. Front. Neuroanat. 2016, 10, 58. [Google Scholar] [CrossRef] [Green Version]
- Thiebaut de Schotten, M.; Ffytche, D.H.; Bizzi, A.; Dell’Acqua, F.; Allin, M.; Walshe, M.; Murray, R.; Williams, S.C.; Murphy, D.G.; Catani, M. Atlasing location, asymmetry and inter-subject variability of white matter tracts in the human brain with MR diffusion tractography. NeuroImage 2011, 54, 49–59. [Google Scholar] [CrossRef]
- Hau, J.; Sarubbo, S.; Houde, J.C.; Corsini, F.; Girard, G.; Deledalle, C.; Crivello, F.; Zago, L.; Mellet, E.; Jobard, G.; et al. Revisiting the human uncinate fasciculus, its subcomponents and asymmetries with stem-based tractography and microdissection validation. Brain Struct. Funct. 2017, 222, 1645–1662. [Google Scholar] [CrossRef]
- Basilakos, A.; Fillmore, P.T.; Rorden, C.; Guo, D.; Bonilha, L.; Fridriksson, J. Regional white matter damage predicts speech fluency in chronic post-stroke aphasia. Front. Hum. Neurosci. 2014, 8, 845. [Google Scholar] [CrossRef] [Green Version]
- Fridriksson, J.; Guo, D.; Fillmore, P.; Holland, A.; Rorden, C. Damage to the anterior arcuate fasciculus predicts non-fluent speech production in aphasia. Brain J. Neurol. 2013, 136, 3451–3460. [Google Scholar] [CrossRef] [Green Version]
- Varriano, F.; Pascual-Diaz, S. When the FAT goes wide: Right extended Frontal Aslant Tract volume predicts performance on working memory tasks in healthy humans. PLoS ONE 2018, 13, e0200786. [Google Scholar] [CrossRef] [Green Version]
- Dick, A.S.; Garic, D.; Graziano, P.; Tremblay, P. The frontal aslant tract (FAT) and its role in speech, language and executive function. Cortex 2019, 111, 148–163. [Google Scholar] [CrossRef]
- Chenausky, K.; Kernbach, J.; Norton, A.; Schlaug, G. White Matter Integrity and Treatment-Based Change in Speech Performance in Minimally Verbal Children with Autism Spectrum Disorder. Front. Hum. Neurosci. 2017, 11, 175. [Google Scholar] [CrossRef]
- Breier, J.I.; Juranek, J.; Papanicolaou, A.C. Changes in maps of language function and the integrity of the arcuate fasciculus after therapy for chronic aphasia. Neurocase 2011, 17, 506–517. [Google Scholar] [CrossRef]
- Hope, T.M.H.; Leff, A.P.; Price, C.J. Predicting language outcomes after stroke: Is structural disconnection a useful predictor? NeuroImage Clin. 2018, 19, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Polanowska, K.E.; Leśniak, M.; Seniów, J.B.; Członkowska, A. No effects of anodal transcranial direct stimulation on language abilities in early rehabilitation of post-stroke aphasic patients. Neurol. Neurochir. Pol. 2013, 47, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Spielmann, K.; van de Sandt-Koenderman, W.M.E.; Heijenbrok-Kal, M.H.; Ribbers, G.M. Transcranial Direct Current Stimulation Does Not Improve Language Outcome in Subacute Poststroke Aphasia. Stroke 2018, 49, 1018–1020. [Google Scholar] [CrossRef] [PubMed]
- Feil, S.; Eisenhut, P.; Strakeljahn, F.; Müller, S.; Nauer, C.; Bansi, J.; Weber, S.; Liebs, A.; Lefaucheur, J.P.; Kesselring, J.; et al. Left Shifting of Language Related Activity Induced by Bihemispheric tDCS in Postacute Aphasia Following Stroke. Front. Neurosci. 2019, 13, 295. [Google Scholar] [CrossRef] [PubMed]
- You, D.S.; Kim, D.Y.; Chun, M.H.; Jung, S.E.; Park, S.J. Cathodal transcranial direct current stimulation of the right Wernicke’s area improves comprehension in subacute stroke patients. Brain Lang. 2011, 119, 1–5. [Google Scholar] [CrossRef]
