The Deficit of Multimodal Perception of Congruent and Non-Congruent Fearful Expressions in Patients with Schizophrenia: The ERP Study
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Ethical Statement
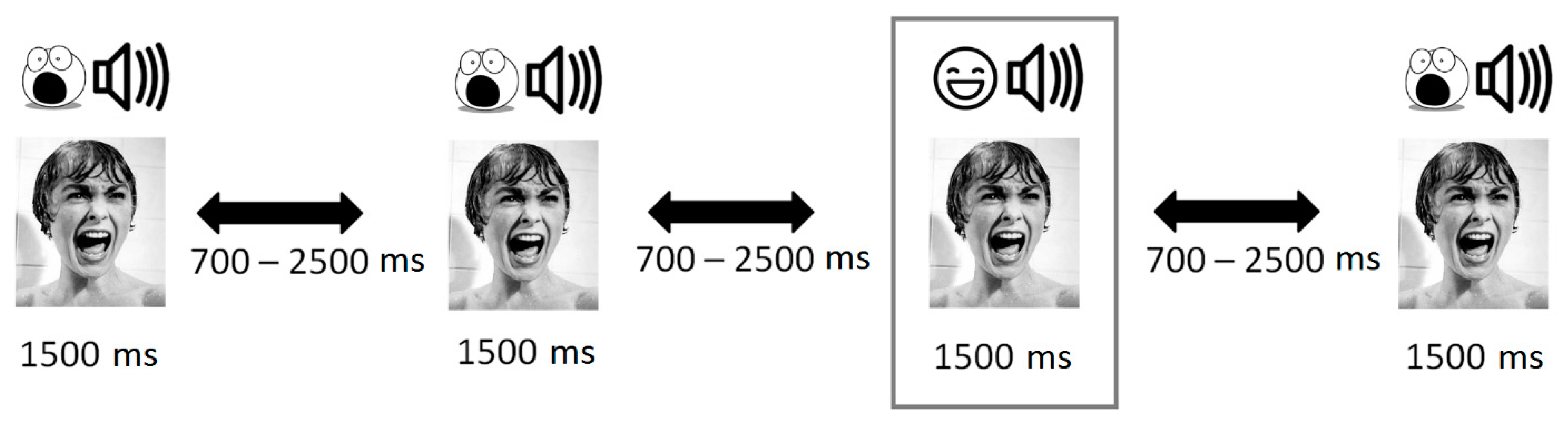
2.3. Stimuli Description
2.4. Procedure
2.5. Self-Assessment
2.6. Subjective Assessment of Stimuli
2.7. EEG Registration
2.8. Data Analysis
2.9. Event-Related Potential (ERP) Analysis
2.10. Statistical Analysis
3. Results
3.1. Differences in Subjective Ratings of Stimuli
3.2. Group Differences in ERP Components to Congruent and Non-Congruent Stimuli
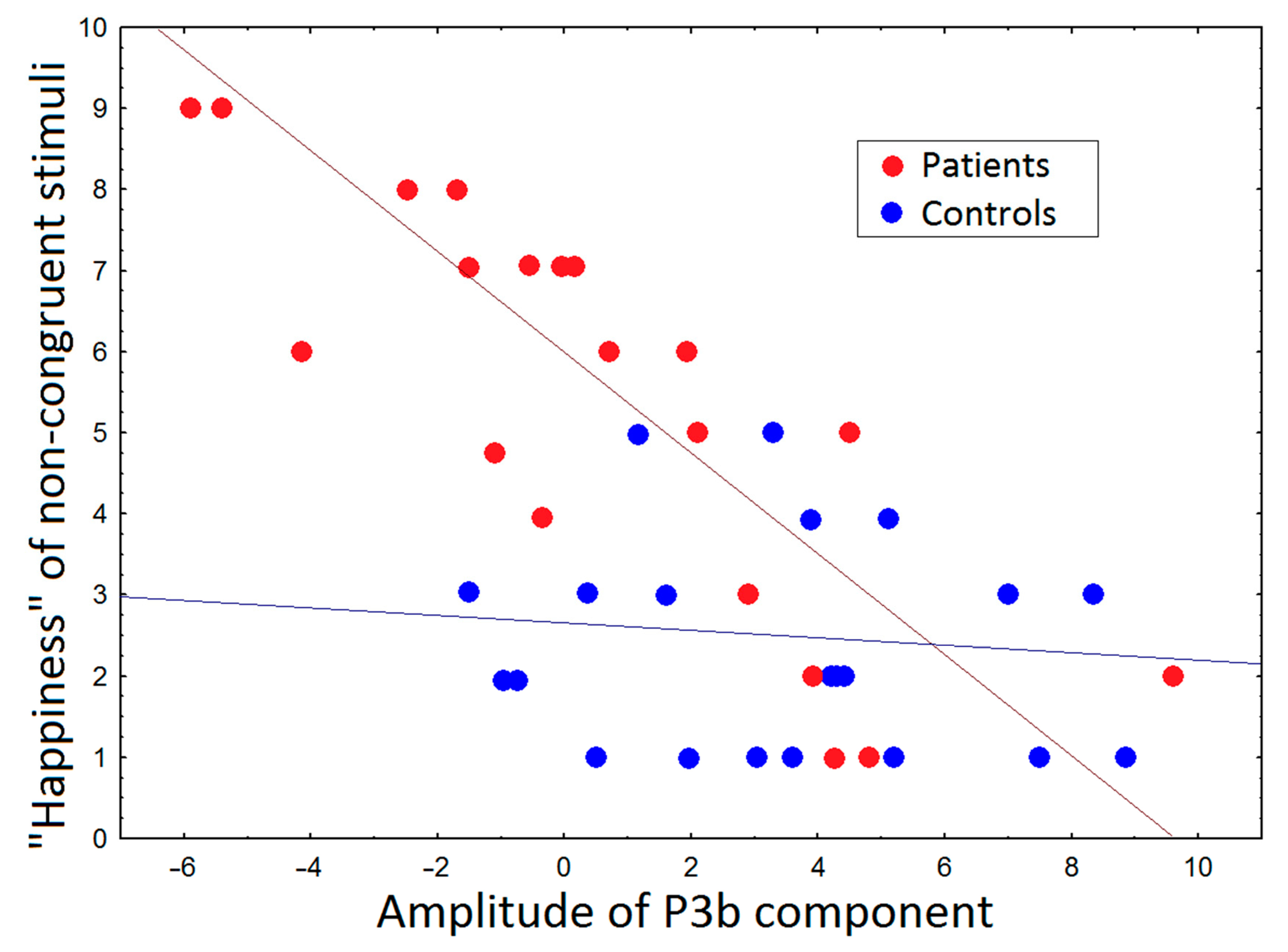
3.3. Correlations between ERP and Subjective Ratings and PANSS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bonfils, K.A.; Ventura, J.; Subotnik, K.L.; Nuechterlein, K.H. Affective prosody and facial emotion recognition in first-episode schizophrenia: Associations with functioning & symptoms. Schizophr. Res. Cogn. 2019, 18, 100153. [Google Scholar] [PubMed]
- Johnstone, T.; Reekum, C.M.; Oakes, T.R.; Davidson, R.J. The voice of emotion: An FMRI study of neural responses to angry and happy vocal expressions. Soc. Cogn. Affect. Neurosci. 2006, 1, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.I.; Kim, J.J.; Seok, J.H.; Chun, J.W.; Lee, S.K.; Park, H.J. Abnormal brain response during the auditory emotional processing in schizophrenic patients with chronic auditory hallucinations. Schizophr. Res. 2009, 107, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Schneider, F.; Gur, R.C.; Gur, R.E.; Shtasel, D.L. Emotional processing in schizophrenia: Neurobehavioral probes in relation to psychopathology. Schizophr. Res. 1995, 17, 67–75. [Google Scholar] [CrossRef]
- Kelley, N.J.; Schmeichel, B.J. The effects of negative emotions on sensory perception: Fear but not anger decreases tactile sensitivity. Front. Psychol. 2014, 5, 942. [Google Scholar] [PubMed]