- Calamuneri, A.; Arrigo, A.; Mormina, E.; Milardi, D.; Cacciola, A.; Chillemi, G.; Marino, S.; Gaeta, M.; Quartarone, A. White Matter Tissue Quantification at Low b-Values Within Constrained Spherical Deconvolution Framework. Front. Neurol. 2018, 9, 716. [Google Scholar] [CrossRef] [PubMed]
- Arrigoni, F.; Peruzzo, D. Characterizing White Matter Tract Organization in Polymicrogyria and Lissencephaly: A Multifiber Diffusion MRI Modeling and Tractography Study. Am. J. Neuroradiol. 2020, 41, 1495–1502. [Google Scholar] [CrossRef] [PubMed]


| Features | Patients Receiving Real tDCS n = 12 | Patients Receiving Sham tDCS n = 7 | p-Value |
|---|---|---|---|
| Age mean ± SD (range)years | 52.58 ± 13.12 (32–68) | 53.71 ± 6.89 (45–61) | 0.809 |
| Gender | 0.830 | ||
| Female number (%) | 4 (33.4) | 2 (28.6) | |
| Male number (%) | 8 (66.6) | 5 (71.4) | |
| Time passed from the onset mean ± SD (range) weeks | 3.08 ± 1.42 (2–6) | 3.14 ± 1.77 (1–5) | 0.93 |
| Total HSS language score Mean ± SD (range) | 17.08 ± 3.17 (9–20) | 18.28 ± 1.60 (17–20) | 0.289 |
| Aphasia type | |||
| Broca’s aphasia number (%) | 4 (33.4) | 3 (42.8) | 0.678 |
| Global aphasia number (%) | 8 (66.6) | 4 (57.2) | |
| NIHSS mean ± SD (range) | 8.52 ± 4.75 (5–17) | 10.0 ± 2.58 (7–13) | 0.313 |
| Nr | Group | Age | Sex | Education/ Years of Education | Risk Factors | NIHSS pre | NIHSS Post | Duration Elapsed from Onset to Session Beginning | Brain Imaging | PRE Total Language HSS Score | Post 10th Session, Total Language HSS Score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Real | 34 | M | Literate/ 12 years | Rheumatic heart disease | 6 | 1 | 4 weeks | Left BG and parietal and temporal operculum | 14 | 9 |
| 2 | Real | 68 | M | Literate/ 6 years | Hypertension, TIA | 9 | 7 | 2 weeks | Left insula and frontal and temporal operculum | 15 | 11 |
| 3 | Real | 58 | M | Literate/ 8 years | Hypertension History of old stroke, IHD | 5 | 2 | 3 weeks | Left Posterior insula, temporal and parietal operculum and post-rolandic region | 18 | 12 |
| 4 | Real | 52 | M | Literate/ 6 years | History of old stroke, hypertension. | 9 | 7 | 3 weeks | Left Posterior insula and parietal operculum | 17 | 12 |
| 5 | Real | 55 | M | Illiterate/ read and write | IHD, TIA | 17 | 15 | 2 weeks | Left insula and operculum | 9 | 7 |
| 6 | Real | 67 | M | Literate/ 6 years | hypertension | 5 | 2 | 2 weeks | Left pre-rolandic region | 18 | 11 |
| 7 | Real | 56 | F | Illiterate | Hypertension DM, history of old stroke | 17 | 14 | 4 weeks | Left full MCA territory | 20 | 18 |
| 8 | Real | 65 | F | Illiterate | Hypertension old stroke IHD | 11 | 11 | 6 weeks | Left insula, frontal operculum and pre-rolandic region. | 17 | 9 |
| 9 | Real | 32 | F | Literate/ 12 years | None | 5 | 1 | 4 weeks | Left full MCA territory | 19 | 13 |
| 10 | Real | 32 | F | Literate/ 12 years | TIA | 2 | 0 | 2 weeks | Left anterior insula and frontal operculum | 18 | 16 |
| 11 | Real | 60 | M | Illiterate/ read and write | Hypertension, TIA | 8 | 6 | 3 weeks | Left Frontal operculum, rolandic and pre-rolandic regions | 20 | 20 |
| 12 | Real | 52 | M | Literate/9 years | Hypertension | 5 | 3 | 2 weeks | Pre-rolandic region | 20 | 20 |
| 13 | Sham | 61 | M | Literate/ 9 years | Hypertension smoker | 7 | 5 | 5 weeks | Full MCA territory | 20 | 18 |
| 14 | Sham | 45 | F | Literate/ 5 years | Hypertension | 7 | 7 | 1 week | BG, pre-rolandic region | 17 | 17 |