- Phelps, E.A.; Ling, S.; Carrasco, M. Emotion facilitates perception and potentiates the perceptual benefits of attention. Psychol. Sci. 2006, 17, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Fellinger, R.; Gruber, W.; Zauner, A.; Freunberger, R.; Klimesch, W. Evoked traveling alpha waves predict visual-semantic categorization-speed. NeuroImage 2012, 59, 3379–3388. [Google Scholar] [CrossRef]
- Globerson, E.; Amir, N.; Kishon, R.L.; Golan, O. Prosody recognition in adults with high-functioning autism spectrum disorders: From psychoacoustics to cognition. Autism Res. 2015, 8, 153–163. [Google Scholar]
- Kring, A.M.; Moran, E.K. Emotional response deficits in schizophrenia: Insights from affective science. Schizophr. Bull. 2008, 34, 819–834. [Google Scholar] [CrossRef]
- Berenbaum, H.; Oltmanns, T.F. Emotional experience and expression in schizophrenia and depression. J. Abnorm. Psychol. 1992, 101, 37. [Google Scholar]
- Deutsch, H. Some forms of emotional disturbance and their relationship to schizophrenia. Psychoanal. Q. 1942, 11, 301–321. [Google Scholar] [CrossRef]
- Pinheiro, A.P.; Re, E.; Mezin, J.; Nestor, P.G.; Rauber, A.; Carley, R.W.; Gonc, A.F.; Niznikiewicz, M.A. Sensory-based and higher-order operations contribute to abnormal emotional prosody processing in schizophrenia: An electrophysiological investigation. Psychol. Med. 2013, 43, 603–618. [Google Scholar] [CrossRef] [PubMed]
- Tüscher, O.; Silbersweig, D.; Pan, H.; Smith, T.; Beutel, M.; Zonana, J.; Erbesh, V.; Weisholtz, D.; Stern, E.; Engelien, A. Processing of environmental sounds in schizophrenic patients: Disordered recognition and lack of semantic specificity. Schizophr. Res. 2005, 73, 291–295. [Google Scholar]
- Shah, D.; Knott, V.; Baddeley, A.; Bowers, H.; Wright, N.; Labelle, A.; Smith, D.; Collin, C. Impairments of emotional face processing in schizophrenia patients: Evidence from P100, N170 and P300 ERP components in a sample of auditory hallucinators. Int. J. Psychophysiol. 2018, 134, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Müller, V.I.; Kellermann, T.S.; Seligman, S.C.; Turetsky, B.I.; Eickhoff, S.B. Modulation of affective face processing deficits in schizophrenia by congruent emotional sounds. Soc. Cogn. Affect. Neurosci. 2014, 9, 436–444. [Google Scholar] [CrossRef]
- Lin, Y.; Ding, H.; Zhang, Y. Multisensory integration of emotion in schizophrenic patients. Multisens. Res. 2020, 33, 865–901. [Google Scholar] [CrossRef]
- Liu, T.; Pinheiro, A.P.; Zhao, Z.; Nestor, P.G.; Carley, R.W.; Niznikiewicz, M. Simultaneous face and voice processing in schizophrenia. Behav. Brain Res. 2016, 15, 76–86. [Google Scholar] [CrossRef]
- Wynn, J.K.; Jimenez, A.M.; Roach, B.J.; Korb, A.; Lee, J.; Horan, W.P.; Green, M.F. Impaired target detection in schizophrenia and the ventral attentional network: Findings from a joint event-related potential–functional MRI analysis. NeuroImage Clin. 2015, 9, 95–102. [Google Scholar] [CrossRef]
- Ahveninen, J.; Jaaskelainen, I.P.; Osipova, D.; Huttunen, M.O.; Ilmoniemi, R.J.; Kaprio, J.; Lonnqvist, J.; Manninen, M.; Pakarinen, S.; Therman, S.; et al. Inherited auditory-cortical dysfunction in twin pairs discordant for schizophrenia. Biol. Psychiatry 2006, 60, 612–620. [Google Scholar] [CrossRef]
- Düring, S.; Glenthøj, B.Y.; Oranje, B. Effects of blocking D2/D3 receptors on mismatch negativity and P3a amplitude of initially antipsychotic naïve, first episode schizophrenia patients. Int. J. Neuropsychopharmacol. 2016, 19, 109. [Google Scholar] [CrossRef]