| 15 | Sham | 55 | M | Illiterate/ read and write | Heavy smoker | 11 | 10 | 2 weeks | Full MCA territor | 17 | 17 |
| 16 | Sham | 51 | M | Illiterate/ read and write | Hypertension smoker | 12 | 10 | 4 weeks | Insular and sub-insular region | 17 | 17 |
| 17 | Sham | 61 | M | Illiterate/ read and write | Hypertension smoker | 8 | 7 | 5 weeks | Left Posterior insula and parietal, operculum | 20 | 19 |
| 18 | Sham | 45 | F | Illiterate/ read and write | NONE | 12 | 12 | 1 week | Full MCA territory | 20 | 18 |
| 19 | Sham | 58 | M | Illiterate/ read and write | Hypertension smoker | 13 | 11 | 4 weeks | Operculum, rolandic and pre-rolandic regions | 17 | 17 |
| Experimental Group (Real tDCS) Post Session p-Value | Control Group (Sham tDCS) | ||||||
|---|---|---|---|---|---|---|---|
| Rating Scales | Pre-Session | Post-Session | p-Value | Pre-Session | Post-Session | p-Value | Two-Way ANOVA Repeated Measure Analysis Time X Groups |
| Total HSS language score | 17.1 ± 3.2 (9–20) | 13.2 ± 4.4 (7–19) | 0.0001 * | 18.3 ± 1.6 (17–20) | 17.6 ± 0.79 (17–18) | 0.09 | F = 9.1, df = 1 (17) 0.008 p = 0.02 |
| Comprehension score | 3.4 ± 2.1 (0–5) | 2.3 ± 1.8 (0–5) | 0.005 * | 3.7 ± 1.6 (2–5) | 3.4 ± 1.4 (2–5) | 0.172 | F = 3.3, df = 1 (17) 0.086 |
| Naming score | 4.2 ± 1.4 (0–5) | 3.3 ± 1.6 (0–5) | 0.005 * | 4.6 ± 1.1 (2–5) | 4.3 ± 1.1 (2–5) | 0.178 | F = 2.1, df = 1 (17) 0.107 |
| Repetition score | 4.7 ± 0.5 (4–5) | 3.6 ± 1.2 (2–5) | 0.002 * | 5 ± 0 (5–5) | 4.9 ± 0.38 (4–5) | 0.356 | F = 6.8, df = 1 (17) p = 0.018 0 |
| Fluency score | 4.8 ± 0.6 (3–5) | 3.9 ± 1.2 (2–5) | 0.017 * | 5.0 ± 0 (5–5) | 5.0 ± 0.0 (3–5) | 1.0 | F = 4.5, df = 1 (17) p = 0.049 |
| ΔFA and ΔMD (Indicating Significant Changes after tDCS Sessions) | ΔHSS Subsets | |||
|---|---|---|---|---|
| Comprehension | Naming | Repetition | Fluency | |
| Mean ΔFA of the right UF | r = −0.800, p = 0.056 | r = −0.241, p = 0.645 | r = −0.254, p = 0.627 | r = −0.811, p = 0.049 * |
| Mean ΔMD of the right FAT | r = 0.334, p = 0.518 | r = −0.461, p = 0.357 | r = −0.333, p = 0.519 | r = −0.480, p = 0.336 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soliman, R.K.; Tax, C.M.W.; Abo-Elfetoh, N.; Karim, A.A.; Youssef, A.; Kamal, D.; Khedr, E.M. Effects of tDCS on Language Recovery in Post-Stroke Aphasia: A Pilot Study Investigating Clinical Parameters and White Matter Change with Diffusion Imaging. Brain Sci. 2021, 11, 1277. https://doi.org/10.3390/brainsci11101277
Soliman RK, Tax CMW, Abo-Elfetoh N, Karim AA, Youssef A, Kamal D, Khedr EM. Effects of tDCS on Language Recovery in Post-Stroke Aphasia: A Pilot Study Investigating Clinical Parameters and White Matter Change with Diffusion Imaging. Brain Sciences. 2021; 11(10):1277. https://doi.org/10.3390/brainsci11101277
Chicago/Turabian StyleSoliman, Radwa K., Chantal M. W. Tax, Noha Abo-Elfetoh, Ahmed A. Karim, Ayda Youssef, Doaa Kamal, and Eman M. Khedr. 2021. "Effects of tDCS on Language Recovery in Post-Stroke Aphasia: A Pilot Study Investigating Clinical Parameters and White Matter Change with Diffusion Imaging" Brain Sciences 11, no. 10: 1277. https://doi.org/10.3390/brainsci11101277
APA StyleSoliman, R. K., Tax, C. M. W., Abo-Elfetoh, N., Karim, A. A., Youssef, A., Kamal, D., & Khedr, E. M. (2021). Effects of tDCS on Language Recovery in Post-Stroke Aphasia: A Pilot Study Investigating Clinical Parameters and White Matter Change with Diffusion Imaging. Brain Sciences, 11(10), 1277. https://doi.org/10.3390/brainsci11101277