- Santos, M.L.; San, J.R.L.M.; Arribas, J.I. A computer-aided diagnosis system with EEG based on the P3b wave during an auditory odd-ball task in schizophrenia. IEEE Trans. Biomed. Eng. 2016, 64, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Cecotti, H.; Kasper, R.W.; Elliott, J.C.; Eckstein, M.P.; Giesbrecht, B. Multimodal target detection using single trial evoked EEG responses in single and dual-tasks. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Hong Kong, China, 30 August–3 September 2011; pp. 6311–6314. [Google Scholar]
- Tanaka, E.; Inui, K.; Kida, T.; Miyazaki, T.; Takeshima, Y.; Kakigi, R. A transition from unimodal to multimodal activations in four sensory modalities in humans: An electrophysiological study. BMC Neurosci. 2008, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Jong, M.C.; Brascamp, J.W.; Kemner, C.; Ee, R.; Verstraten, F.A. Implicit perceptual memory modulates early visual processing of ambiguous images. J. Neurosci. 2014, 34, 9970–9981. [Google Scholar] [CrossRef] [PubMed]
- Tsalamlal, M.Y.; Rizer, W.; Martin, J.C.; Ammi, M.; Ziat, M. Affective communication through air jet stimulation: Evidence from event-related potentials. Int. J. Hum. Comput. Interact. 2018, 34, 1157–1168. [Google Scholar] [CrossRef]
- Jackson, G.M.; Swainson, R.; Cunnington, R.; Jackson, S.R. ERP correlates of executive control during repeated language switching. Bilingualism 2001, 4, 169. [Google Scholar]
- Giard, M.H.; Peronnet, F. Auditory-visual integration during multimodal object recognition in humans: A behavioral and electrophysiological study. J. Cogn. Neurosci. 1999, 11, 473–490. [Google Scholar] [CrossRef]
- Lebib, R.; Papo, D.; Bode, S.; Baudonnière, P.M. Evidence of a visual-to-auditory cross-modal sensory gating phenomenon as reflected by the human P50 event-related brain potential modulation. Neurosci. Lett. 2003, 341, 185–188. [Google Scholar]
- Boutros, N.N.; Trautner, P.; Rosburg, T.; Korzyukov, O.; Grunwald, T.; Schaller, C.; Kurthen, M. Sensory gating in the human hippocampal and rhinal regions. Clin. Neurophysiol. 2005, 116, 1967–1974. [Google Scholar] [CrossRef]
- Aine, C.J.; Bockholt, H.J.; Bustillo, J.R.; Cañive, J.M.; Caprihan, A.; Gasparovic, C.; Liu, J. Multimodal neuroimaging in schizophrenia: Description and dissemination. Neuroinformatics 2017, 15, 343–364. [Google Scholar]
- Gomez, G.C.M.; Clark, V.P.; Fan, S.; Luck, S.J.; Hillyard, S.A. Sources of attention-sensitive visual event-related potentials. Brain Topogr. 1994, 7, 41–51. [Google Scholar]
- Eimer, M.; Holmes, A. Event-related brain potential correlates of emotional face processing. Neuropsychologia 2007, 45, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Campanella, S.; Montedoro, C.; Streel, E.; Verbanck, P.; Rosier, V. Early visual components (P100, N170) are disrupted in chronic schizophrenic patients: An event-related potentials study. Neurophysiol. Clin. Clin. Neurophysiol. 2006, 36, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Jetha, M.K.; Zheng, X.; Goldberg, J.O.; Segalowitz, S.J.; Schmidt, L.A. Shyness and emotional face processing in schizophrenia: An ERP study. Biol. Psychol. 2013, 94, 562–574. [Google Scholar] [CrossRef]
- Stroganova, T.A.; Kozunov, V.V.; Posikera, I.N.; Galuta, I.A.; Gratchev, V.V.; Orekhova, E.V. Abnormal pre-attentive arousal in young children with autism spectrum disorder contributes to their atypical auditory behavior: An ERP study. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Thoma, P.; Bauser, D.S.; Norra, C.; Brüne, M.; Juckel, G.; Suchan, B. Do you see what I feel?–Electrophysiological correlates of emotional face and body perception in schizophrenia. Clin. Neurophysiol. 2014, 125, 1152–1163. [Google Scholar] [CrossRef]
- Hermens, D.F.; Ward, P.B.; Hodge, M.A.R.; Kaur, M.; Naismith, S.L.; Hickie, I.B. Impaired MMN/P3a complex in first-episode psychosis: Cognitive and psychosocial associations. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 822–829. [Google Scholar] [CrossRef]
- Atkinson, R.J.; Michie, P.T.; Schall, U. Duration mismatch negativity and P3a in first-episode psychosis and individuals at ultra-high risk of psychosis. Biol. Psychiatry 2012, 71, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Schultze, L.F.; Klosterkötter, J.; Michel, C.; Winkler, K.; Ruhrmann, S. Personality disorders and accentuations in at-risk persons with and without conversion to first-episode psychosis. Early Interv. Psychiatry 2012, 6, 389–398. [Google Scholar] [CrossRef]
- Delplanque, S.; Silvert, L.; Hot, P.; Sequeira, H. Event-related P3a and P3b in response to unpredictable emotional stimuli. Biol. Psychol. 2005, 68, 107–120. [Google Scholar] [CrossRef]
- Delplanque, S.; Silvert, L.; Hot, P.; Rigoulot, S.; Sequeira, H. Arousal and valence effects on event-related P3a and P3b during emotional categorization. Int. J. Psychophysiol. 2006, 60, 315–322. [Google Scholar] [CrossRef]
- Moran, Z.D.; Williams, T.J.; Bachman, P.; Nuechterlein, K.H.; Subotnik, K.L.; Yee, C.M. Spectral decomposition of P50 suppression in schizophrenia during concurrent visual processing. Schizophr. Res. 2012, 140, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Campanella, S.; Bruyer, R.; Froidbise, S.; Rossignol, M.; Joassin, F.; Kornreich, C.; Verbanck, P. Is two better than one? A cross-modal oddball paradigm reveals greater sensitivity of the P300 to emotional face-voice associations. Clin. Neurophysiol. 2010, 121, 1855–1862. [Google Scholar] [CrossRef] [PubMed]
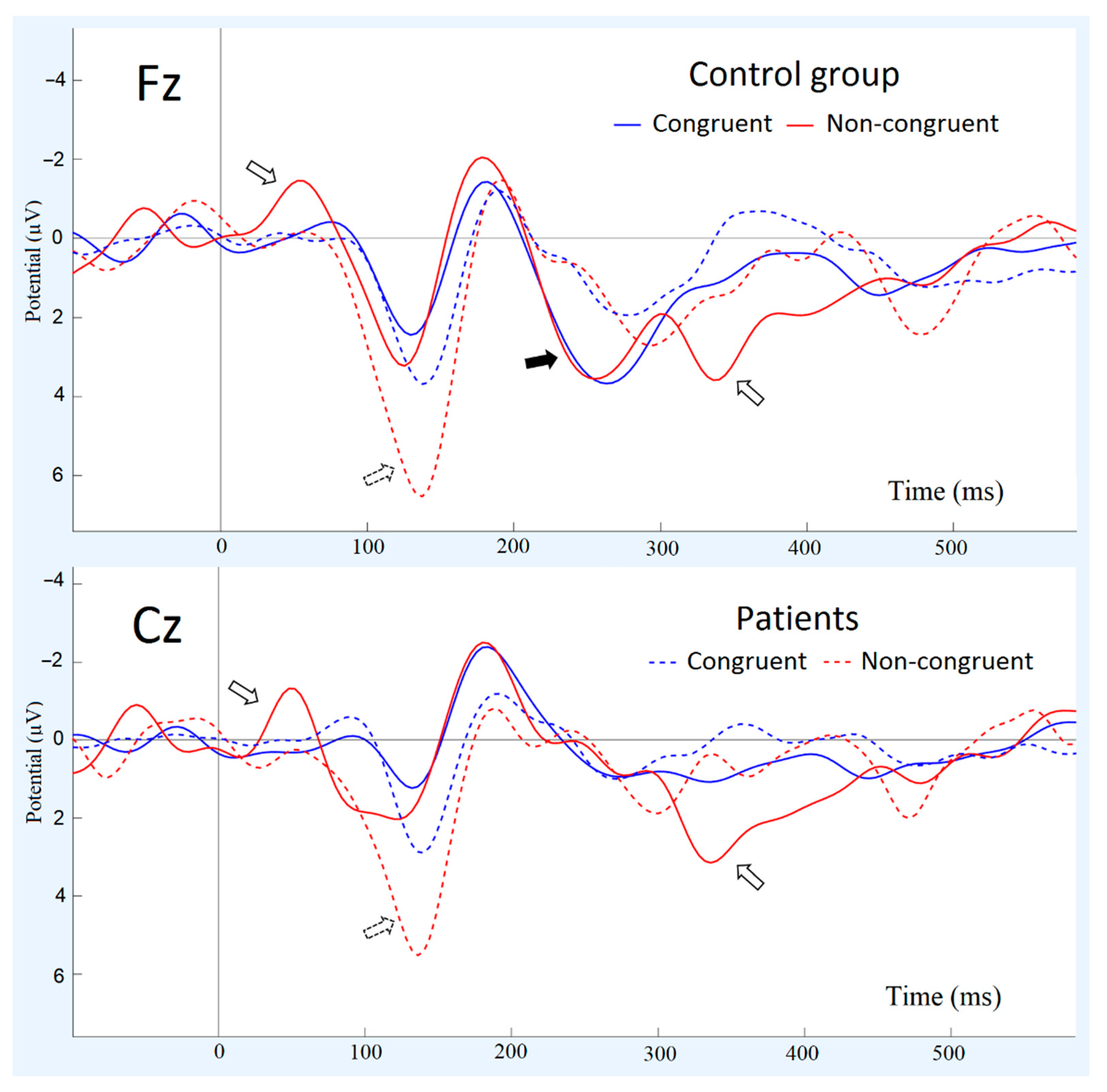
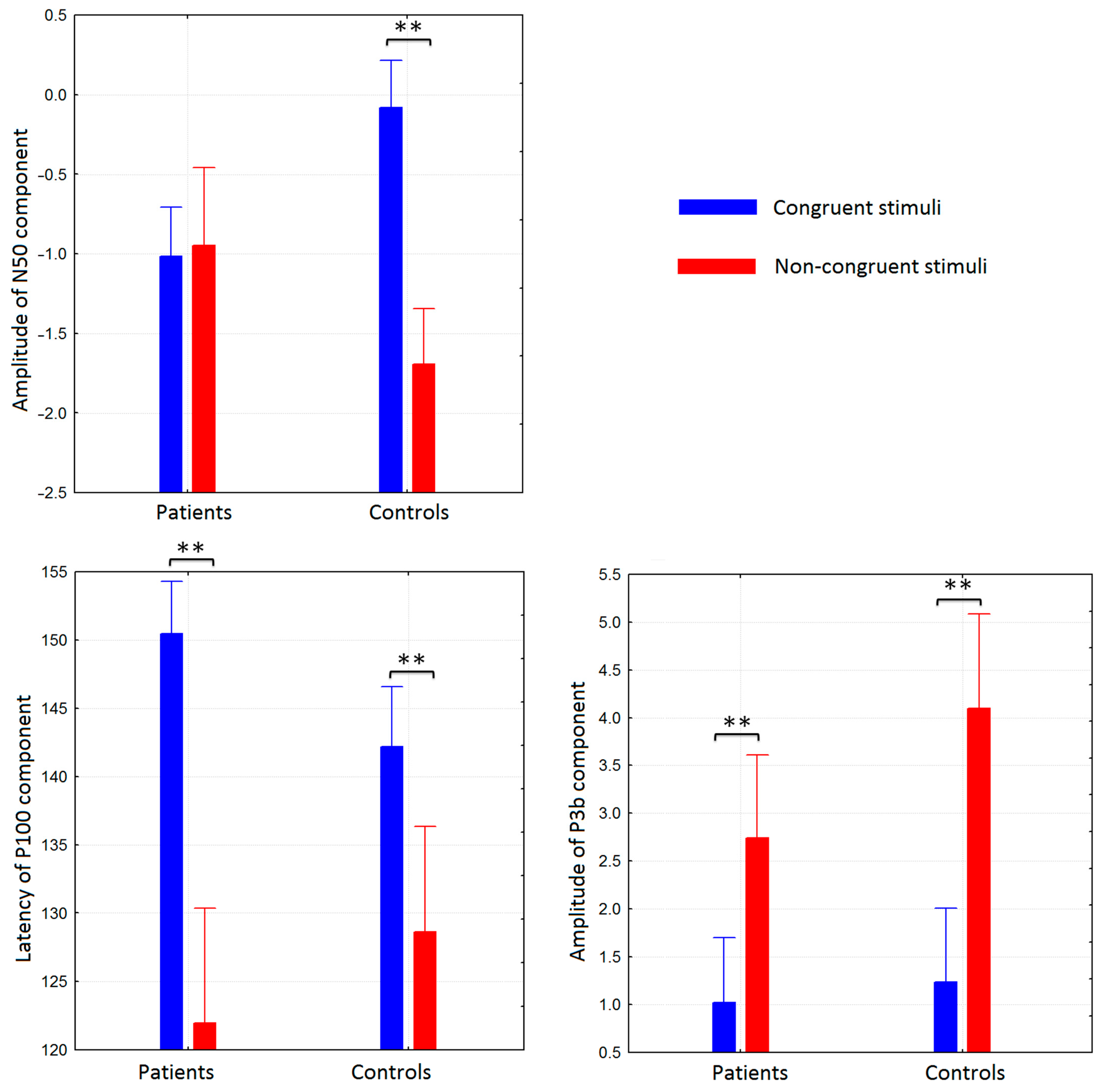
| N50 | P100 | N200 | P3a | P3b | ||
|---|---|---|---|---|---|---|
| Control group | ||||||
| Congruent stimuli | amplitude | −0.1 ± 0.4 | 1.9 ± 0.5 | −3.9 ± 0.7 | 2.7 ± 0.7 | 1.2 ± 0.5 |
| latency | 62 ± 9.7 | 142.2 ± 22 | 188.8 ± 31 | 266.0 ± 32 | 337.2 ± 35 | |
| non-congruent stimuli | amplitude | −1.6 ± 0.3 | 3.8 ± 0.6 | −4.3 ± 1.0 | 2.6 ± 0.6 | 4.1 ± 1.2 |
| latency | 49.6 ± 8.8 | 128.6 ± 29 | 200.2 ± 37 | 273.1 ± 44 | 312.9 ± 49 | |
| Patients with schizophrenia | ||||||
| Congruent stimuli | amplitude | −0.2 ± 0.5 | 4.6 ± 0.6 | −3.3 ± 0.4 | 0.7 ± 0.4 | 1.0 ± 0.7 |
| latency | 88.8 ± 12 | 152.5 ± 45 | 214.9 ± 39 | 274.8 ± 43 | 371.8 ± 58 | |
| non-congruent stimuli | amplitude | −0.2 ± 0.4 | 7.6 ± 1.3 | −2.8 ± 1.1 | 0.5 ± 1.0 | 2.7 ± 0.8 |
| latency | 66.2 ± 15 | 122.9 ± 27 | 207.1 ± 36 | 260.6 ± 45 | 356.1 ± 55 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Portnova, G.V.; Maslennikova, A.V.; Zakharova, N.V.; Martynova, O.V. The Deficit of Multimodal Perception of Congruent and Non-Congruent Fearful Expressions in Patients with Schizophrenia: The ERP Study. Brain Sci. 2021, 11, 96. https://doi.org/10.3390/brainsci11010096
Portnova GV, Maslennikova AV, Zakharova NV, Martynova OV. The Deficit of Multimodal Perception of Congruent and Non-Congruent Fearful Expressions in Patients with Schizophrenia: The ERP Study. Brain Sciences. 2021; 11(1):96. https://doi.org/10.3390/brainsci11010096
Chicago/Turabian StylePortnova, Galina V., Aleksandra V. Maslennikova, Natalya V. Zakharova, and Olga V. Martynova. 2021. "The Deficit of Multimodal Perception of Congruent and Non-Congruent Fearful Expressions in Patients with Schizophrenia: The ERP Study" Brain Sciences 11, no. 1: 96. https://doi.org/10.3390/brainsci11010096
APA StylePortnova, G. V., Maslennikova, A. V., Zakharova, N. V., & Martynova, O. V. (2021). The Deficit of Multimodal Perception of Congruent and Non-Congruent Fearful Expressions in Patients with Schizophrenia: The ERP Study. Brain Sciences, 11(1), 96. https://doi.org/10.3390/brainsci11010096




